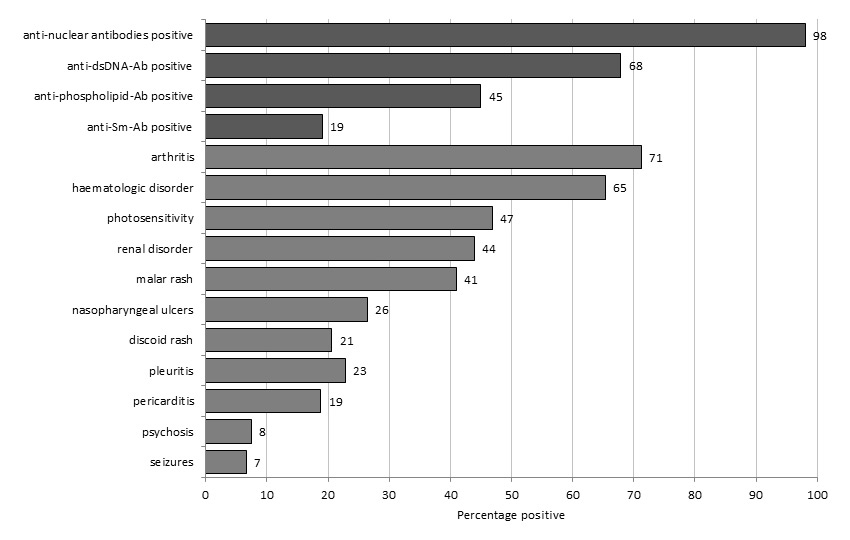
Figure 1
American College of Rheumatology Criteria fulfilled at inclusion in 255 patients with systemic lupus erythematosus.
DOI: https://doi.org/10.4414/smw.2014.13990
Systemic lupus erythematosus (SLE) is a chronic disease with a waxing and waning course, mainly affecting woman in their fertile years. Loss of tolerance to a variety of auto-antigens including, but not exclusively, double stranded DNA and nucleosomes is characteristic of SLE and reflects defective T and B cell threshold of activation. Cells of the innate immune system and their receptors are also thought to play a major role in disease initiation and flares. Environmental cues interacting with a predisposed genetic background are considered fundamental for setting in motion the immunological alterations that will result in overt disease.
The estimated incidence of the disease in western European countries ranges from 2.2 to 5 / 100,000 inhabitants and the prevalence from 20.5 to 91 /100,000 [1]. Clinical presentation may involve tissues and organs as diverse as the skin, the kidneys, the central and peripheral nervous system, the haematological system and the joints. Thus, patients with SLE are taken care of by different medical specialities. The care of SLE patients by various medical disciplines contributes to major differences in assessing disease activity, damage and treatment. The Swiss SLE Cohort Study (SSCS) aims at identifying the clinical characteristics of SLE patients followed in Switzerland, their disease burden, and treatment. We report here the results from a cross-sectional study of the first 255 patients with definite SLE included in this cohort, focusing on the differences in the prescription of SLE-specific drugs between medical disciplines and their impact on global disease activity.
A cross-sectional collection of data was performed between April 2007 and March 2012 in the Swiss SLE Cohort Study (SSCS) [2]. We prospectively included 255 adult patients with prevalent SLE defined according to the American College of Rheumatology (ACR) criteria [3, 4]. The cohort study was approved by the ethics review boards of all participating institutions and all patients gave their written informed consent. Patients included originated from Clinical Immunology, Internal Medicine, Nephrology, and Rheumatology tertiary centres located both in the French and German-speaking regions of Switzerland.
Data on patient’s age, sex, ethnicity and family history of SLE, dates of first lupus manifestation and diagnosis, clinical and biological characteristics at baseline, disease activity, laboratory parameters, treatment modalities and co-morbidity were collected. SLE manifestations were defined using the 1982 ACR classification criteria, which were updated in 1997 [3, 4]. Disease activity was assessed by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score with the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) modification [5]. Laboratory parameters were assessed by the individual centres. For the purpose of the SELENA-SLEDAI, positivity for anti-dsDNA antibodies and reduced complement levels were defined independently of the methodology used by a test result above and below normal range, respectively. Furthermore, we used a Physician’s Global Assessment (PGA) score with a 4–point-scale of disease activity, ranging from 0 (inactive disease) to 3 (very active disease). Both scores were used with a 30–day window [6]. Medication was detailed for disease-modifying drugs (DMARD's) taken. DMARD's were classified in three groups: systemic corticosteroids, anti-malarial drugs and immunosuppressive agents. Regular use of these medications was defined by prescription at study visit, during the preceding 4 weeks and previously. The anonymous clinical data were stored in an online database (https://www.slec.ch).
Our primary outcome was the assessment of global disease activity by the SELENA-SLEDAI score at study visit. Secondary outcomes were the 4–point PGA scale and the erythrocyte sedimentation rate (ESR) at study visit. Variables for SLE disease activity were dichotomised as follows: SELENA-SLEDAI <4 (inactive) and >= 4 (active), in analogy to what has been published for the SLEDAI-2000 score, (7) PGA <1 (inactive) and >= 1 (moderately active to very active), and ESR <20 mm/1st hour and >= 20 mm/1st hour.
Quantitative variables were expressed as the mean ± standard deviation (SD). We compared quantitative variables by presence/absence of the outcome using Student’s t-test or nonparametric tests depending on the application criteria. We also compared the mean values of activity indices by DMARD use. Categorical variables were compared by outcome and also by the type of DMARD's using the chi-square test or, when appropriate, Fisher’s exact test. We provided Pearson’s correlation coefficients between the SLEDAI and PGA scores, SLEDAI score and ESR and also between PGA and ESR.
We assessed the association between the use of specific drugs and the activity of the disease, using SELENA-SLEDAI, PGA and ESR. We first created propensity scores for the three different treatments in order to adjust on potential confounders for the use of anti-malarials, corticosteroids and immunosuppressive agents [8]. We used the same confounders for the three propensity scores: age, disease duration, gender, disease characteristics as defined by ACR Criteria (renal disease, neuropsychiatric SLE, haematologic involvement, arthritis, serositis and photosensitivity), and renal insufficiency defined by serum creatinine levels above 100 umol/l.
As the cohort included patients from 8 different Swiss hospitals, we expected some variability between centres. Thus, we used a mixed effect logistic regression model with a random effect on the centre in order to assess the association between disease activity and DMARD type used after adjustment on the relative propensity score categorised following their quantiles. p-values <0.05 (two-sided) were judged significant. Statistical analysis was performed using STATA Version 12.0 software (StataCorp LP, College Station, TX).
The 255 prospectively included patients with prevalent SLE had their first study visit between April 11th, 2007 and March 21st, 2012. Patient’s baseline characteristics are shown in table 1. ACR criteria present prior to or at inclusion are shown in figure 1. Renal disease, as defined by the ACR criteria, was present in 112 (44%) of patients, of which 4 were on dialysis and 2 had received renal transplants. A total of 29 (11%) patients had either psychosis or seizures as a neuropsychiatric SLE manifestation. The median SELENA-SLEDAI score at the time of visit was 4 (IQR 2–10). It scored less than 4 points in 103 (40%) patients. According to PGA, SLE was recorded as “inactive” in 128 (51%) of patients. Correlation between SELENA-SLEDAI and PGA was high (r = 0.5, p <0.001). ESR at visit was measured in 202 patients, with a median value of 14 (IQR 6–30) millimetres in the first hour (mm/1st hr). There was moderate correlation between ESR and SELENA-SLEDAI (r = 0.22, p = 0.02) and between ESR and PGA (r = 0.34, p <0.001). No significant correlation was found between disease duration and SELENA-SLEDAI or ESR. Complement levels at the time of visit were low in 86/219 (39%) and anti-dsDNA antibodies present in 97/217 (45%) of the patients.

Figure 1
American College of Rheumatology Criteria fulfilled at inclusion in 255 patients with systemic lupus erythematosus.
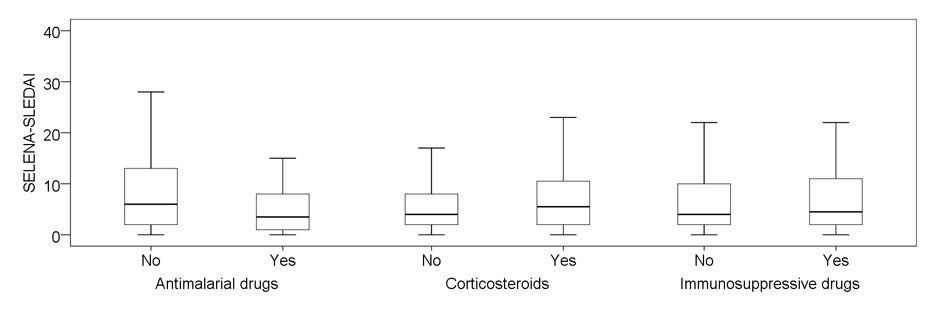
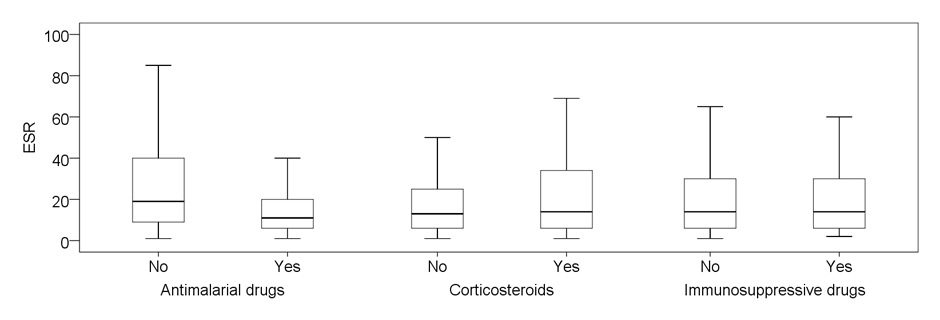
Figure 2
Disease activity in relation to treatment preceding visit (past month and before).
A: SELENA-SLEDAI score at study visit. Differences are statistically significant for antimalarial drugs (p <0.001) and corticosteroids (p = 0.024).
B: Erythrocyte sedimentation rate at study visit, in millimeter 1st hour. Differences are statistically significant for antimalarial drugs (p = 0.002)
SLE care was provided by internists and immunologists in 130 (51%), rheumatologists in 90 (35%) and by nephrologists in 35 (14%) of the cases. Patient’s characteristics by contributing medical specialty are shown in table 2. Age at diagnosis was a median of 31.6 (inter-quartile range (IQR) 23.3–44.2) years for patients from Internal Medicine and Immunology, 24.4 (IQR 21–38.4) years for patients from Nephrology and 39.9 (IQR 28.4–51.2) years for patients from Rheumatology (p = 0.001). First manifestations of SLE had occurred at a median of 30 (IQR 20.9–41.1) years in patients from Internal Medicine and Immunology, 23.4 (IQR 19.1–38.3) years from Nephrology and 39.3 (IQR 25.5–51.7) years in patients from Rheumatology (p = 0.003). Median time from first SLE manifestations to diagnosis was 3 (IQR 0–12) months and did not differ between disciplines.
Disease manifestations are compared to other SLE cohorts in table 3.
Disease-specific treatment is shown in table 4. Of the 244 patients in whom SLE medication was detailed at the time of study visit and previously, 43 (17.6%) were on anti-malarial drugs (AM) alone, 40 (16.4%) on AM, systemic corticosteroids (CS) and immunosuppressive agents (IS) in association, 35 (14.4%) on AM and CS, 21 (8.6%) on CS alone, 20 (8.2%) on CS and IS, 12 (4.9%) on IS and AM, and 4 (1.6%) on IS alone. A total of 62 (25.4%) patients had either stopped SLE specific medication previous to the visit or had been newly treated, and 7 (2.8%) had never been treated for SLE. Anti-coagulants and platelet inhibitors were taken by 44.7% of patients (acetylsalicylic acid in 25.2%, coumarine derivatives in 18.3%, clopidogrel in 2.8% and low molecular weight heparin in 2.4%). The use of these agents was twice as frequent in the 112 patients with anti-phospholipid antibodies.
Regular use of AM was noted in 130 (53%) patients, with prescription rates ranging from 68 (76%) patients in the care of a rheumatologist to 61 (48%) patients in the care of internists and immunologists, and 1 (3%) patient in the care of nephrologists (p <0.001). Of the 126 patients in whom the type of AM was known, 120 (95%) had hydroxychloroquine and 6 (5%) chloroquine.
IS were regularly taken by 43 (48%) patients from Rheumatology, 29 (23%) patients from Internal Medicine and Immunology and 6 (18%) patients from Nephrology (p <0.001). The choice of IS was similar across specialties with the exception of more frequent use of azathioprine (AZA) and reciprocal lesser use of mycophenolate mofetil (MMF) by rheumatologists, when compared to other specialties (49% AZA and 28% MMF for Rheumatology vs. 28% AZA and 55% MMF for Internal Medicine and Immunology vs. 17% AZA and 83% MMF for Nephrology). CS were regularly taken by 44 (49%) patients from Rheumatology, 65 (52%) patients from Internal Medicine and Immunology and 7 (24%) patients from Nephrology. The ratio of patients treated with IS and AM to IS without AM was 4:1 in Rheumatology and 2:1 in Internal Medicine and Immunology. In Nephrology, no patient had a combination of IS and AM.
The association of regular use of AM, CS and IS to disease activity is shown in figure 2. Patients regularly using AM had lower disease activity scores: median SELENA-SLEDAI score of 3.5 (IQR 1–8) versus 6 (IQR 2–13) (p <0.001) and median PGA score of 0 versus 1 (p = 0.09) in those without AM. The association of AM with lower disease activity was particularly evident in patients with no additional IS or CS: SELENA-SLEDAI <4 in 26/43 (61%) of patients with AM versus 25/69 (36%) without AM (p = 0.012).
In contrast, patients regularly receiving CS had a higher disease activity: median SELENA-SLEDAI score of 5.5 (IQR 2–10.75) versus 4 (IQR–2–8 (p = 0.024) and median PGA 1 versus 0 (p <0.001) in those without CS. For regular use of IS, no significant change in global disease activity was found compared to those not receiving IS: median SELENA-SLEDAI score of 4 (IQR 2–10.5) versus 4 (IQR 2–9.75). When investigated for the type of IS, patients under continuous treatment with MMF had a higher disease activity (median SLEDAI 8 versus 4; p = 0.006) than those treated with other drugs. These differences were not seen for patients treated with AZA.
When assessing patients treated with a combination of CS and IS, those with additional AM also had less active disease than those without AM: SELENA-SLEDAI <4 in 15/40 (38%) with AM compared to 2/20 (10%) without AM (p = 0.034).
Median ESR at the study visit was 11 (IQR 6–20) mm/1st hr in patients regularly taking AM, compared to 19.5 (IQR 9–41.5) in those without this treatment (p = 0.002). On the other hand, patients regularly treated with CS or IS had no significant differences in ESR values compared with those without these treatments.
In order to avoid bias due to the comparison of disease activity in patients treated with anti-malarial drugs to a non-randomised control group and also to centre- or discipline-specific differences in the prescription of AM (e.g. patients with more severe SLE being prone to higher disease activity and also more susceptible to be treated in centres and by specialists with lower prescription rates for AM), we generated a propensity score to adjust the drug use to potential confounders and secondarily performed a mixed-effect logistic regression model with a random effect on the centre. Using the SELENA-SLEDAI as the dependent variable in this model, the random effect on the centre was significant (p = 0.0016). For AM use, the odds ratio to have a SELENA-SLEDAI value >= 4 was 0.49 (0.26–0.95 confidence interval (CI); p = 0.034). Using the ESR as dependent variable in this model, the random effect on the centre was not significant (p = 0.99). For AM use, the odds ratio to have an ESR value ≥20 mm/1st hour was 0.37 (0.19–0.73 CI; p = 0.004).
| Table 1: Disease characteristics at baseline in 255 patients with systemic lupus erythematosus (SLE). | |
| Sex, female/male (%) | 210/45 (82/18) |
| Ethnicity | |
| Caucasian, no. (%) | 210 (82.4) |
| African, no. (%) | 9 (3.5) |
| Asian, no. (%) | 19 (7.5) |
| Other, no. (%) | 6 (2.4) |
| Unknown, no. (%) | 11 (4.3) |
| First-degree relatives with SLE, no. (%) | 25 (12.9)a |
| Age at SLE diagnosis, mean ± SD (range) years | 36.4 ± 15.5 (12–87) |
| Age at first SLE manifestation, mean ± SD (range) years | 34.5 ± 16.0 (9–86) |
| Body mass index at time of visit, mean ± SD (range) | 25.4 ± 5.1 (16.9–41.0) |
| Active smoker at time of visit, N (%) | 44 (22.3)b |
| Disease activity at the time of visit and past 4 weeks | |
| SELENA-SLEDAI, mean ± SD (range) | 7.1 ± 8.0 (0–42) |
| Physician's global assessment, mean ± SD (range) | 0.7 ± 0.8 (0–3) |
| Biological values at the time of visit | |
| Erythrocyte sedimentation rate, mean ± SD (range), mm/1st hr | 22.8 ± 24.2 (1–150) |
| Plasma creatinine, mean ± SD (range), μmol/l | 87 ± 69.4 (36–716) |
| SD = Standard Deviation, SELENA-SLEDAI = Systemic Lupus Erythematosus Disease Activity Index score with the Safety of Estrogens in Lupus Erythematosus National Assessment modification a in 194 patients; b in 197 patients | |
| Table 2:Disease characteristics by medical specialties in 255 patients with systemic lupus erythematosus (SLE). | |||||||
| Medical specialty | n | Age at inclusion, mean ± SD (range), years | SLE duration, median (IQR), years | n (%) with disease onset after 50 years | ACR Criteria fulfilled, median (IQR) | n (%) with renal disease | SELENA-SLEDAI, median (IQR) |
| Clinical Immunology & Internal Medicinea | 130 | 43.5 ± 14.5 (18–85) | 5.3 (1.2–12.5) | 21/125 (17) | 5 (5–6) | 62 (48) | 6 (2–11.25) |
| Nephrologyb | 35 | 39.6 ± 12.3 (21–68) | 10.5 (4–14.2) | 3/35 (9) | 6 (5–8) | 29 (83) | 6 (2–13) |
| Rheumatologyc | 90 | 48.3 ± 14.4 (20–89) | 3.5 (2–10) | 17/67 (25) | 4 (4–5) | 21 (23) | 2 (0–6) |
| Total | 255 | 44.8 ± 14.4 (18–89) | 5.2 (1.7–12.8) | 41/227 (18) | 5 (4–6) | 112 (44) | 4 (2–10) |
| a Immunology and Allergy Geneva and Sion (N = 65), Internal Medicine Basel (N = 51), Immunology and Allergy Lausanne (N = 14). b Nephrology Bern (N = 21), Nephrology Zurich (N = 14). c Rheumatology Sankt Gallen (N = 78), Rheumatology Schaffhausen (N = 12). The number of subjects at each center corresponds to the number of patients with full dataset at the time of evaluation and at least 4 components of the American College of Rheumatology (ACR) diagnostic criteria for Systemic Lupus Erythematosus (SLE). SD = Standard Deviation; SELENA-SLEDAI = Systemic Lupus Erythematosus Disease Activity Index with the Safety of Estrogens in Lupus Erythematosus National Assessment modification | |||||||
| Table 3:Disease characteristics at baseline in comparison with other cohorts. | |||||
| Authors | Petri et al. [31] | Wang et al. [32] | Alarcon et al. [33] | Cervera, et al. [10] | Present study |
| Number of subjects | 574 | 539 | 555 | 1000 | 255 |
| Region | USA | Asia | USA | Europe | Switzerland |
| Malar rash, no. present (%) | 331 (58) | 410 (76) | 322 (58) | 579 (58) | 104 (41) |
| Discoid lesions, no. present (%) | 162 (28) | 30 (6) | 107 (19) | 104 (10) | 52 (20) |
| Photosensitivity, no. present (%) | 335 (58) | 222 (41) | 334 (60) | 453 (45) | 119 (47) |
| Oral ulcers, no. present (%) | 219 (38) | 185 (34) | 293 (53) | 238 (24) | 67 (26) |
| Arthritis, no. present (%) | NR | 272 (51) | 489 (88) | 840 (84) | 181 (71) |
| Nephropathy, no. present (%) | 319 (56) | 399 (74) | 223 (40) | 393 (39) | 112 (44) |
| Serositis, no. present (%) | NR | NR | NR | 364 (36) | 74 (29) |
| Neurologic involvement, no. present (%) | NR | 123 (23) | 67 (12) | 268 (27) | 29 (11)a |
| ANA positive, no. positive (%) | NR | NR | NR | 963 (96) | 250 (98) |
| Anti-dsDNA antibodies, no. positive (%) | NR | NR | NR | 779 (78) | 173 (68) |
| Anti-phospholipid antibodies, no. positive (%) | NR | NR | NR | 112 (44) | |
| Anti-Sm antibodies, no. positive (%) | NR | NR | NR | 105 (10) | 47 (18) |
| a based on history of psychosis or epilepsy related to SLE ANA = Anti-nuclear antibodies, dsDNA = doubles-stranded DNA, NR = not reported | |||||
| Table 4: Treatment in 255 patients with systemic lupus erythematosus. | ||
| Medication | Used during past month and before | |
| Systemic corticosteroids, no. (%) | 116/244 (48) | |
| Oral daily prednisone equivalent at study visit, mean ± SD (range), mg | 12.3 ± 11.2 (2–60) | |
| Parenteral corticosteroids in the preceding month, no. (%) | 3 (3) | |
| Antimalarial drugs, N (%) | 130/247 (53) | |
| Hydroxychloroquine, no. (%) | 120 (95) | |
| Chloroquine, no. (%) | 6 (5) | |
| Immunosuppressants, no. (%) | 78/252a (31) | |
| Mycophenolate, no.b | 33 | |
| Azathioprine, no. | 29 | |
| Methotrexate, no. | 9 | |
| Cyclophosphamide, no. | 3 | |
| Rituximab, no. | 1 | |
| Other, no. | 4 | |
| Non-steroidal anti-rheumatic drugs on a daily basis, no. (%) | 40/231 (16.6) | |
| a one patient had more than one immunosuppressive drugb includes mycophenolic acid SD = Standard deviation | ||
The main objectives of this study were to characterise patients included in the Swiss SLE cohort and to assess differences in SLE management among medical specialties. Our SLE cohort is the first multidisciplinary one in Switzerland. The subjects originated from eight different centres. All the investigators were based at tertiary devoted academic centres. Physicians involved were internists and/or clinical immunologists in 51%, rheumatologists in 35% and nephrologists in 14% of the cases. The largest European cohort so far is the Euro-Lupus cohort with 1000 patients, whose baseline characteristics are here used as reference [9]. A total of 86% of our patients were of European ancestry, compared with 97% in the Euro-lupus cohort. Additionally, 82% of our patients were women, in accordance with the female preponderance reported by others. With a mean age of 45 years at inclusion, patients were an average of 7 years older than in the Euro-lupus cohort, but very similar to the mean age of 44.3 years reported in the CaNIOS/1000 faces of SLE cohort [10]. In SSCS we did not include paediatric patients, and in addition 41/227 (18%) of our patients developed SLE after the age of 50 years, compared to 9% in the Euro-lupus cohort, 10.5% in the CaNIOS cohort, and up to 39.3% in Lugo, Spain [11]. Whether the larger proportion of late-onset SLE in our cohort reflects changes with time in SLE expression in Europe remains to be established. We also found a significant difference in age at disease onset depending on the medical specialty involved. In patients included by nephrologists, first manifestations of SLE had occurred on average 15 years earlier than in patients from Rheumatology. Also, renal involvement was reported to be 83% in patients from Nephrology, compared to 23% of patients from Rheumatology. This is in accordance with the observation that in SLE, nephritis affects mostly young individuals and occurs early during the disease course [12]. Consistently with previous reports, the female predominance in this group of elderly SLE patients was less pronounced, with a female/male ratio of 2/1 [13]. The prevalence of most disease characteristics matched with other cohorts, in particular the proportion of patients suffering from photosensitivity, oral ulcers, nephropathy, arthritis, serositis and haematological involvement. Regarding serological findings, 98% of patients had antinuclear antibodies (ANA), 68% had antibodies to double-stranded DNA and 19% anti-Sm antibodies. These proportions of auto-antibodies are also consistent with those found in the Euro-lupus cohort. Positivity of anti-phospholipid antibodies defined by either antibodies to cardiolipin or beta2–glycoprotein or presence of a lupus anticoagulant prior or at the time of study visit, was found in 45% of our patients. Thus, aside from the older age at inclusion, patient characteristics in our cohort are comparable to those in the Euro-lupus cohort. Although SLE duration in our cohort was highly variable, we were able to capture several patients with active disease after many years of evolution. SELENA-SLEDAI and ESR values were not correlated with disease duration, thus reinforcing the concept that SLE has not the tendency to wane with time.
We focused on the SLE-specific treatment. Overall, 53% of patients in our study were taking anti-malarial drugs on a regular basis, with hydroxychloroquine (HCQ) being prescribed in 95% of cases. This proportion is comparable to major SLE trials, in which 50% of patients were treated with anti-malarial drugs [14]. Of interest, HCQ prescription varied significantly between centres and medical specialties. In particular, 76% of patients in the care of rheumatologists were taking anti-malarial drugs, compared to 3% in the care of nephrologists. Others have reported important differences in anti-malarial drug prescription rates, which are particularly high in patients treated by rheumatologists and low in non-rheumatologist disciplines [15–18]. It could be argued that patients in the care of rheumatologists had less severe SLE and were more likely to be treated with anti-malarial drugs, thus introducing an indication bias. However, anti-malarials were evenly prescribed in individuals taking or not immunosuppressive agents and/or systemic corticosteroids. While anti-malarials appeared particularly associated with low disease activity in patients without other treatments, there was still an association with lower SELENA-SLEDAI values when combined with immunosuppressive agents and systemic corticosteroids. The disparity of anti-malarial drug prescription may be explained by the fact that rheumatologists were introduced earlier to these agents and that their guidelines recommend to consider them for all SLE patients [19, 20]. Indeed, anti-malarial drugs have been traditionally regarded as indicated in mild SLE with skin and joint involvement [21] and as ineffective in more severe disease. However, newer data suggest that all SLE patients may benefit from early and long-lasting treatment with anti-malarial drugs [22].
The present study found that the regular use of HCQ was associated with lower disease activity, as expressed by SELENA-SLEDAI values below 4 and an ESR below 20 mm/1st hour. This contrasted with regular use of systemic corticosteroids, which was associated with more active SLE. Although an elevated ESR is not specific for active SLE, this simple and inexpensive laboratory parameter has a very good sensitivity [23]. Furthermore, ESR has been shown to be significantly associated with SLE flares in the subsequent year [24]. The SELENA-SLEDAI score on the other hand depends on the training and the experience of the assessing physician. Putting a random effect on the centre in the regression model assessing the SELENA-SLEDAI had a statistically significant effect, suggesting that there are major differences in this variable between centres arising either from utilisation of this tool or SLE severity. However after adjustment there was still a tendency to lower SELENA-SLEDAI values in patients treated with anti-malarial drugs, possibly pointing to a therapeutic effect. PGA values were also lower in patients treated with anti-malarial drugs, although the association was not significant. We used a 4–point Likert-like scale for PGA and it is likely that this tool was not sensitive enough to assess lower disease activity. Other limitations of our study include the lack of precise information on the duration of corticosteroids and immunosuppressive treatment received since SLE diagnosis as well as differences in the methodologies used to assess immunologic parameters, which was done by individual centres. Of interest, in our cohort the use of immunosuppressant agents was not associated with lower disease activity. While at first sight this may be surprising, it reflects the experience of other centres in which the use of immunosuppressant agents was independently associated with chronically active or relapsing remitting disease [25]. In particular, higher disease activity was recorded specifically among individuals taking MMF, which may reflect the preferential use of this compound in most severe cases, for instance in individuals with renal disease, as reflected by higher frequency of MMF use among nephrologists.
Others have shown that HCQ is associated with lower SLE activity. A Canadian randomised placebo-controlled study of 47 SLE patients receiving HCQ showed an increased risk of clinical flares of 2.5 (95%-CI 1.08–5.58) in the 6 months after HCQ discontinuation [26]. In patients treated with mycophenolate mofetil for membranous lupus nephritis, HCQ was an independent predictor of complete renal remission, with a remission rate 5.2 times higher than those not receiving HCQ (95% CI 1.2–22.2) [27]. Besides controlling disease activity, HCQ may have several other beneficial effects in SLE patients, such as prevention of cardiovascular events [28], thrombotic events [29], diabetes [30] decreased bone loss due to corticosteroids [31], and reducing the risk of malignancy [32]. Thus, HCQ reduces the risk of overall damage accrual [33], and appears to increase survival in SLE patients [34, 35]. Most SLE patients are women in child-bearing age and for them, HCQ is of particular interest in pregnancy and lactation, as it is considered safe and prevents SLE flares during this critical period [22]. It is therefore important to consider the use of anti-malarials to improve the treat-to-target strategy in the context of SLE [36].
In summary, our study has documented substantial differences in the rate of prescription of anti-malarials for SLE across medical disciplines in Switzerland and provides circumstantial evidence that anti-malarials are associated with lower SLE disease activity. A longitudinal study is planned to confirm the impact of HCQ treatment on SLE activity and damage accrual in our cohort population.
SLE patients with renal involvement have an earlier disease onset.
Anti-malarial drugs for SLE are infrequently used by non-rheumatologists.
SLE disease activity is lower in patients treated with anti-malarial drugs.
1 D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369(9561):587–96.
2 Chizzolini C, Cohen CD, Eisenberger U, Hauser T, Hunziker T, Leimgruber A, et al. Towards the Swiss systemic lupus erythematosus cohort study (SSCS). Rev Med Suisse. 2009;5(199):808–11.
3 Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
4 Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7.
5 Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8.
6 Touma Z, Urowitz MB, Ibanez D, Gladman DD. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus. 2011;20(1):67–70.
7 Yee CS, Farewell VT, Isenberg DA, Griffiths B, Teh LS, Bruce IN, et al. The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford).50(5):982–8.
8 D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81.
9 Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore). 1993;72(2):113–24.
10 Lalani S, Pope J, de Leon F, Peschken C, Members of Ca NFoL. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37(1):38–44.
11 Alonso MD, Martinez-Vazquez F, de Teran TD, Miranda-Filloy JA, Dierssen T, Blanco R, et al. Late-onset systemic lupus erythematosus in Northwestern Spain: differences with early-onset systemic lupus erythematosus and literature review. Lupus. 2012;21(10):1135–48.
12 Seligman VA, Lum RF, Olson JL, Li H, Criswell LA. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med. 2002;112(9):726–9.
13 Cervera R, Abarca-Costalago M, Abramovicz D, Allegri F, Annunziata P, Aydintug AO, et al. Systemic lupus erythematosus in Europe at the change of the millennium: lessons from the “Euro-Lupus Project”. Autoimmun Rev. 2006;5(3):180–6.
14 Costedoat-Chalumeau N, Leroux G, Piette JC, Amoura Z. Why all systemic lupus erythematosus patients should be given hydroxychloroquine treatment? Joint Bone Spine. 2010;77(1):4–5.
15 Lerang K, Gilboe IM, Gran JT. Differences between rheumatologists and other internists regarding diagnosis and treatment of systemic lupus erythematosus. Rheumatology (Oxford). 51(4):663–9.
16 Schmajuk G, Yazdany J, Trupin L, Yelin E. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 62(3):386–92.
17 Molina MJ, Mayor AM, Franco AE, Morell CA, Lopez MA, Vila LM. Utilization of health services and prescription patterns among lupus patients followed by primary care physicians and rheumatologists in Puerto Rico. Ethn Dis. 2008;18(2 Suppl 2):S2–205–10.
18 Yazdany J, Gillis JZ, Trupin L, Katz P, Panopalis P, Criswell LA, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthritis Rheum. 2007;57(4):593–600.
19 Scherbel AL. Use of synthetic antimalarial drugs and other agents for rheumatoid arthritis: historic and therapeutic perspectives. Am J Med. 1983;75(1A):1–4.
20 Wallace DJ. The history of antimalarials. Lupus. 1996;5(Suppl 1):S2–3.
21 D’Cruz D. Antimalarial therapy: a panacea for mild lupus? Lupus. 2001;10(3):148–51.
22 Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol.7(12):718–29.
23 Vila LM, Alarcon GS, McGwin G, Jr., Bastian HM, Fessler BJ, Reveille JD. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXIX. Elevation of erythrocyte sedimentation rate is associated with disease activity and damage accrual. J Rheumatol. 2005;32(11):2150–5.
24 Mirzayan MJ, Schmidt RE, Witte T. Prognostic parameters for flare in systemic lupus erythematosus. Rheumatology. 2000;39(12):1316–9.
25 Zen M, Bassi N, Nalotto L, Canova M, Bettio S, Gatto M, et al. Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study. Clin Exp Rheumatol. 2012;30(6):856–63.
26 A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991;324(3):150–4.
27 Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus. 2006;15(6):366–70.
28 Munro R, Morrison E, McDonald AG, Hunter JA, Madhok R, Capell HA. Effect of disease modifying agents on the lipid profiles of patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56(6):374–7.
29 Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68(2):238–41.
30 Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187–93.
31 Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus. 2005;14(2):106–12.
32 Ruiz-Irastorza G, Ugarte A, Egurbide MV, Garmendia M, Pijoan JI, Martinez-Berriotxoa A, et al. Antimalarials may influence the risk of malignancy in systemic lupus erythematosus. Ann Rheum Dis. 2007;66(6):815–7.
33 Fessler BJ, Alarcon GS, McGwin G, Jr., Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52(5):1473–80.
34 Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007;66(9):1168–72.
35 Ruiz-Irastorza G, Egurbide MV, Pijoan JI, Garmendia M, Villar I, Martinez-Berriotxoa A, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–83.
36 Doria A, Gatto M, Zen M, Iaccarino L, Punzi L. Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmunity reviews. 2014.
Funding / potential competing interests: This work was supported by the Gebert-Rüf Foundation (grant number GRS-027/07). None of the authors have conflict of interest, financial disclosures, and financial interests to declare related to the present manuscript.