Efficacy of anti-fungal but not anti-bacterial prophylaxis in intensive primary AML therapy: A real-world, retrospective comparative single-centre study
DOI: https://doi.org/10.4414/smw.2014.13985
Bernhard
Gerber, Jan
Köppel, Michaela
Paul, Thi Dan Linh
Nguyen-Kim, Thomas
Frauenfelder, Gayathri
Nair, Urs
Schanz, Markus G.
Manz
Summary
QUESTIONS UNDER STUDY: The optimal strategy of anti-infectious prophylaxis in patients with acute leukaemia undergoing intensive chemotherapy remains a matter of debate. We assessed the impact of primary prophylaxis with posaconazole and levofloxacin on the incidence of invasive fungal infections (IFI) and bacteraemia.
METHODS: A retrospective single-centre study including two groups of adult patients with AML receiving intensive chemotherapy. Group one without anti-infective prophylaxis (September 2008 – February 2010), and group two with anti-infective prophyalaxis (March 2010 – April 2011). The primary end-point was IFI according to the EORTC/MSG 2008 definitions and bacteraemia.
RESULTS: Baseline characteristics were similar in the non-prophylaxis (n = 43 patients; 99 chemotherapy cycles) and the prophylaxis (n = 45; 104 chemotherapy cycles) group. IFI were significantly reduced in the prophylaxis group (55.3% vs. 88.9%; p = 0.0032) and there was a trend of the projected IFI-free survival at 100 days to be increased (50.1% vs. 25%; p = 0.0526). One-hundred day overall survival (84.4% and 88.4%, p = 0.35) and 2-year overall survival (64.4% and 58.1%; p = 0.64) were unaffected. No difference in the occurrence of bacteraemia was observed (32.3% vs. 34.6%; p = 0.8). A total of two (3.6%) patients in the non-prophylaxis and three (6.7%) in the prophylaxis group died due to IFI, and two (3.6%) in the non-prophylaxis and none in the prophylaxis group patients had to stop leukaemia treatment due to IFI.
CONCLUSIONS: The anti-infective prophylaxis with posaconazole and levofloxacin resulted in a significant reduction of ‘possible’ IFI with a number-needed to treat to prevent one IFI of only 3 but did not result in a reduction of the incidence of bacteraemia.
List of abbreviations
AML Acute myeloid leukaemia
IFI Invasive fungal infection
MDS Myelodysplastic syndrome
EORTC/MSG European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group
CT Computed tomography
PCR Polymerase chain reaction
ESBL Extended-spectrum beta-lactamase
MRSA Methicillin-resistant Staphylococcus aureus
APL Acute promyelocytic leukaemia
HOVON Stichting Hemato-Oncologie voor Volwassenen Nederland
CFU colony-forming unit
ANC absolute neutrophil count
WHO World health organization
GEE General estimation equation
SAKK Schweizerische Arbeitsgemeinschaft für klinische Krebsforschung
MSD Merck Sharp & Dohme AG
NOS not otherwise specified
t(x;x) translocation
inv(x) inversion
NPM1 Nucleophosmin 1
CEBPA CCAAT/enhancer-binding protein alpha
tAML therapy-related acute myeloid leukaemia
AML MRC Acute myeloid leukaemia with myelodysplasia-related changes
RAEB2 Refractory anaemia with excess blasts 2
Introduction
Supportive therapy for patients with acute myeloid leukaemia (AML) and high-risk myelodysplastic syndrome (MDS) undergoing intensive chemotherapy has improved significantly during the last decades [1]. However, infections remain a major cause of morbidity and mortality among this severely immunosuppressed patient population. Without anti-infective prophylaxis, bacteraemia occurs in up to 34% and invasive fungal infections (IFI) in up to 51.7% of all patients undergoing intensive chemotherapy [2–7]. IFI have high-rates of morbidity and mortality and can require prolonged antifungal treatment [8].
Different strategies have been developed to reduce the risk of infections, especially IFI: (i) the empirical approach relies on clinical parameters such as the presence of persistent fever despite use of broad-spectrum antibiotics to trigger antifungal therapy; (ii) the pre-emptive strategy is based on standardised screening tests such as periodical computed tomography (CT) scans, fungal polymerase chain reaction (PCR), and galactomannan serum measurements as well as bronchoalveolar lavage to guide antifungal treatment; (iii) the prophylactic approach with the administration of anti-infective chemotherapeutics in all patients from the beginning of chemotherapy [9–13]. Each of these approaches has distinct advantages and weaknesses: The empirical treatment does not prevent infection and is known to result in overtreatment. The pre-emptive strategy can delay the diagnosis, under-diagnose infections, and can lead to an excess in morbidity and mortality. On the other hand, the prophylactic approach raises concerns about the emergence of chemo-resistant pathogens and associated costs [10, 14].
The optimal anti-infective strategy depends on many factors such as the local incidence of infections, local microbial resistance pattern, quality and availability of screening methods, structural characteristics of the facility (e.g. high efficiency particulate air filtration), as well as financial aspects.
At the University Hospital of Zurich an empirical approach was traditionally followed to guide anti-microbial therapy in patients with haematological malignancies. The bacterial resistance pattern in this institution is still of relatively little concern. Data from our microbiological surveillance database showed that in 2008 28% of all Escherichia coli isolates were resistant to ciprofloxacine, the extended-spectrum beta-lactamase (ESBL) rate among E.coli and Klebsiella pneumoniae was 10%, and approximately 4% of all S.aureus isolates were identified as methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin-resistent Enterococci are very uncommon. Importantly, a resistance analysis from 2009 restricted to the leukaemia ward, revealed a 100% susceptibility to ciprofloxacine for all gram-negative isolates from positive blood cultures (n = 17). However, the rate of possible IFI in our haematologic patient population seemed to exceed the level that would be expected from the literature [3, 8]. Thus, considering our local infection and resistance pattern, in March 2010 we implemented a prophylactic anti-fungal strategy with posaconazole, and a prophylactic anti-bacterial approach with levofloxacin. The change of our strategy was based on the known efficacy of levofloxacine and posaconazole in this setting, and was in line with current international recommendations (e.g. European Conference on Infections in Leukaemia, ECIL) [2, 11, 15, 16]. Simultaneously, dexamethasone was introduced to prevent chemotherapy-induced nausea and possibly reduce cytarabine-induced skin toxicity. Here, we analyse the outcome with respect to IFI and bacteraemia before and after implementation of this new standard.
Methods
Study population and protocol
This retrospective single-centre study was performed in the leukaemia ward and the haematopoietic stem cell transplantation unit of the University Hospital Zurich. Patients were included between September 1st 2008 and April 30th 2011. Two patient groups were analysed, one prior to initiation of the anti-infective prophylaxis in March 2010 (‘non-prophylaxis’) and one after its introduction (‘prophylaxis’), respectively. The end of follow up was May 31st 2013. Patients were eligible if they were older than 18 years and received intensive chemotherapy for AML, acute promyelocytic leukaemia (APL) or high-risk MDS (IPSS >1.5). A total of 100 patients were screened, and 12 patients with chemotherapy cycles during both treatment periods were excluded. Therefore, 88 patients were included in the final analysis. Patients with AML and MDS were treated according to the standard arm of the HOVON 42 protocol unless they participated in the HOVON 92 trial (from 09th of October 2009 until 18th of December 2009), or the HOVON 102 trial (starting from 14th October 2010) [17]. All trials were registered at the HOVON Data Centre. Treatment protocols are available online (http://www.hovon.nl). Patients with APL were treated according to the APL2000 study (NCT00591526) until January 2010 and according to the APL2006 protocol (NCT00378365) starting from February 2010. All these trials were approved by the local Ethical Committee (Kantonale Ethikkomission Zürich) and informed consent was provided prior to inclusion. The local Ethical Committee (Kantonale Ethikkomission Zürich) also approved the present study and waived the requirement for written informed consent due to its retrospective character.
Anti-infective prophylaxis
We did not use anti-bacterial or anti-fungal prophylaxis until March 2010. In March 2010, we introduced an anti-infective chemoprophylaxis scheme in combination with an antiemetic prophylaxis with dexamethasone for all patients undergoing intensive chemotherapy. Anti-fungal chemoprophylaxis consisted of oral amphotericine B (200 mg QID) during chemotherapy and oral posaconazole (200 mg TID) after completion of chemotherapy and until neutrophil recovery, defined as a sustained neutrophil count >0.5 G/l or until therapeutic antifungal therapy was started. No intravenous substitution for the anti-fungal prophylaxis was provided if the oral formula was not tolerated and no posaconazole dose adaption was performed in the case of low serum concentrations. Anti-bacterial prophylaxis with levofloxacin (500 mg OD) was given from the start of therapy until neutrophil recovery or until intravenous antibiotic treatment was needed, whatever occurred first. Antiemetic prophylaxis and prophylaxis against cytarabine-induced skin toxicity were intensified by adding dexamethasone (8 mg BID) during cytarabine-containing chemotherapy. Prophylaxis against Pneumocystis jirovecii infection was started in all chemotherapy cycles containing dexamethasone prophylaxis, and consisted of trimethoprime/sulfamethoxazole 800/160 mg three times a week. Anti-fungal prophylaxis was not routinely used during or after allogeneic stem cell transplantation throughout the study period.
Anti-infective treatment
The empirical approach to treatment of patients with neutropenic fever remained unchanged throughout the whole study period: Blood cultures were taken and a broad-spectrum intravenous antibiotic therapy was started when fever occurred. Fever was defined as a core temperature of >38.4 °C once or >38.0 °C twice, when measured by a tympanic thermometer. Initial empirical antibacterial treatment consisted of cefepime 2 g TID or tazobactam/piperacillin 4.5 g TID, the latter being used if an abdominal focus was suspected. A low-dose chest CT exam was performed and an empirical antifungal treatment with intravenous amphotericine B-deoxycolate (1 mg/ kg body weight by continuous infusion over 24 hours) was started if fever persisted for more than 48 to 72 hours after initiation of the intravenous antibiotic or if fungal infection of other sites was suspected (sinus, skin, hepatosplenic) [18]. Anti-fungal therapy could be withheld according to the treating physicians’ discretion. No systematic screening for fungal infections using serological essays (e.g. galactomannan) or anti-fungal-PCR was employed and bronchoalveolar lavage or transthoracic puncture of suspected fungal lesions were performed rarely and only in highly-selected cases (e.g. therapy-refractory fungal infections).
Facility
The leukaemia ward is situated in a historical building (built in the middle of last century) with standard ventilation. In contrast, the haematopoietic stem cell transplantation ward was built within the structure in 1995 and is equipped with high efficiency particulate air filtration and positive air pressure. Construction work was ongoing in some areas of the hospital throughout the observation period but did not directly affect the haematologic units. Most AML patients were treated on the leukaemia ward and not on the stem cell transplantation ward. Exceptions were due to a shortage of hospital beds or organisational reasons. Cycles of treatment on the transplantation ward are indicated separately.
Local fungus surveillance programme
Our local surveillance programme consists of sporadic environmental air sampling for quantitative and qualitative identification of filamentous fungi.
Measurements and definitions
Therapy cycles lasted from the first day of chemotherapy until neutrophil recovery (absolute neutrophil count (ANC) >0.5 G/L for 3 consecutive days). Aplasia time lasted from the first day of an ANC <0.5 G/l until ANC recovery >0.5 G/l.
Risk assessment of AML and MDS were made according to the HOVON 102 protocol, the APL2000/2006 protocols, and the IPSS score, respectively. Diagnosis was made according to the WHO 2008 Classification of Tumours of Haematopoietic and Lymphoid Tissues [19].
Primary end points: Total number of fungal infections and bacteraemias per therapy cycle were defined as the primary end-points. Patients with IFI at diagnosis were excluded from the analysis and the rate of fungal infections was expressed in proportion to patients at risk. Patients with bacterial infections at the beginning of a chemotherapy cycle were excluded from the analysis and the rate of proven bacteraemia was expressed in proportion to cycle numbers.
The radiological presence of IFI was assessed by two independent radiologists who were blinded for the patient’s clinical data. The radiological diagnosis of IFI required at least one of the following 3 signs on CT: (i) Dense, well-circumscribed lesions(s) with or without a halo sign, (ii) air-crescent sign, (iii) cavitation. Discordant interpretations were resolved by consensus in a second readout. Invasive fungal infections were then classified as ‘possible’, ‘probable’ or ‘proven’ according to the EORTC/MSG criteria [20].
Secondary end-points were the overall survival at 100 days and 2 years, time from initiation of chemotherapy to onset of IFI, use of intravenous and oral antifungal and antibacterial therapy, and total duration of anti-fungal and anti-bacterial medication defined as the sum of prophylactic and therapeutic anti-infective therapy days. Additionally, the fever days, and the time in the intensive care unit per therapy cycle as well as the proportion of therapy cycles complicated by antiviral treatment, the occurrence of cytarabine-related skin toxicity, and the incidence of neutropenic enterocolitis were assessed.
Statistical analysis
Baseline characteristics are displayed as frequencies and percentages for categorical data and as means (standard deviation) for continuous data. Since patients typically had more than one therapy cycle, the correlation between cycles for the same patient has to be taken into account when comparing cycle-specific variables between prophylaxis and non-prophylaxis periods. To account for this dependency, generalised estimation equations (GEE) models with each patient considered as a separate cluster were applied to test for differences. For each variable, an unstructured correlation matrix was assumed in a Poisson and logistic GEE for count and binary variables, respectively. Patient-specific differences in IFI occurrence after the first and last cycle were compared between the prophylaxis and non-prophylaxis groups using a chi-square test. The time-to-IFI was defined as the time between initiation of chemotherapy and the onset of IFI. If a patient did not develop IFI, he or she was censored at the end of the observation period (May 31st2013) or at date of death. Survival functions were estimated using the Kaplan-Meier estimate and were compared between the prophylaxis and non-prophylaxis group using a log-rank test. Survival at 100-days and two-years was compared using logistic regression. Multivariate analysis for the occurrence of IFI per therapy cycle was performed using mixed effects logistic regression with a random intercept for each patient. Correction for multiple testing in these exploratory analyses was not performed. All tests were performed at a significance level of α = 0.05 and confidence intervals were computed at a confidence level of 95%. Statistical analysis was conducted using R software [21].
Results
Baseline characteristics
The study comprised 88 patients receiving a total of 203 therapy cycles. Baseline characteristics were equally distributed among the patients in the non-prophylaxis and the prophylaxis periods with the exception of chemotherapy cycles on the transplantation ward and duration of aplasia as shown in table 1 and discussed below. The majority of patients was treated for acute myeloid leukaemia (AML) (n = 80, 90.9%), the remaining patients were diagnosed with acute promyelocytic leukaemia (APL) (n = 7; 8%), or MDS (refractory anaemia with excess blasts) (n = 1; 1.1%). When applying the SAKK/HOVON 102 risk stratification scheme for AML, 30 (37.5%) patients were stratified in the ‘good risk’, 12 (15%) in the ‘intermediate risk’, 28 (35%) in the ‘poor risk’, and 10 (12.5%) in the ‘very poor risk’ category, respectively. Intensive chemotherapy cycles consisted of induction cycles I and II (138, 68%), conventional consolidation (49, 24.1%), and relapse salvage therapy (16, 7.9%). There was a statistically non-significant trend to more salvage therapy cycles in the non-prophylaxis versus the prophylaxis group, with 13 (15.1%) and 3 (2.9%), respectively (p = 0.077). The number of chemotherapy cycles per patient excluding stem cell transplantation were one (n = 15, 17.1%), two (n = 37, 42%), three (n = 30, 34.1%) or four (n = 6, 6.8%), respectively. The mean aplasia time was significantly different in the prophylaxis and the non-prophylaxis group (18.3 and 21 days, respectively; p = 0.011). A total of 41 (20.2%) cycles were administered in the stem cell transplantation unit with statistically significantly more patients in the non-prophylaxis than in the prophylaxis group, respectively (29.3%; and 9.6% p = 0.012). A total of 35 patients (39.8%), of whom 15 in the non-prophylaxis and 20 in the prophylaxis group, underwent allogeneic hematopoietic stem cell transplantation after the second (n = 21), the third (n = 6), and after salvage therapy (n = 8), respectively.
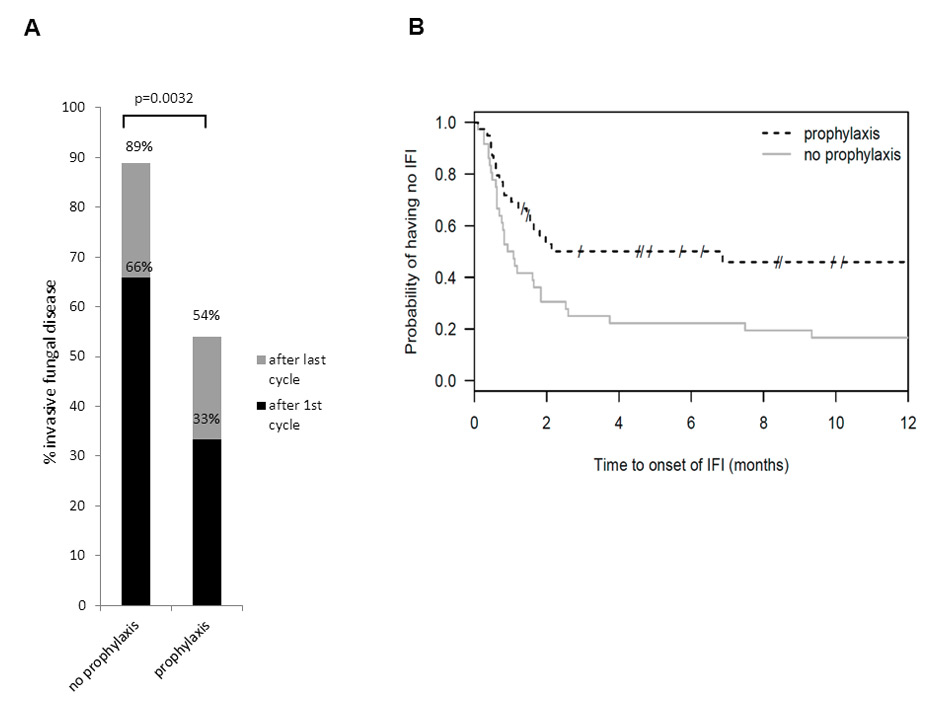
Figure 1
Invasive fungal infections and fungal-free survival.
(A) Patients with invasive fungal infection (IFI) after first cycle (non-prophylaxis 65.8% vs. prophylaxis 33.3%; p = 0.0088) and after the last cycle of chemotherapy (non-prophylaxis 88.9% vs. prophylaxis 53.9%; p = 0.0032), respectively. (B) Kaplan-Meier plot for fungal-free survival: Log rank test p = 0.0194. The projected IFI-free survival at 100 days was increased (50.1% vs. 25%; p = 0.0526).
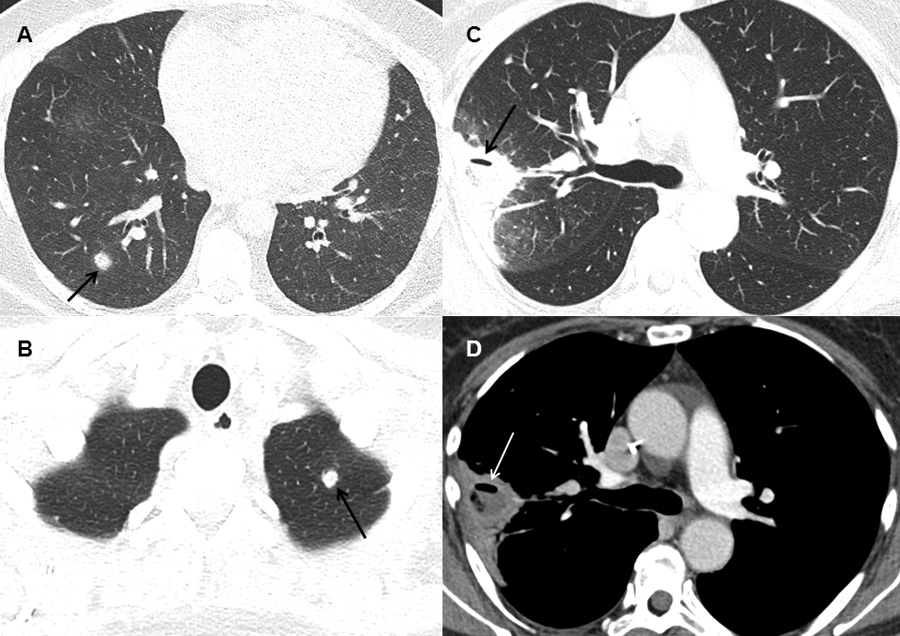
Figure 2
Typical findings on computed tomography.
(A) Dense, well-circumscribed lesion with a halo (arrow) in the right lower lobe in a patient with possible invasive fungal infection (IFI) (B) Dense, well-circumscribed lesion in the left upper lobe (arrow) in a patient with possible IFI (C and D) Dense lesion with an air-crescent sign (arrows) in the right upper lobe in a patient with proven IFI (mucorales spp.).
Adherence to the prophylaxis scheme and screening measures
In the non-prophylaxis group, posaconazole, levofloxacin, and dexamethasone were given in a total of 1.9%, 2.2% and 1% of all cycles, and in the prophylaxis group, in 81.7%, 69.5% and 90.5% of all evaluable cycles, respectively (table 2). Posaconazole was given during 0.3 (0–17) days/cycle in the non-prophylactic and 6.8 (0–30) days/cycle in the prophylactic group. Posaconazole serum concentration was measured in 67.9% of all evaluable cycles in the prophylaxis period, and resulted in a mean concentration of 0.38 mg/L (0–0.82). Galactomannan measurement was performed during 13.1% of all cycles in the non-prophylaxis and 3.8% in the prophylaxis period (p = 0.087). A total of 91 low-dose chest CT exams were done in the non-prophylaxis and 84 in the prophylaxis group (p = 0.07).
Local fungus surveillance programme:
We obtained environmental samples from three different periods from the leukaemia ward. In March and April 2007 the median airborne Aspergillus fumigatus and Aspergillus niger conidia levels were 2 to 24 colony-forming units (CFUs) and 0 to 6 CFUs per cubic meter, respectively. The same fungi were measured in March 2010 with 2 to 18 CFUs, and 2 to 4 CFUs, and in December 2010 with 0 to 4 CFUs, and 2 to 32 CFUs per cubic meter, respectively.
Fungal infections
IFI were significantly less common in the prophylaxis group after the first chemotherapy cycle (13 (33.3%) vs. 25 (65.8%); p = 0.0088), as well as after the last chemotherapy cycle (21 (53.9%) vs. 32 (88.9%); p = 0.0021) (fig. 1A). The absolute risk reduction for IFI was 35% in the prophylaxis group. The total days of anti-fungal medication (prophylaxis and therapy combined) per cycle did not differ between the two groups (table 2). The Kaplan-Meier plot shows a higher fungal-free survival in the prophylaxis group (log rank test p = 0.0194) (fig. 1B). The majority of IFI were classified as possible according the EORTC/MSG 2008 criteria. Most IFI were pulmonary, and in most cases the fungus type was not identified (table 3)
Bacterial infections
The incidence of chemotherapy cycles, that were complicated by bacteraemia was 34.6% during the prophylaxis period and 32.3% in the non-prophylaxis period (p = 0.8) and the total number of positive blood cultures were 50 and 43, respectively (table 4). There was a statistically non-significant trend to more gram-negative infections in the non-prophylaxis-group (42% vs. 14%; p = 0.073) and to more gram-positive infections in the prophylaxis group (86% vs. 58%; p = 0.092). Coagulase-negative Staphylococci and Enterococcus faecium accounted for 70% of the positive blood cultures during the prophylaxis period (table 4).
Secondary end points
Overall survival at 100 days and 2 years, mean ICU days, the occurrence of neutropenic enterocolitis, as well as the use of antiviral medication did not differ between the two groups (table 5). However,fewer fever days (5.6 vs. 9.2; p = 0.00032) and less cytarabine skin toxicity (18.3% vs. 35%; p = 0.025) were observed during the prophylaxis period.
Death and termination of therapy due to IFI
In the non-prophylaxis group four patients experienced severe adverse events due to IFI: Patient one had chemotherapy-resistant AML and died due to autoptically confirmed septic emboli (mucorales spp.); patient two had a relapse of the AML and possible pulmonary IFI, and died of pulmonary haemorrhage; patient three had to stop AML treatment after the second induction chemotherapy due to possible pulmonary IFI; patient four had chemotherapy-resistant AML and had to stop AML treatment after the second induction chemotherapy due to probable pulmonary IFI. In the prophylaxis group three patients experienced severe adverse events due to IFI: Patient one had very poor-risk AML and died of clinically and radiologically diagnosed septic emboli, possibly related to mucorales (no autopsy was performed); patient two had a relapse of the AML after stem cell transplantation and died of autoptically confirmed pulmonary IFI with aspergillus spp; patient three had chemotherapy-resistant AML and died of autoptically confirmed disseminated infection with mucorales spp. and aspergillus spp (table 3).
Multivariate analysis
Posaconazole prophylaxis remained significantly associated with a lower occurrence of IFI when controlled for potential confounders, such as duration of neutropenia, treatment in the stem cell transplantation unit, and occurrence of neutropenic enterocolitis (table 6). On average, the patient-specific odds for occurrence of IFI are reduced by a factor of 0.092 in the prophylaxis group compared to the non-prophylaxis group (95%-CI [0.03, 0.31], p <0.0001). Patient-specific odds for occurrence of IFI are also significantly reduced for patients treated in the stem cell transplantation unit when compared to the regular leukaemia ward (OR = 0.174, 95%-CI [0.04, 0.70], p = 0.014).
|
Table 1: Patients baseline characteristics. |
|
|
Non-prophylaxis
|
Prophylaxis
|
p-value
|
| Number of patients |
43 |
45 |
|
| Mean age (years) |
49.8 |
53.5 |
|
| Male gender (%) |
22 (51.2) |
23 (51.1) |
|
| WHO diagnosis (%)
NOS
t(8;21)
inv16; t(16;16)
mutated NPM1
mutated CEBPA
t(9;11)
t(6;9)
inv3
tAML
AML MRC
RAEB2
APL |
12 (27.9)
4 (9.3)
2 (4.7)
6 (13.9)
0 (0)
1 (2.3)
0 (0)
1 (2.3)
1 (2.3)
12 (27.9)
1 (2.3)
3 (7) |
19 (42.2)
3 (6.7)
2 (4.4)
7 (15.6)
1 (2.2)
0 (0)
1 (2.2)
1 (2.2)
1 (2.2)
6 (13.3)
0 (0)
4 (8.9) |
|
| SAKK/HOVON 102 risk stratification (%)
Non applicable
Good risk
Intermediate risk
Poor risk
Very poor risk |
4 (9.3)
14 (32.6)
8 (18.6)
11 (25.6)
6 (14) |
4 (8.9)
16 (35.6)
4 (8.9)
17 (37.8)
4 (8.9) |
|
| Number of chemotherapy cycles |
99 |
104 |
|
| Chemotherapy cycles per patient (%)
1
2
3
4 |
8 (18.6)
17 (39.5)
15 (34.9)
3 (7) |
7 (15.6)
20 (44.4)
15 (33.3)
3 (6.7) |
|
| Cytarabine cycles (%)
No cytarabine
Standard-dose cytarabine
Intermediate/high-dose cytarabine |
16 (16.2)
40 (40.4)
43 (43.6) |
22 (21.2)
43 (41.4)
39 (37.4) |
|
| Chemotherapy cycles (%)
Induction cycles I and II
Conventional consolidation
Salvage |
61 (61.6)
25 (25.3)
13 (13.1) |
77 (74)
24 (23.1)
3 (2.9) |
0.077a
|
| Chemotherapy cycles in transplantation ward (%) |
29 (29.3) |
10 (9.6) |
0.012b
|
| Aplasia duration in days per cycle$
|
21 (10) |
18.3 (8.8) |
0.011c
|
|
$Mean (Standard deviation); aLogistic GEE reinduction vs. other; bLogistic GEE; normal vs. tpl ward; cPoisson GEE |
|
Table 2: Adherence to anti-bacterial and anti-fungal prophylaxis, duration of anti-fungal prophylaxis/medication, IFI screening measures, and posaconazole serum concentrations. |
|
|
Non- prophylaxis
|
Prophylaxis
|
p-value
|
| Chemotherapy cycles with (%)
posaconazole prophylaxis
levofloxacin prophylaxis
dexamethasone |
1.9
2.2
1 |
81.7
69.5
90.5 |
|
| Anti-fungal medication days per cycle$
Posaconazole prophylaxis
Total antifungal medication |
0.3 (0–17)
25.3 (20.5) |
6.8 (0–30)
20.3 (9.4) |
0.066a
|
| Galactomannan measurement per cycle (%)
None
any |
86 (86.9)
13 (13.1) |
100 (96.2)
4 (3.8) |
0.087a
|
| Chemotherapy cycles with CT scans (%) |
91 (91.1) |
84 (80) |
0.07a
|
| Posaconazole serum concentration (mg/L)$
|
NA |
0.38 (0–0.82) |
|
|
$Mean (min-max); aLogistic GEE at least one measurement/scan vs. none |
|
Table 3: Invasive fungal infections: Severe complications, probability according to EORTC/MSG 2008, site and fungus type. |
| |
|
No prophylaxis
|
Prophylaxis
|
p-value
|
| Death or termination of therapy due to IFD (%) |
4 (9.3) |
3 (6.7) |
0.79c
|
| Fungal infection probability according EORTC/MSG 2008 |
Possible
Probable
Proven |
30
1
1 |
18
1
2 |
|
| Fungal infection site |
Lung
Sinus
Hepatosplenic |
30
1
1 |
19
0
2 |
|
| Fungus type |
Unknown
Aspergillus spp
Candida glabrata
Candida albicans
Mucorales
|
29
1
NA
1
1 |
17
2
1
NA
1 |
|
|
cLogistic GEE yes vs. no |
|
Table 4: Duration of antibacterial prophylaxis/medication, incidence of bacteraemia and bacterial strains. |
|
|
Non- prophylaxis
|
Prophylaxis
|
p-value
|
| Anti-bacterial medication days per cycle$
levofloxacin prophylaxis
total anti-bacterial medication |
0.2 (1.1)
23.6 (17.8) |
10.3 (37.4)
28.7 (36.6) |
0.31a
|
| Chemotherapy cycles with bacteraemia (%) |
32 (32.3) |
36 (34.6) |
0.8a
|
|
Positive blood cultures (%)
|
50 (100)
|
43 (100)
|
0.59a
|
| Gram positive bacteria
Staphylococcus, coagulase-negative
Staphylococcus aureus
Staphylococcus haemolyticus
Enterococcus faecium
Enterococcus faecalis
Streptococcus pneumoniae
Streptococcus mitis
Streptococcus salivarius
Bacillus sp
Corynebacteria
|
29 (58)
9 (18)
–
1 (2)
8 (16)
1 (2)
–
5 (10)
2 (4)
1 (2)
2 (4) |
37 (86)
16 (37)
1 (2)
1 (2)
14 (33)
–
1 (2)
2 (5)
1 (2)
1 (2)
– |
0.092a
|
| Gram negative bacteria
Escherichia coli
Escherichia coli, ESBL
Burkholderia cepacea
Klebsiella oxytoca
Klebsiella pneumoniae
Pseudomonas aeruginosa
Enterobactercloacae
Acinetobacter baumanii
Serratia marescens
Morganella morganii
Sphingopyxi ssp
Fusobacterium nucleatum
|
21 (42)
4 (8)
–
–
3 (6)
2 (4)
3 (6)
4 (8)
1 (2)
2 (4)
1 (2)
1 (2)
– |
6 (14)
3 (7)
2 (5)
1 (2)
–
–
–
–
–
–
–
–
1 (2) |
0.073a
|
|
$Mean (Standard deviation); aPoisson GEE |
|
Table 5: Secondary endpoints: Overall survival and complications. |
|
|
Non-prophylaxis
|
Prophylaxis
|
p-value
|
| Overall survival at day 100 |
88.4% |
84.4% |
0.35a
|
| Overall survival at 2 years |
58.1% |
64.4% |
0.64a
|
| Fever days/therapy cycle$
|
9.2 (8.0) |
5.6 (5.1) |
0.00032b
|
| ICU days/therapy cycle$
|
2.4 (13) |
1.7 (5.2) |
0.66b
|
| Cytarabine skin toxicity (%)#
|
29 (35) |
15 (18.3) |
0.025c
|
| Anti-viral medication (%)&
|
63 (62.6) |
54 (51.9) |
0.38c
|
| Neutropenic enterocolitis (%)&
|
28 (28.3) |
24 (23.1) |
0.51c
|
|
$Mean (Standard deviation); aLogistic regression; bPoisson GEE; #percentage of cytarabine-containing therapy cycles; dLogistic GEE; &percentage of all therapy cycles |
|
Table 6: Multivariate analysis. Impact of posaconazole prophylaxis, aplasia duration, neutropenic enterocolitis and the infrastructure on the occurrence of IFI. |
|
|
Odds ratio (OR)
|
95% confidence interval for OR
|
p-value
|
| Posaconazole prophylaxis |
0.092 |
[0.03, 0.31] |
0.0001 |
| Duration of Aplasia |
1.071 |
[0.99, 1.16] |
0.079 |
| Neutropenic enterocolitis |
1.016 |
[0.37, 2.81] |
0.98 |
| Transplantation ward |
0.174 |
[0.04, 0.70] |
0.014 |
Discussion
This real-world study aimed at investigating the effect of a newly introduced combined anti-infective prophylaxis with posaconazole and levofloxacin in patients undergoing intensive chemotherapy for AML, APL and MDS. The study demonstrated a positive effect of prophylaxis on the occurrence of invasive fungal infections (IFI), but no effect with respect to bacteraemias.
The data on the efficacy of posaconazole in the prophylaxis setting is in line with prior reports, however the net benefit is surprisingly high, with a number-needed to treat of less than three patients to prevent one ‘possible’ IFI [6, 11]. As we had anticipated, the rate of IFI in our institution was higher, when compared to other published data: More than 80% of patients without antifungal prophylaxis developed ‘possible’, ‘probable’ or ‘proven’ IFI at the end of induction and consolidation chemotherapy, compared to 5–51.7% in prior studies [3, 6, 8]. However, a combined endpoint of mortality and termination of the therapy due to IFI in our institution was lower than 10% in both our treatment groups, which is considerably less than what would be expected from the literature, where a mortality rate between 27% and >50% depending on the type and the site of the fungus is reported with a downward trend in the more recent publications [22–24]. Several factors might have contributed to this favourable outcome. (1) our empirical approach to guide antifungal treatment as well as early detection of small lesions in the CT might be an effective clinical strategy, (2) the inclusion of possible IFI could have led to over-diagnosis of IFI, and might therefore not reflect the true outcome of IFI, (3) the choice of the empirical and salvage regimen (amphotericin B dexocholate as a 24h continuous infusion) might be very effective, and (4) todays anti-fungals are known to be more effective than older agents. The overall survival at 2 years was around 60% in both our treatment groups which is certainly in line or better with what could be expected from the literature [25].
The following factors might explain the relatively high prevalence of IFI as reported in our study: Most importantly, the inclusion of ‘possible’ IFI in this analysis has most probably led to over-diagnosis of IFI, as ‘possible’ IFI were almost exclusively diagnosed based on a patient criterion (prolonged neutropenia) and on the computed tomography (CT) findings (fig. 3). CT scans were usually triggered by persistent fever >48 to 72 hours despite broad-spectrum antibiotics and false-positive results can occur. In this context it is important to note, that there was not a significant difference between the numbers of CT scans performed in our two treatment groups. Secondly, our data suggest that infrastructural characteristics might play a role, as there were statistically less IFI in patients treated in the transplantation ward, which is equipped with high efficiency particulate air filtration and positive air pressure ventilation. Air filtration and positive pressure ventilation are known to provide a higher degree of protection against IFI [26]. However, and in contrast to what might be expected, even though significantly more patients in the non-prophylaxis group were treated in the stem cell transplantation ward, still the pharmacological approach to prophylaxis led to a statistically significant reduction of IFI in the prophylaxis group. Thirdly, local differences such as the climate and building activities are known to have an impact on the incidence of IFI and might in part explain some of the reported differences [27]. Our surveillance data on airborne CFU are in line with what could be expected from the literature [28–30]. However, measurement of airborne CFU is not very well standardised and studies are therefore difficult to compare.
Despite the possible over-diagnosis of IFI in our real-world-analysis, we decided to include all ‘possible’ IFI as they represent an important and critical everyday problem for physicians caring for AML patients under intensive chemotherapy. The diagnosis of ‘probable’ or ‘proven’ invasive fungal infections remains notoriously difficult and the yield of even an extensive diagnostic work-up including biopsies and bronchoalveolar lavage is limited. However, given the high morbidity and mortality of IFI we would not withhold antifungal treatment in a persistently febrile neutropenic patient on broad-spectrum antibiotics with a computed tomography scan compatible with an IFI despite negative galactomannan measurement and a negative bronchoalveolar lavage. This is why we strongly believe that ‘possible’ IFI are a real-world problem and that ‘possible’ IFI have an impact on further patient management as they tend to occur early in the course of the disease and lead to prolonged antifungal treatment, especially following allogeneic stem cell transplantation. Hence, considerable treatment costs are being shifted to the outpatient setting. Preventing rather than treating IFI can reduce long-term antifungal treatment and preclude possible side effects and interactions [31–33].
Also, the high prevalence of IFI might have added to the efficacy of the prophylactic approach in our patient population. We like to stress, however, that reduction in IFI was achieved even though (i) overall adherence to posaconazole prophylaxis was only 81.7%; (ii) mean days of antifungal prophylaxis was no longer than 6.7 days covering only one third of the total aplasia time; (iii) mean posaconazole serum concentration was low (0.38 ug/ml); and (iv) more cycles in the non-prophylaxis group were applied in air-filtered rooms. Thus, the results rather underestimate the effect, and longer treatment and higher serum concentrations might lead to even more favourable results [34].
In today’s tight budgetary environment, the cost effectiveness of a prophylactic or therapeutic intervention is a relevant consideration. In our institution with a relatively high incidence of ‘possible’ IFI and a net drug cost of € 130/day for posaconazole prophylaxis and € 120/day for intravenous amphotericin B deoxycholate, the prophylactic approach seems to be cost effective. ‘Prophylaxis’ (6.7 days of posaconazole and 13.5 days of amphotericinB per therapy cycle) and ‘non-prophylaxis’ (0.2 days of posaconazole and 25.1 days of amphotericin B deoxycholate per therapy cycle) resulted in a total drug expense of approximately € 2500 and € 3000, respectively. The relevance of this might be even more pronounced if liposomal amphotericine B (€ 1700/day for an 80 kg patient with a dose of 4 mg/kg/day) or alternative i.v. antimycotic drugs are used. This is in line with the findings from other groups [3, 6, 35].
In contrast to previously published studies, antibacterial prophylaxis with levofloxacin did not seem to be beneficial in our patient population as it did not lead to a significant reduction of bacteraemia [2, 16]. The reduction of Gram-negative bacteraemia in the levofloxacin-prophylaxis group was counterbalanced by an increase in Gram-positive bacteraemia. Enterococcus faecium and coagulase-negative Staphylococci accounted for 70% of all blood-stream infections in the levofloxacin prophylaxis group. However, the study groups might have been too small and the adherence to levofloxacin in the prophylaxis group too low to show a statistically significant difference.
Regarding the secondary endpoints, no difference in the 100 day overall survival was observed between the non-prophylaxis and the prophylaxis group. Fever-days were reduced significantly during the prophylaxis period and cytarabine-induced skin toxicity was significantly less common in the prophylaxis group, the latter being most likely a consequence of adding dexamethasone to the prophylaxis scheme.
There are some limitations to the current study: Firstly, the retrospective design. Secondly, the two patient groups were not perfectly matched regarding the aplasia duration and the treatment in a ward with air filtration, which might influence the final results. However, the possible biases were distributed in a counterbalanced way: (i) more patients during the non-prophylaxis period received treatment in the modern stem cell transplantation unit, a factor that was associated with a lower incidence if IFI in the multivariate analysis. (ii) The duration of aplasia was longer in the non-prophylaxis group, and even though the duration was not significantly associated with the occurrence of IFI in our study, the duration of severe neutropenia is generally known as a risk factor to favour infections [22].
Conclusions
We conclude that in our institution, which likely reflects many hospital settings, posaconazole prophylaxis is highly efficient in reducing possible invasive fungal infections, with a number-needed to treat to prevent one IFI of only 3, and it is cost-efficient, while a clinically relevant benefit of the anti-bacterial prophylaxis with levofloxacin was not observed.
Authors’ contributions: BG and MGM designed the study. JK and BG collected the data. TF and TDLN performed the radiological readout. MP performed the statistical analysis. FH, GN, US, BG and MGM were involved in the clinical patient management and data generation. BG wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved its final version.
Acknowledgement: We thank our colleagues Dr. Annelies Zinkernagel, Dr. Hugo Sax and Dr. Stefan Kuster from the Division of Infectious Diseases and Hospital Epidemiology of the University Hospital Zurich for the kind collaboration and for providing us with the data on the local fungus surveillance programme and the microbiological surveillance database.
Funding / potential competing interests: This study was supported by an unrestricted research grant from MSD. MSD did not provide medication, had no influence on the study design, had no insight in the original patient data and was not involved in the data analysis and writing of the manuscript. MSD received the final version of the manuscript before submission in order to be able to comment on it.
References
1 Hahn-Ast C, Glasmacher A, Muckter S, Schmitz A, Kraemer A, Marklein G, et al. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 2006. J Antimicrob Chemother. 2010;65(4):761–8.
2 Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977–87.
3 Slobbe L, Polinder S, Doorduijn JK, Lugtenburg PJ, el Barzouhi A, Steyerberg EW, et al. Outcome and medical costs of patients with invasive aspergillosis and acute myelogenous leukemia-myelodysplastic syndrome treated with intensive chemotherapy: an observational study. Clin Infect Dis. 2008;47(12):1507–12.
4 Kjellander C, Bjorkholm M, Cherif H, Kalin M, Giske CG. Hematological: Low all-cause mortality and low occurrence of antimicrobial resistance in hematological patients with bacteremia receiving no antibacterial prophylaxis: a single-center study. Eur J Haematol. 2012 Feb 15.
5 Syrjala H, Ohtonen P, Kinnunen U, Raty R, Elonen E, Nousiainen T, et al. Blood stream infections during chemotherapy-induced neutropenia in adult patients with acute myeloid leukemia: treatment cycle matters. Eur J Clin Microbiol Infect Dis. 2010;29(10):1211–8.
6 Girmenia C, Frustaci AM, Gentile G, Minotti C, Cartoni C, Capria S, et al. Posaconazole prophylaxis during front-line chemotherapy of acute myeloid leukemia: a single-center, real-life experience. Haematologica. 2012;97(4):560–7.
7 Neofytos D, Lu K, Hatfield-Seung A, Blackford A, Marr KA, Treadway S, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis. 2013;75(2):144–9.
8 Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26(4):781–803; quiz 4–5.
9 Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–60.
10 Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48(8):1042–51.
11 Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59.
12 Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41(9):1242–50.
13 Hebart H, Klingspor L, Klingebiel T, Loeffler J, Tollemar J, Ljungman P, et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009;43(7):553–61.
14 Pagano L, Caira M, Nosari A, Cattaneo C, Fanci R, Bonini A, et al. The use and efficacy of empirical versus pre-emptive therapy in the management of fungal infections: the HEMA e-Chart Project. Haematologica. 2011;96(9):1366–70.
15 Gafter-Gvili A, Fraser A, Paul M, van de Wetering M, Kremer L, Leibovici L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2005(4):CD004386.
16 Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:CD004386.
17 Lowenberg B, van Putten W, Theobald M, Gmur J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–52.
18 Eriksson U, Seifert B, Schaffner A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. BMJ. 2001;322(7286):579–82.
19 Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC, 2008.
20 De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21.
21 Team RDC. R: A language and environment for statistical computing. In: Computing RFfS, ed. Vienna, Austria., 2012.
22 Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95(4):644–50.
23 Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32(3):358–66.
24 Even C, Bastuji-Garin S, Hicheri Y, Pautas C, Botterel F, Maury S, et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica. 2011;96(2):337–41.
25 Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89.
26 Benet T, Nicolle MC, Thiebaut A, Piens MA, Nicolini FE, Thomas X, et al. Reduction of invasive aspergillosis incidence among immunocompromised patients after control of environmental exposure. Clin Infect Dis. 2007;45(6):682–6.
27 Panackal AA, Li H, Kontoyiannis DP, Mori M, Perego CA, Boeckh M, et al. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis. 2010;50(12):1588–97.
28 Sautour M, Sixt N, Dalle F, L’Ollivier C, Fourquenet V, Calinon C, et al. Profiles and seasonal distribution of airborne fungi in indoor and outdoor environments at a French hospital. Sci Total Environ. 2009;407(12):3766–71.
29 Hospenthal DR, Kwon-Chung KJ, Bennett JE. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol. 1998;36(3):165–8.
30 Anaissie EJ, Stratton SL, Dignani MC, Lee CK, Summerbell RC, Rex JH, et al. Pathogenic molds (including Aspergillus species) in hospital water distribution systems: a 3–year prospective study and clinical implications for patients with hematologic malignancies. Blood. 2003;101(7):2542–6.
31 Gerber B, Guggenberger R, Fasler D, Nair G, Manz MG, Stussi G, et al. Reversible skeletal disease and high fluoride serum levels in hematologic patients receiving voriconazole. Blood. 2012;120(12):2390–4.
32 Cowen EW, Nguyen JC, Miller DD, McShane D, Arron ST, Prose NS, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62(1):31–7.
33 Cronin S, Chandrasekar PH. Safety of triazole antifungal drugs in patients with cancer. J Antimicrob Chemother. 2010;65(3):410–6.
34 Jang SH, Colangelo PM, Gobburu JV. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88(1):115–9.
35 Bohme A, Atta J, Mousset S, Ehlken B, Shlaen M, Bug G, et al. Antifungal management and resource use in patients with acute myeloid leukaemia after chemotherapy – retrospective analysis of changes over 3 yr in a German hospital. Eur J Haematol. 2012;88(1):68–77.

