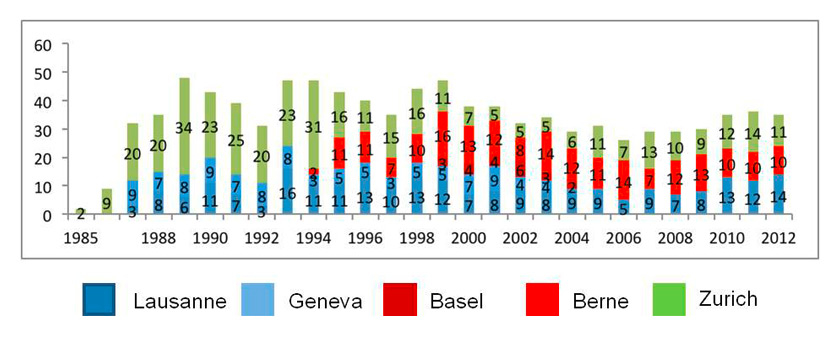
Figure 1
Heart transplantation activity in Switzerland (adapted from Swisstransplant annual reports).
DOI: https://doi.org/10.4414/smw.2014.13977
Abbreviations
ACC American College of Cardiology
AHA American Heart Association
BMI body mass index
CAV cardiac allograft vasculopathy
CMR cardiac magnetic resonance
CNI calcineurin-inhibition
EMB endomyocardial biopsy
HF heart failure
HTx heart transplantation
ISHLT International Society of Heart and Lung Transplantation
MCS mechanical circulatory support
MMF mycophenolate mofetil
NHLB National Heart, Lung, and Blood Institute
STCS Swiss Transplant Cohort Study
TAVI transcatheter aortic valve implantation
It has been over 40 years since Christian Barnard performed the first human-to-human heart transplantation. After early enthusiasm subsided, dedicated and steady research has ultimately led to an era with encouraging long-term results in heart transplantation: median survival after HTx increased significantly from 8.3 years in the 80’s to 10.4 years in the 90’s. Analysis of outcome data of the ISHLT registry (n = 80038) suggests another non-significant increase in survival for the period 2000 to June 2008 [1]. For Switzerland, respective data are not available. Figure 1 represents HTx activity as documented by Swisstransplant.

Figure 1
Heart transplantation activity in Switzerland (adapted from Swisstransplant annual reports).
The ACCF/AHA guidelines for the diagnosis and management of HF in adults have more recently presented another approach to the classification of HF, emphasising both clinical and structural characteristics of the disease [2]. In doing so, four stages were identified with stage D designating patients with truly refractory HF despite maximal medical therapy. Hallmarks of these patients are recurrent hospitalisation, and the requirement of specialised interventions such as continuous or periodic treatment with positive inotropic drugs, re-synchronisation, cardiac surgery, TAVI for symptomatic aortic stenosis, MitralClip® placement for symptomatic mitral valve insufficiency, MCS, heart transplantation, or palliative care. Ideally, a local “Heart team” representing respective expertise should discuss the different treatment options in every patient with advanced heart failure. Because of the painful shortage of donor hearts, this discussion is likewise recommended even when patients are potential candidates for heart transplantation.
The main indication of HTx is persistent advanced chronic heart failure despite optimal medical treatment of left ventricular dysfunction. For candidacy, the patients will have to fulfill various criteria (table 1) with cardiopulmonary exercise testing playing the central role. Nevertheless, the decision on a candidacy for HTx should take into account all clinical data rather than focusing on peak oxygen uptake alone [3], and, usually, is obtained after multidisciplinary discussion.
The timing of referral is of central importance and, ideally, potential HTx candidates should be presented to a respective centre ahead of the manifestation of other organ dysfunctions related to heart failure (e.g., cardiorenal syndrome, pulmonary hypertension, liver dysfunction, metabolic syndrome). Indicators that should prompt consideration for referral are indicated in table 2.
| Table 1: Criteria for heart transplantation. |
| Common criteria |
| 1. Impaired left ventricular systolic dysfunction |
| 2. NYHA III (e.g., patient cannot climb one flight of stairs without dyspnoea) or IV symptoms |
| 3. Receiving optimal medical treatment on maximal dosage tolerated |
| 4. CRT, ICD or CRTD implanted when indicated |
| 5. Evidence of poor prognosis on the basis of cardiopulmonary exercise testing (VO2max <12 ml/kg/min if on β-blockade, and <14 ml/min/kg if not on β-blockade, ensuring respiratory equivalent ratio ≤1.05) markedly elevated NT-proBNP (or BNP) serum levels despite of optimal medical treatment established composite prognostic scoring system (HFSS, SHFM) |
| Uncommon criteria |
| 1. Persisting haemodynamically compromising ventricular arrhythmia refractory to all usual treatment |
| 2. Refractory angina where there is clear evidence of recurrent debilitating myocardial ischaemia not amenable to conventional treatment |
| 3. Restrictive and hypertrophic cardiomyopathy with persistent NYHA III and IV symptoms and/or recurrent hospitalisation for with acute heart failure. |
| BNP = brain natriuretic peptide; CRT = cardiac resynchronisation; CRTD = CRT and ICD treatment; HFSS = heart failure survival score; ICD = internal cardiac defibrillator; NT-proBNP = N-terminal pro-BNP; NYHA = New York Heart Association; SHFM = Seattle heart failure model. |
| Table 2: Clinical indicators that should prompt consideration for referral. |
| 1. Two or more admissions for treatment of decompensated heart failure within the last 12 months |
| 2. Persistent clinical evidence of overt heart failure despite of optimised heart failure treatment |
| 3. Calculated score suggesting a 1-year mortality ≥20% |
| 4. Echocardiographic evidence of right ventricular dysfunction and increasing pulmonary artery pressure on optimal medical treatment |
| 5. Anaemia, involuntary weight loss, liver dysfunction, hyponatremia attributable to heart failure |
| 6. Deteriorating renal function attributable to heart failure or inability to tolerate diuretic dosages sufficient to clear congestion without change in renal function |
| 7. Significant episodes of ventricular arrhythmia despite of full drug and electrophysiological/device treatment |
| 8. Increasing BNP / NT-proBNP levels despite optimal medical treatment. |
HTx aims both at the improvement of symptoms and the prolongation of patient survival. Therefore, other organ dysfunction not likely to improve, posttransplant, may preclude HTx in the individual patient. In contrast, other organ dysfunction often does not preclude HTx if related with the severity of HF and thus likely to improve after HTx.
Advanced heart failure is most often associated with renal, hepatic, pulmonary, and metabolic dysfunction. Discrimination of reversible from irreversible other organ dysfunction requires careful medical work-up and subspecialty expertise. Some more general recommendations are described below.
Cardiorenal syndrome:Impaired renal function is an independent risk factor of mortality both in HF [4] and after HTx [5]. The international guidelines for the care of HTx candidates recommend precluding potential HTx candidates from single organ transplantation when the estimated glomerular filtration rate is <40 ml/min/1.73 m2 [3, 6].
Liver dysfunction is a predictor of adverse outcome after HTx. Abnormal standard liver function tests are common in HF and elevated bilirubin has been related to increased mortality after HTx [1, 7]. In patients with chronic right HF and venous hypertension or refractory ascites extensive diagnostic work-up is obligatory in order to assess the individual potential of liver dysfunction reversibility [3].
Secondary pulmonary hypertension without adequate response to pharmacological pulmonary vasodilation presents a contraindication to orthotopic HTx due to increased mortality posttransplant [3, 6, and ref. there]. Several studies indicate successful treatment of this therapy-resistant secondary pulmonary hypertension with permanent left ventricular MCS treatment [8] suggesting that this treatment option can be discussed on a case by case basis.
Metabolic dysfunctionis common with advanced heart failure and not an absolute contraindication when glycaemia is under control (glycosylated haemoglobin <7.5%) [5, 6]. In general, microvascular complication other than non-proliferative retinopathy is usually considered as absolute contra-indication to HTx. A pre-transplant BMI >30/m2 is a relevant risk factor, and obese patients are required to lose weight before listing [6]. In the UK, patients with >32/m2are unlikely to be accepted [3].
Common co-morbidities presenting absolute contra-indication to HTx are listed in table 3. Progress in medicine is fast and may, in the individual patient, permit treatment of non HF related comorbidities generally considered as contra-indication to HTx [6]. Therefore, discussion with experts in the respective subspecialty is recommended.
| Table 3: Contraindication to heart transplantation. |
| Absolute contraindication |
| 1. Sepsis and active infection |
| 2. Recent pulmonary embolism |
| 3. Active malignancy other than localised non-melanoma skin cancer |
| 4. Infiltrative cardiac disease with extensive extracardiac manifestation |
| Relative contraindication |
| 1. symptomatic peripheral or cerebrovascular disease |
| 2. chronic viral infection |
| 3. autoimmune disorder |
| 4. skeletal myopathy with good mid-term outlook |
| 5. substance abuse ( tobacco, excessive alcohol consumption) |
| 6. history of prior non-adherence |
In the past decade, recipient demographics have shown a shift in the leading diagnosis for which HTx was performed from ischaemic cardiomyopathy to dilated cardiomyopathy. Furthermore, the proportion of patients in their 60’s is increasing despite an unchanged median age of HTx recipients [1]. In addition, the load of comorbidity is rising, and the number of patients bridged to transplant with MCS support has grown from about 20% in the past 10 years to 30% in 2009 [1]. Respective data of Swiss HTx recipients are missing but may be available from the STCS prospectively collecting data of all HTx recipients as of May 2008.
In recent years, the gap has widened between the number of allocated donor hearts and the number of patients awaiting HTx in Switzerland. Subsequently, the number of patients on the waiting list as well as the time of HTx candidacy has increased in Switzerland (fig. 2) and elsewhere [1, 5]. In addition, mortality on the waiting list has increased in some countries [1, 5] while remaining stable in Switzerland (fig. 2).
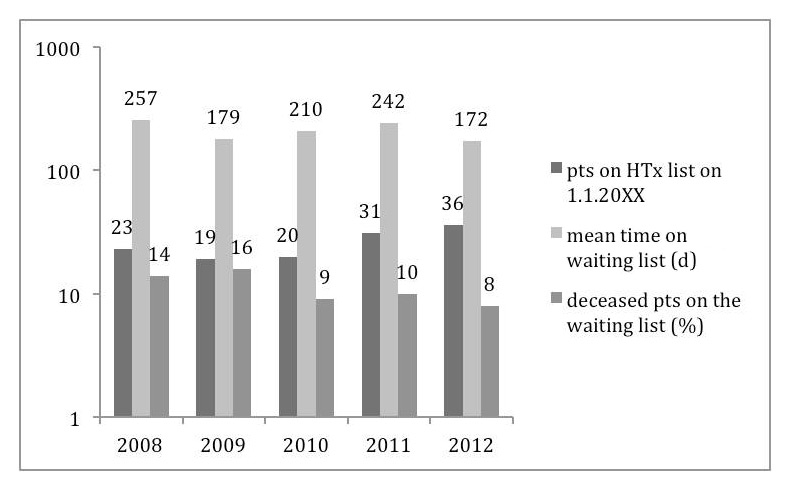
Figure 2
HTx waiting list characteristics (adapted from annual Swisstransplant reports).
In an attempt to decrease mortality on the waiting list, the donor heart allocation algorithm was adapted in the United Network of Organ Sharing in the United States. This adaptation favours allocation of donor hearts for patients waiting in urgent status and increased the respective percentage from 74% to 92% [9]. While decreasing mortality on the waiting list by 17% [10], this strategy prioritises allocation of donor hearts to patients waiting on the urgent list; setting patients on the normal waiting list at a disadvantage.
The Swiss transplantation law mandates equality of organ attribution for all patients on the waiting list. Therefore, the delay between time of listing and HTx is in Switzerland, at large, a function of the time on the waiting list provided that other criteria of the allocation algorithm such as blood group and donor/recipient body weight match. Since urgent listing bypasses the normal waiting list, several criteria are defined in the enactment of the transplantation law (article 4) demanding that candidates:
– receive positive inotropic or vasoactive support and remain under continued medical surveillance (paragraph 1a);
– are on mechanical circulatory support complicated by inherent disorder (e.g., mechanical disorder, secondary haematologic disorder, and others) (paragraph 1b);
– present recalcitrant transplant rejection (paragraph 1c)
– show co-morbidity associated with dismal prognosis comparable to the preceding conditions (paragraph 1d).
Between 2000 and 2009, the percentage of HTx in urgent status increased in Switzerland to a peak of 63% in 2009. In parallel, the overall time on the waiting list increased from a minimum of 79 days in 2002 [11] to a maximum of 257 days in 2008 (fig. 2) [12] suggesting non-equality of donor heart attribution. In anticipation of growing disadvantage of HTx candidates on the normal waiting list, the Swisstransplant Audit Group Heart (STAH) implemented in 2010 an unsolicited consensus aiming to limit HTx in urgent status to a 30% level of the annual total of all HTx. This consensus being active as of 2011 has been widely respected (2011: 8 urgent HTx /total of 36 HTx; 2012: 8/35; 2013: 7/33) without concomitant increase in waiting list mortality (fig. 2).
The advances in adult heart transplantation are largely based on the contemporary immunosuppression consisting of CNI (cyclosporine or tacrolimus) (Recommendation Class IIa, level of evidence A), inhibitors of purine synthesis such as MMF or azathioprine, and corticosteroids in the immediate postoperative phase (Recommendation Class IIa, level of evidence B) [13].
The mammalian target of rapamycin (mTOR) is a regulator protein playing an important role in growth-factor driven proliferation of a number of cell types, such as smooth vascular smooth muscle cells and lymphocytes. De novo immunosuppression with mTOR inhibitors sirolimus and everolimus is shown to effectively prevent acute cellular rejection when combined with standard dose cyclosporine [14, 15]. Recently, reduced cyclosporine dose with everolimus at 1.5 mg/d was shown to be non-inferior to standard-dose cyclosporine with 3 g/d MMF [16] in a multicentre, open-label, randomised–controlled trial in de novo HTx recipients using a larger composite endpoint (biopsy-proven rejection, acute rejection with haemodynamic compromise, graft loss/retransplant, and death or loss of follow-up). A pre-specified subgroup analysis of this study focusing on renal function revealed no significant difference between standard treatment and everolimus treatment at 1.5 mg/d with cyclosporine dose achieving reduced cyclosporine levels [16]. Overall, patients on everolimus at 1.5 mg/d with reduced cyclosporine dose presented more non-fatal severe adverse events (70.3% vs 55.6%; 95% CI 1.11–1.44) [16]. Similar to previous reports [14, 15], progression of cardiac allograft vasculopathy (CAV) was slowed in patients receiving everolimus at 1.5 mg/d and reduced cyclosporine [16]. Case reports and small series had suggested an increased incidence of incisional wound healing complications in de novo HTx recipients receiving mTOR inhibition. However, a meta-analysis of de novo HTx recipients on everolimus treatment reviewing 1,009 patients from three randomised controlled trials did not confirm this observation [17].
In maintenance HTx patients, switching immunosuppressants to mTOR inhibition was proven to improve outcome in HTx recipients with renal dysfunction [18], CAV [19], or malignancy [20, 21].
In acknowledgement of the promising profile of mTOR inhibitors, the NHLB working group on future directions of clinical research in HTx recommends clinical investigation of CNI-free regimen or early weaning of CNI in de novo and maintenance HTx recipients [22].
The 2010 guidelines for the care of HTx recipients recommend inclusion of sirolimus and everolimus in contemporary immunosuppressive regimens [13].
The tremendous progress in transplantation medicine bases on our understanding of cell-mediated acute rejection and the development of immunosuppressive drugs targeting crucial signaling pathways of T-cell activation [23]. More recently, regulatory T-cells (Treg) [24] were shown to suppress immune response to foreign antigens in the context of transplantation [25], and, therefore, may mediate prevention from rejection.
So far, B cells were largely considered as simple antibody secreting cells with a role in antibody-mediated acute rejection. While there is evidence that regulatory B-cells play a role in autoimmune disease [26], their role in transplantation is unknown.
First experience with specific tolerance was obtained in kidney transplant recipients using donor haematopoietic cell transplantation for nonmyeloablative induction of mixed chimerism. Results from a respective protocol of human leucocyte antigen-mismatched kidney allografts are promising [27], however, results from a clinical trial in HTx recipients conducted at the University of Louisville, USA, are pending [28].
CD28 is an important co-stimulatory molecule involved in T-cell proliferation at the time of antigen recognition. This suggests that inhibition of CD28/B7 T cell co-stimulatory receptors may present a highly specific way of immunosuppression [29]. In animal models, inhibition of CD28 results in reduced T cell proliferation and prolonged graft survival. Betalacept is a humanised immunoglobulin detecting a human homologue of CD28 and has been approved by the US Food and Drug Administration for use in renal transplantation based on its non-inferiority to standard cyclosporine combination therapy [30]. However, patients with betalacept treatment are at increased risk for lymphoproliferative disorder predominantly involving the central nervous system, furthermore, tuberculosis is observed more often [31].
Rituximab, a chimeric anti-CD20 (anti-B-cell) monoclonal antibody, in combination with intravenous immune globulin has been proven to be effective as a desensitisation regimen in kidney transplantation candidates with allosensitisation [32]. A clinical trial applying rituximab in HTx candidates is currently ongoing [33].
Plasmapheresis in conjunction with bortezomib treatment decreases antibody production in sensitised heart transplantation candidates without adequate response to treatment with intravenous immunoglobulin and rituximab [34]. However, use of bortezomib is associated with an increased risk for infection and peripheral neuropathy [31, 34].
Eculizumab is a humanised monoclonal antibody directed against the terminal complement protein C5. Binding of eculizumab blocks the cleavage of C5 and halts formation of the membrane attack complex. In renal transplantation, eculizumab has been shown to decrease the incidence of early antibody-mediated rejection in recipients with high levels of donor-specific alloantibody [35].
The incidence of multiorgan transplantation including heart-kidney or heart-liver, and heart-lung has increased steadily over the years but remains low overall [1]. Survival after multiorgan transplantation is comparable to heart transplantation on the basis of the Organ Procurement and Transplantation Network in the United States. Surprisingly, a lower incidence of acute cellular rejection and CAV is observed in multiorgan transplant recipients suggesting protective immune modulation [36]. Results from a recent meta-analysis suggest that simultaneous heart- and kidney transplantation should be considered in HTx candidates with more severe kidney disease because of prolonged survival of the renal allograft when compared to sequential transplantation [37].
CAV is a significant contributor to posttransplant mortality. The ISHLT registry documents a small but significant decrease in the cumulative incidence of CAV in the years 2002–2008 when compared to preceding decades. Nevertheless, the incidence of CAV remains significant with 8% at 1 year, 20% at 3 years, 30% at 5 years, and more than 50% over 10 years [1]. In general, CAV is most relevant for long-term mortality, nevertheless, diagnosis of CAV is associated with 10% mortality within the following 12 months. Factors known to increase the risk of CAV are donor and recipient age, donor history of diabetes mellitus, hypertension, as well as mismatch of body size or donor/recipient human leucocyte antigen. Finally, a significant interaction between CAV and donor/recipient gender mismatch has been observed [1]. The guidelines for perioperative-postoperative care of HTx recipients [13] recommend:
1. Use of statins to reduce CAV and improve long-term outcome. Recommendation Class I, level of evidence A
2. Prevention of CAV on the basis of strict control of cardiovascular risk factors as well as strategies to prevent CMV infection. Recommendation Class I, level of evidence C
3. Annual or biannual coronary angiography to assess development of CAV especially in patients with renal insufficiency. Recommendation Class I, level of evidence C
4. Follow-up coronary angiography after percutaneous coronary intervention at 6 months interval. Recommendation Class I, level of evidence C
5. In HTx recipients with established CAV the substitution of MMF or AZA by mTOR inhibition. Recommendation Class IIa, level of evidence B
6. Percutaneous intervention with drug-eluting stents for treatment of appropriate discrete lesions. Recommendation Class IIa, level of evidence C
Since long-term survival after HTx is improving, there is a growing need for evidence-based strategies that reduce long-term mortality resulting from non-immunological risk [1]. Based on the actual understanding of cardiovascular risk factors in the population, preservation of renal function, prevention and treatment of lipid disorder and glycaemia, as well as blood pressure control should have the potential for significant impact on long-term outcome after HTx. However, respective evidence in the form of randomised controlled trials is largely missing as emphasised recently by the Working Group of the NHLB Institute [28].
Arterial hypertension is common after HTx.
1. Antihypertensive treatment has benefits in HTx recipients similar to those in the general population. Treatment of hypertension after HTx should achieve the same goals recommended for the general population. Recommendation Class I, level of evidence C
2. Choice of antihypertensive drug treatment remains empiric with wide use of calcium channel blockers while ACE inhibition and ARB may be preferred in HTx patients with diabetes. Recommendation Class I, level of evidence C
3. Lifestyle modification as well as modification of concomitant cardiovascular risk factors are appropriate adjuncts to facilitate blood pressure control after HTx. Recommendation Class I, level of evidence C
4. Adjustment of immunosuppressive therapy, especially corticosteroid weaning, may be helpful in the management of hypertension in HTx recipients. Recommendation Class I, level of evidence C.
1. Glomerular filtration rate (GFR) with the modified diet in renal disease (MDRD) equation, urine analysis, and spot urine/albumin ratio should be obtained at least yearly after HTx. Measurement of serum creatinine for estimation of GFR should be obtained more often in patients with GFR <60 ml/min/1.73 m2, and in HTx recipients with fast GFR decline in the past (>4 ml/min/1.73 m2 per year). Recommendation class I, level of evidence C.
2. HTx recipients with an estimated GFR <30 ml/min/1.73 m2, proteinuria >500 mg/d (or albumin/creatinine ratio >500 mg/g), or rapidly declining GFR (see above) should be referred to a nephrologist for management of metabolic abnormalities and other complications of renal insufficiency and consideration of renal transplantation. Recommendation class I, level of evidence C.
3. CNI exposure should be lowered in HTx recipients to the minimum level required for effective immunosuppression. Recommendation class I, level of evidence B.
4. Interventions proven to slow progression of chronic kidney disease in the general population should be considered in all HTx recipients. These include strict glucose and blood pressure control and respective medical treatment based on the respective guidelines. Recommendation Class I, level of evidence C.
5. Haemoglobin levels should be measured at least once per year in all HTx recipients with chronic kidney disease. If anaemia is detected (Hb <13.5 g/dl in adult men; <12 g/dl in adult females), iron status should be controlled and erythropoiesis-stimulating treatment considered in order to maintain a haemoglobin level between 11 to 13 g/dl. Recommendation class I, level of evidence C.
1. Prevention, early detection, and appropriate therapy for diabetes should be considered as an important component of patient care after HTx. Recommendation Class I, level of evidence C.
2. Regular screening for diabetes after HTx is recommended by measuring fasting blood glucose levels and/or HbA1c, as appropriate. Recommendation Class I, level of evidence C.
3. Therapies for short-term peri-operative and long-term glycaemic control should be based on respective international recommendations. Recommendation Class I, level of evidence C.
4. CNI-sparing or CNI-free regimens should be used as appropriate. Recommendation class I, level of evidence C.
5. Annual screening for diabetic complications should be performed (ophthalmology, podiatry, peripheral vascular disease, and others). Recommendation Class I, level of evidence C.
The risk of malignancy in solid organ transplant recipients is increased when compared to the general population. This is believed to be a result of long-term immunosuppression comprising the patient’s immune system. Skin cancer is the most prevalent malignancy in HTx recipients with an incidence of 1% at 1 year, 10% at 5 years, 20% at 10 years, and 29% at 14 years [1]. Lymphoproliferative malignancies are much less common and available treatments are much less likely to result in a cure. The incidence of lymphoproliferative malignancy is 1%, 2%, 4%, 5% at 1, 5, 10, and 14 years post-transplant [1]. A recent study suggests a protective effect of statin treatment [38].
1. Recommendations for screening for colon, breast, and prostate cancer in the general population should be followed in HTx recipients. Recommendation Class I, level of evidence C
2. Close skin cancer surveillance with at least annual dermatologic exam. Recommendation Class I, level of evidence C.
3. Initial evaluation and a therapeutic plan for treatment of malignancy should be done by physicians familiar with transplant-associated malignancies. Recommendation Class I, level of evidence C.
Bisphosphonates suppress bone re-absorption, however, it is not known whether pre-operative administration of these drugs can prevent bone loss developing with the introduction of corticosteroids after HTx. Furthermore, the predictive value of bone mass density (BMD) measurement for fracture risk remains unproven in HTx recipients. Several studies describe a beneficial effect of bisphosphonates and vitamin D analogues on bone density in HTx recipients. Nevertheless, none of these studies was powered to detect a decrease in fracture rate.
1. Adult HTx candidates should be screened for pre-existing bone disease, preferably at the time of placement on the waiting list. Recommendation Class I, level of evidence C.
2. All HTx candidates should have the recommended daily allowance for 1000 to 1500 mg calcium depending on age and menopausal status, and vitamin D (500–1000 IU or as necessary to maintain serum 25–OH vitamin D level >30 ng/ml). Recommendation Class I, level of evidence C.
3. All adult HTx patients should begin therapy with bisphosphonates early after HTx and continue it at least during the first postoperative year. Recommendation Class I, level of evidence B.
4. Bisphosphonates can be used to treat bone-loss in the long-term after HTx and should be used in addition to calcium and vitamin D. Recommendation Class I, level of evidence C.
5. Proximal femur and lumbar spine BMD should be assessed by DEXA scanning in all adult patients at 1 year after HTx. Thereafter, annual controls are wise in patients receiving long-term treatment with bisphosphonates or corticosteroids. It is reasonable to repeat BMD measurement every 2 years in patients with osteopenia and every 3 years in patients with normal bone density. Recommendation Class IIa, level of evidence C.
In summary, posttransplant complications such as chronic kidney disease, hypertension, and hyperglycaemia need non-immunological strategies. Individualisation of immunosuppressive therapy may help to minimise non-immunologic adverse events, however, they should not risk the beneficial effect of immunological treatment on long-term outcomes. Clinical studies are necessary to provide evidence-based non-immunological treatment strategies after HTx.
The goal of HTx management includes prevention of acute cellular and antibody-mediated rejection, cardiac allograft vasculopathy, and optimal preservation of the integrity and function of the donor organ. The standard current posttransplantation protocol for detection of allograft rejection consists of echocardiographic control in conjunction with endomyocardial biopsy procurement (EMB). EMB is invasive, uncomfortable, and associated with potential damage to the tricuspid valve. In addition, sampling error limits the sensitivity of the EMB to 70% [39] while specificity suffers from interobserver variation in the histological interpretation even when performed by most experienced pathologists.
The FDA has recently approved two biomarker-based diagnostic blood tests monitoring acute rejection after HTx. The functional immunoassay ImmuKnow, Cylex® exploits the measurement of intracellular ATP that depends on T-cell activation, hypothesising that the activation status of T-cells identify patients at high risk for rejection or at high risk due to over- or under-immunosuppression [39]. The AlloMap blood test (AlloMap, XDx®) applies a mathematical algorithm for detection of acute cellular rejection on the basis of the expression of a panel of eleven different genes [40]. Both tests permit detection of acute cellular rejection with a high negative predictive value, however, detection of acute rejection should be achieved using a method with a high positive predictive value.
Recently it has been shown that cardiac magnetic resonance (CMR) imaging may provide a non-invasive method for detection of acute rejection with a high positive predictive value. Hallmark of CMR imaging is its ability to reproduce quantitative measurement of cardiac size and function, as well as myocardial ischaemia, fibrosis, and edema. Acute rejection is a host cell mediated reaction to donor antigens resulting in local inflammation and oedema [42]. Measurement of the T2 relaxation time permits quantification of interstitial free water because of an increase of the length of the decay time constant of the respective MR signal which is in proportion to the magnitude of myocardial oedema [for detailed explanations see 43 and ref. there]. More recently, translation of promising results from animal research into the human was obtained using latest 3T MRI technology in combination with a new software algorithm [44]. This novel technology achieves real time acquisition of T2 relaxation time and allows for three-dimensional mapping of the left ventricle with detection of interstitial edema at small scale volume (signal volume of 1.7 μl). This suggests that T2 mapping should be able to detect mild acute rejection with high sensitivity and specificity. The applicability of this method in the clinical setting is actually under investigation (Swiss National Fund 320030_147121/1).
1 Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The registry of the International Society of Heart and Lung transplantation: twenty-seventh official adult heart transplant report-2010. J Heart Lung Transplant. 2011;29:1089–103.
2 Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adulta: a report from the American College of Cardiology/American Heart Association Task Force on practice guidelines: developed in collaboration with the international Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016.
3 Banner NR, Bonser RS, Clark AL, Clark S, Cowburn PJ, Gardner RS, et al. UK guidelines for referral and assessment of adults for heart transplantation. Heart. 2011;97:1520–2.
4 Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8.
5 Ganesh JS, Rogers CA, Banner NR, Bonser RS, Steering Group of the UK Cardiothoracic Transplant Audit. Development and validation of a model to predict perioperative mortality following heart transplantation in the UK. J Heart Lung Transplant. 2004;23(2 suppl):S118–9.
6 Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. Listing criteria for heart transplantation: International Society of Heart and Lung Transplantation guidelines for care of cardiac transplant cadidates-2006. J Heart Lung Transplant. 2006;25:1024–42.
7 Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, et al. Liver function abnormalities and outcome in patients chronic heart failure: data from the Candesartan in heart failure: Assessment of mortality and morbidity in the CHARM program. Eur J Heart Fail. 2009;1:170–7.
8 Torre Amione G, Southard RE, Loebe MM, Youker KA, Bruckner B, Estep JP, et al. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29:195–200.
9 Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN / SRTR 2010 Annual Data Report. Rockwell MD: Department of Health and Human Services, Health Resources and Services Administration. Healthcare Systems Bureau, Division of Transplantation: 2011:89–106.
10 Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader sharing of donor hearts. Circ Heart Fail. 2012;5:249–58.
11 Swisstransplant Jahresbericht 2005; page 30.
12 Swisstransplant Jahresbericht 2012; page 12
13 Costanzo MR. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:114–56.
14 Keogh A, Richardson RM, Rugyrok P, Spratt P, Galbraith A, O’Driscoll G, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized controlled trial. Circulation. 2004;110:2694–700.
15 Eisen HJ, Tuczu EM, Dorent R, Kobashigawa J, Mancini D, Valentine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac transplant recipients. N Engl J Med. 2003;349:847–58.
16 Eisen HJ, Kobashigawa J, Startling RC, Pauly DF, Kfoury A, Ross H, et al. Everolimus vs. myocophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1206–13.
17 Zuckermann A, Wang SS, Ross H, Frigerio M, Eisen HJ, Bara C, et al. Impact of de novo everolimus based immunosuppression on incisional complications after heart transplantation. Transplantation. 2011;92:594–600.
18 Kushwaha SS, Kalpey Z, Frantz RP, Rodeheffer RJ, Clavell AL, Daly RC, et al. Sirolimus in cardiac transplantation: use as a primary immunosuppressant in calcineurin inhibitor-induced nephrotoxicity. J Heart Lung Transplant. 2005;24:2129–36.
19 Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, et al. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708–20.
20 Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–9.
21 Rubio-Viquera B, Hidalgo M. Targeting mTOR for cancer treatment. Adv Exp Med Biol. 2006;587:309–27.
22 Shah MR, Starling RC, Schwartz Longacre L, Mehra MR, on behalf of the working groups participants. Heart transplantation research in the next decade-a goal to achieving evidence-based outcomes. J Am Coll Cardiol. 2012;59:1263–9.
23 Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29.
24 Fontenot JD, Gavin MA, Rudensky AY. FoxP3 programs the development of and function of CD41CD251 regulatory T cells. Nat Immunol. 2003;3:330–6.
25 Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210.
26 Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–43.
27 Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transplant Int. 2008;21:1118–35.
28 National Institute of Health. Bone marrow transplant to induce tolerance in heart transplant recipients. Available at: http://clinicaltrials.gov/ct2/show/NCT00497757. Accessed 2013.
29 Kokko KE, Newell KA, Pearson TC, Larsen CP. Enhanced immunosuppression enhanced by cytotoxic T lymphocyte antigen-4 immunoglobulin. Curr Opin Organ Transplant. 2005;10:265–9.
30 Archdeacon P, Dixon C, Belen O, Albrecht R, Meyer J. Summary of the US FDA approval of betalacept. Am J Transplant. 2012;12:554–62.
31 Kobashigawa JA. The future of heart transplantation. Am J Transplant. 2012;12:2875–91.
32 Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–51.
33 National institute of Allergy and Infectious Diseases. Prevention of cardiac allograft vasculopathy using rituximab (rituxan) therapy in cardiac transplantation. Available at: http://clinicaltrials.gov/ct2/show/NCT01278745. Accessed 12/2013.
34 Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011;30:1320–6.
35 Locke E, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–5.
36 Pinderski LJ, Kirklin JK, McGiffin KD, Brown D, Naftel DC, Young KR, et al. Multi-organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant. 2005;24:1828–33.
37 Bloom RD, Doyle AM. Kidney disease after heart and lung transplantation. Am J Transplant. 2006;6:671–9.
38 Fröhlich GM, Rufibach K, Enseleit F, Wolfrum M, von Babo M, Frank M, et al. Statins and the risk of cancer after heart transplantation. Circulation. 2012;126:440–7.
39 Nakleh RE, Jones J, Goswitz JJ, Anderson EA, Titus J. Correlation of endomyocardial biopsy findings with autopsy findings in human cardiac allografts. J Heart Lung Transplant. 1992;11:479–85.
40 Rossano JW, Denfield SW, Kim JJ, Price JF, Jefferies JL, Decker JA, et al. Assessment of the Cylex ImmuKnow cell function assay in pediatric heart transplant recipients. J Heart Lung Transplant. 2009;28:26–31.
41 Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;310:1890–900.
42 Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20.
43 Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Mag Res. 2009;11:7–18.
44 van Heeswijk RB, Feliciano H, Bonanno G, Coppo S, Lauriers N, Locca D, et al. Quantitative Free-Breathing 3T T2–mapping of the heart designed for longitudinal Studies. J Cardiovasc Magn Reson. 2012,14(Suppl 1):O14.
Funding / potential competing interests: The author receives financial support from Swiss National Fund 320030–147121/1, Swiss Heart Foundation, Swiss Transplant Cohort Study, the Fondation Muschamps, PFIZER, and NOVARTIS for a clinical research project. No other potential conflict of interest relevant to this article was reported.