Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH1–24 injection in the diagnosis of adrenal insufficiency
DOI: https://doi.org/10.4414/smw.2014.13987
Thomas
Zueger, Marlen
Jordi, Markus
Laimer, Christoph
Stettler
Summary
BACKGROUND: In clinical practise the high dose ACTH stimulation test (HDT) is frequently used in the assessment of adrenal insufficiency (AI). However, there is uncertainty regarding optimal time-points and number of blood samplings. The present study compared the utility of a single cortisol value taken either 30 or 60 minutes after ACTH stimulation with the traditional interpretation of the HDT.
METHODS: Retrospective analysis of 73 HDT performed at a single tertiary endocrine centre. Serum cortisol was measured at baseline, 30 and 60 minutes after intravenous administration of 250 µg synthetic ACTH1–24. Adrenal insufficiency (AI) was defined as a stimulated cortisol level <550 nmol/l.
RESULTS: There were twenty patients (27.4%) who showed an insufficient rise in serum cortisol using traditional HDT criteria and were diagnosed to suffer from AI. There were ten individuals who showed insufficient cortisol values after 30 minutes, rising to sufficient levels at 60 minutes. All patients revealing an insufficient cortisol response result after 60 minutes also had an insufficient result after 30 minutes. The cortisol value taken after 30 minutes did not add incremental diagnostic value in any of the cases under investigation compared with the 60 minutes’ sample.
CONCLUSIONS: Based on the findings of the present analysis the utility of a cortisol measurement 30 minutes after high dose ACTH injection was low and did not add incremental diagnostic value to a single measurement after 60 minutes.
Introduction
Despite many attempts at improvement, the diagnosis of adrenal insufficiency remains a challenge and is, consequently, often delayed [1]. While a variety of diagnostic methods are presently available (e.g insulin tolerance test, metyrapone test, high dose ACTH stimulation test, Low dose ACTH stimulation test, morning cortisol value), there is an ongoing discussion on which test to use preferably [2–6]. While the insulin tolerance test (ITT) has been suggested to represent a gold standard, it has a number of contraindications, requires close supervision, and is, therefore, demanding for patients and medical staff [2, 7].
The ACTH stimulation test offers a less complex alternative to the ITT. While historically, it has been debated whether low-dose ACTH stimulation may have advantages over the high-dose stimulation test (HDT), the current literature does not support this notion anymore [8–11]. As a consequence, the HDT is well established in many centres for specific indications and has been shown to provide a reliable prognostic accuracy in the diagnosis of adrenal insufficiency [6, 12, 13]. The test is comparably easy to perform, has been shown to be cost-effective and is well tolerated by the majority of patients [7].
In general the protocol for the HDT encompasses blood samples for cortisol at time-points 0, 30, and 60 minutes after administering 250µg of synthetic ACTH1–24 [12, 14]. However, there is uncertainty about whether the diagnosis of AI based on HDT may be facilitated by focussing on a single cortisol value, thereby potentially further reducing associated resources. Previous studies have suggested that a single value taken after 30 minutes may adequately reflect adrenocortical response [2, 6, 13, 15]. Other reports conversely found the value after 60 minutes to provide an accurate predictor of adrenal function [4, 16, 17]. Such discrepancies may at least be partially explained by differences in study participants (e.g., age, ethnicity, etc.) as well as by underlying pathologies.
The aim of the present study was to assess whether cortisol measurement 30 minutes after HDT provides additional diagnostic value over a single sample after 60 minutes in the evaluation of AI in a Caucasian population.
Methods
Study design and patients
This was a retrospective single-centre study at a tertiary endocrine referral centre. All procedures were carried out in accordance with the local ethical guidelines and informed consent was obtained for each test.
Data of all consecutive HDT performed between January 2012 and August 2013 at the Division of Endocrinology, Diabetes and Clinical Nutrition of the University Hospital of Bern (Inselspital), Switzerland were analysed.
HDT was not performed less than 3 months after intervention in patients with pituitary surgery and not less than 24 months after intervention in case of radiotherapy for pituitary disease. In case of an ongoing glucocorticoid replacement therapy (e.g. due to suspected but not confirmed AI) all patients were switched to hydrocortisone prior to the HDT to avoid interference due to longer half-life of other compounds (e.g., prednisone, dexamethasone, etc.) and hydrocortisone was stopped at least 12 hours before testing. In case of pituitary disease, deficiencies of additional hypothalamic-pituitary axis hormones had to be under stable and adequate replacement therapy.
HDT protocol
Total serum cortisol was measured at baseline as well as 30, and 60 minutes after intravenous administration of 250 µg synthetic ACTH1–24 (Synacthen, Novartis Pharma Schweiz AG, Switzerland). Determination of cortisol was performed using the Roche Modular Analytics E170 electrochemiluminescence immunoassay (Roche Diagnostics, Rotkreuz, Switzerland). The intra- and inter-assay coefficients of variation were 1.7% and 1.8%, the recovery within 90–110% of serum value.
Statistical methods
Values are expressed as mean ± standard deviation (SD), unless otherwise specified. After testing for normal distribution (Shapiro-Wilk Test and Q-Q plots), continuous variables were analysed for significant differences by two-tailed t tests or ANOVA. In case of repeated measures a repeated-measure-ANOVA model was used and Bonferroni-correction was applied to correct for multiple comparison. We used a cut off level of 550 nmol/l to define AI, i.e., serum levels <550 nmol/l were interpreted as AI [2]. A p-value <0.05 was considered statistically significant. All analyses were performed using Stata 12.1 (Stata Corporation, College Station, TX, USA).
Results
A total of 73 individuals with suspected adrenal insufficiency (40 female and 33 male patients) were included in the analysis. Mean age was 51.2 ± 16.2 years. The indication for the ACTH stimulation test was a suspicion of primary adrenal disease in 9 (12.3%), suspected secondary (e.g., hypothalamic or pituitary) AI in 26 (35.6%), AI due to exogenous corticosteroids in 27 (37%), and other indications (e.g., unspecific symptoms like fatigue, dizziness, etc.) in 11 (15.1%) of the patients, respectively (table 1). Of the 73 patients 34 (46.6%) had received glucocorticoid-replacement therapy with an average hydrocortisone equivalent dose of 14.5 ± 9.6 mg per day in the period before the test. The main reasons for pre-existing glucocorticoid therapy were either pre-emptive temporary coverage after pituitary surgery or radiotherapy (e.g., until formal testing), or permanent glucocorticoid treatment due to an underlying disease (e.g., chronic inflammatory disease, rheumatoid disorders, etc.).

Figure 1
Cortisol values immediately before (0 min = baseline) and 30 minutes (30 min) and 60 minutes (60 min) after ACTH injection. Patients with adrenal insufficiency (AI) are plotted in dark grey; patients with normal adrenal function (NO) are plotted in light grey. * p <0.001 for comparison with baseline values.
Figure 1 depicts cortisol values before and after ACTH stimulation. In 20 of the 73 patients (27.4%) an AI was diagnosed based on the results of HDT (using either of the available measurements). Mean serum cortisol levels at baseline (immediately before ACTH injection) were 165.5 ± 110.4 nmol/l for patients suffering from AI and 346.2 ± 118.2 nmol/l for patients with intact adrenal function (NO), respectively (p <0.001). At 30 minutes after ACTH stimulation mean cortisol values were 332.3 ± 151.7 nmol/l for AI patients and 662.6 ± 119.8 nmol/l for NO, respectively (p <0.001). At 60 minutes after ACTH mean cortisol was 383.8 ± 158.2 nmol/l for AI patients and 778.7 ± 144.3 nmol/l for NO, respectively (p <0.001).
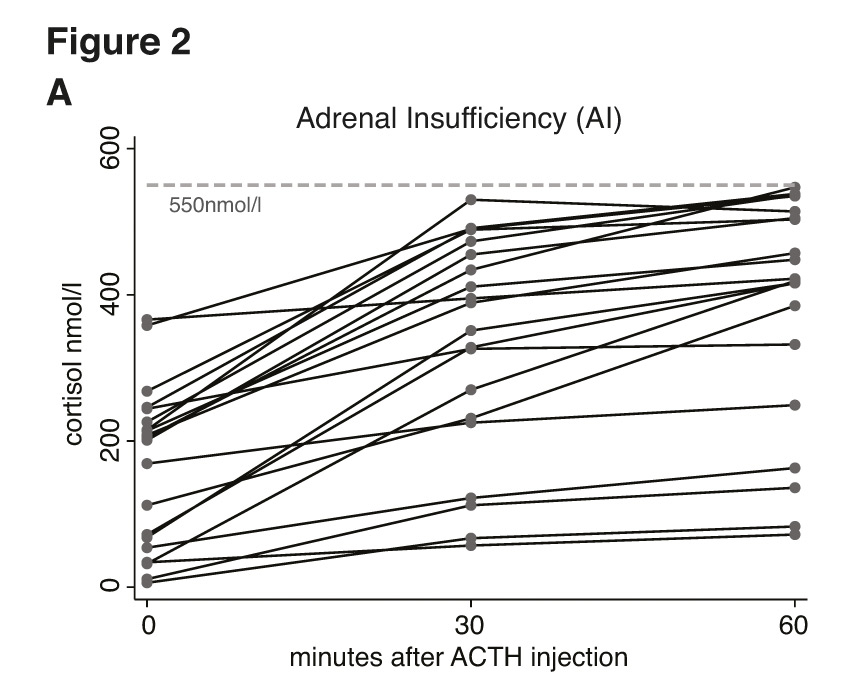
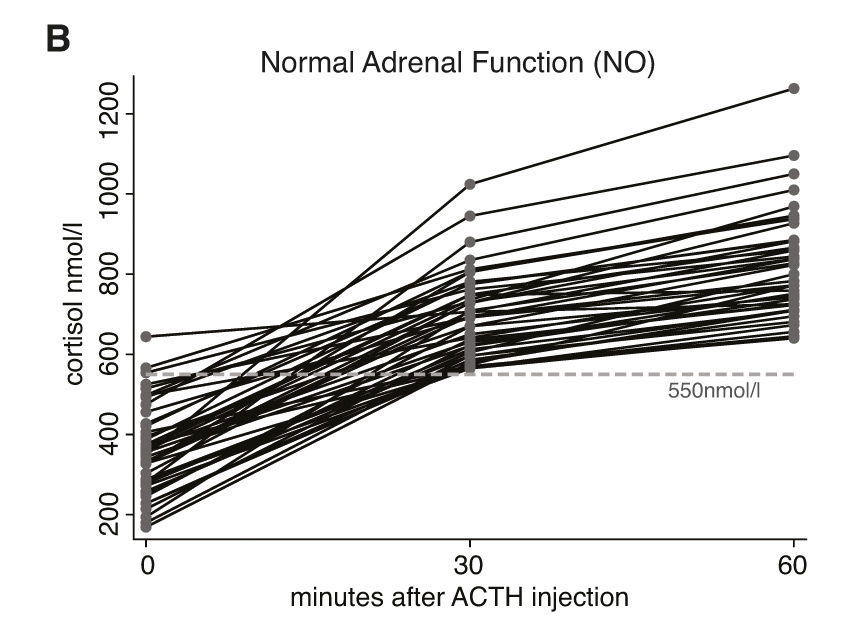
Figure 2 a-c depict the cortisol values of all included individuals at time points 0, 30, and 60 minutes after injection of ACTH. Figure 2a shows the results of individuals where all measurements were consistently <550 nmol/l. Figure 2b shows the results of those individuals where cortisol values were ≥550 nmol/l at both time-points (30 and 60 minutes). Figure 2c finally depicts the cortisol values of those individuals with discrepant results at 30 and 60 minutes (e.g., where the 30 minute result was <550 nmol/l but where the 60 minute value was ≥550 nmol/l). In none of the 73 individuals would the cortisol value taken 30 minutes after ACTH have added incremental diagnostic value to the interpretation of the HDT. Conversely, if interpretation of the HDT results had been based on the 30 minute cortisol value, exclusively, 10 out of 73 patients would have been diagnosed with AI although reaching sufficient cortisol levels 60 minutes after stimulation.
|
Table 1: Patient characteristics |
|
|
Total
|
NO
|
AI
|
p-value
|
| Tests (n) |
73 |
53 (72.6%) |
20 (27.4%) |
|
| Age (y) |
51.2±16.2 |
49.9±16.5 |
54.7±15.4 |
0.26 |
| BMI (kg/m2) |
27.9±5.8 |
27.7±6.7 |
28.3±3.4 |
0.71 |
| Sex
M
F |
33 (45.2%)
40 (54.8%) |
21 (39.6%)
32 (60.4%) |
12 (60%)
8 (40%) |
0.12 |
| Test-indication
primary ins.
second. ins.
exog. GC
other |
9 (12.3%)
26 (35.6%)
27 (37%)
11 (15.1%) |
8 (15%)
19 (35.9%)
17 (32.1%)
9 (17%) |
1 (5%)
7 (35%)
10 (50%)
2 (10%) |
0.48 |
| NO, normal adrenal function; AI, adrenal insufficiency; BMI, body mass index; m, male; f, female; test-indication, purpose of test based on the presumed diagnosis; primary ins., primary adrenal insufficiency; second. ins., secondary adrenal insufficiency (=central adrenal insufficiency); exog. CG, exogenous glucocorticosteroid medication |
Discussion
The main finding of the present analysis is that a cortisol measurement taken 30 minutes after stimulation with a high dose of ACTH does not offer additional diagnostic value in the diagnosis of AI. As a consequence, if HDT is chosen to evaluate a patient with potential AI in clinical practise, the present results suggest that the HDT protocol may be simplified to a single cortisol measurement 60 minutes after ACTH stimulation, thereby reducing labour and costs. Conversely, restricting the analysis to a single value after 30 minutes would have translated into a substantial misclassification of patients.
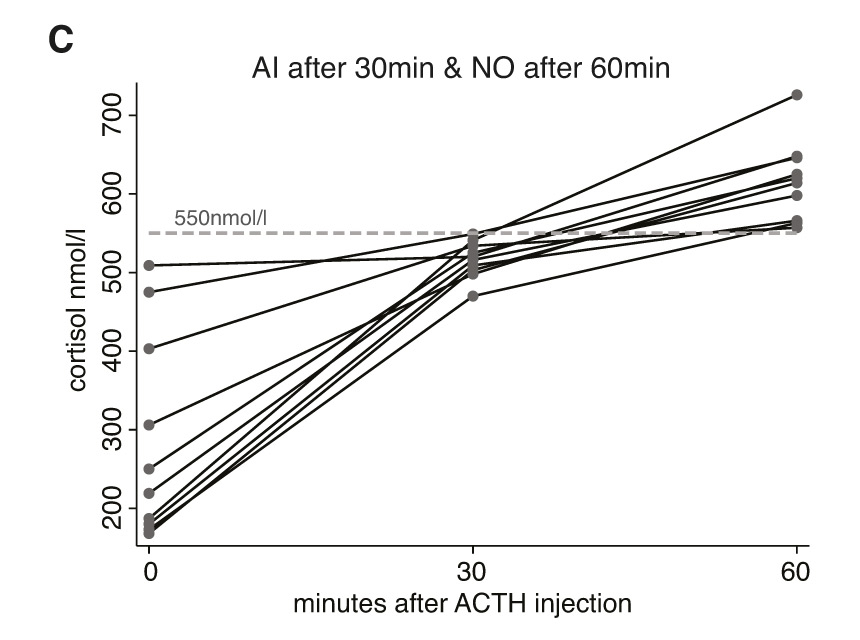
Figure 2
Individual cortisol values at time points 0, 30, and 60 minutes after injection of ACTH; grouped by patients with adrenal insufficiency (fig. 2a), normal adrenal function (fig. 2b) and patients with discrepant adrenal response at 30 and 60 minutes (fig. 2c).
The present findings are well in line with a retrospective study performed in South Asian patients [17] as well as a recent analysis investigating Caucasian individuals [16]. Similarly to the present analysis Chitale et al. found a significant proportion of patients being inappropriately diagnosed to suffer from AI if decisions were based on the 30 minute sample, exclusively, while no individual was found to pass the 30 minute sampling and to fail at 60 minutes [16]. These considerations go along with earlier studies investigating the kinetics of peak-cortisol response after ACTH administration, showing an early cortisol rise within the first 30 minutes, however followed by an additional increase between 30 and 60 minutes after ACTH injection [8, 16, 18–21].
While the gold standard in the diagnosis of AI may still be attributed to the ITT [1], this test is time-consuming and potentially cumbersome for patients and investigators. Furthermore, there are several contraindications for the ITT (as for instance coronary artery disease or seizure disorders). Indeed, the indication to perform HDT in the present study was essentially the presence of accepted contraindications to ITT or refusal of patients to undergo more invasive testing. Of note, the HDT has been validated against the ITT in numerous studies generally revealing a high agreement [13, 15, 18, 22–24]. As a consequence, and despite the well-known fact there are some reports showing discrepancies between results of the ITT and HDT [15, 23], especially in case of secondary AI [12], the HDT is an accepted screening method for AI due to its advantages in feasibility and comparably low associated risks. Interestingly, studies comparing the findings in the ITT with cortisol values taken 60 minutes after ACTH stimulation reported similar correlation as for the values after 30 minutes [8, 21]. Discrepant findings in studies comparing ITT to HDT may be ascribed to different cortisol assays, varying cut off values, as well as different patients under investigation (e.g., pre-test probability, ethnicity, etc). In general, for HDT cortisol cut off levels between 500–600 nmol/l are chosen [4, 12, 25]. Raising the cut off level would improve the sensitivity of the test, however, by simultaneously increasing the risk of false positive results, translating into over-diagnosis and over-treatment [12, 13, 16]. As a consequence, the present study used a widely accepted cut off level of 550 nmol/l [2, 16, 23, 26]. Of importance, the present study was performed in a mixed patient population of a tertiary endocrine referral centre encompassing both individuals with a suspicion of primary (adrenal) as well as secondary (central or exogenous) AI. Although one may criticise that this may reduce generalisability of the present findings, this also reflects daily clinical practise. And it is noteworthy that an incremental value of the 30 minute sample could not been found in any of the potential subgroups. We are entirely aware that results of HDT must be interpreted with caution in patients after pituitary surgery or radiotherapy due to pituitary disease, and testing should not be performed directly after such interventions since accuracy may be substantially reduced for secondary AI [12]. However, if HDT is performed later in the follow-up previous reports have corroborated high predictive values in excluding clinically significant secondary AI, thereby again recommending HDT as a primary screening test also for secondary AI [2, 13]. As a consequence, the present study was careful not to perform HDT less than 3 months after pituitary surgery and not less than 24 months after pituitary irradiation therapy, thereby minimising confounding. Still, it has to be emphasised that if AI is still clinically suspected after a first negative HDT result, other stimulation tests (ITT, Metyrapone) should clearly be added to detect possible false negative HDT results [1, 12–14].
We acknowledge several limitations concerning the present study. First, analyses were based on retrospective data and will formally have to be validated in an independent prospective set of individuals. Second, the aim of the study was not to compare the diagnostic value of the HDT against other test modalities but solely to assess whether a cortisol measurement 30 minutes after HDT provides additional diagnostic value over a single sample after 60 minutes. To further substantiate these findings, a validation against an external gold standard (e.g. ITT) would be highly interesting and valuable. However, if in a clinical situation the HDT is chosen as a diagnostic tool in the evaluation of AI, then the present data at least suggest that independent of correlations with ITT or other test modalities the HDT protocol may potentially be simplified. Third, the present study encompassed a mixed patient population at a tertiary endocrine referral centre including individuals with suspected adrenal as well as central AI or AI secondary to exogenous glucocorticoids. As a consequence our findings apply to these patient characteristics, exclusively, and generalisability is clearly restricted. On the other hand, the findings of the present study are applicable to all subgroups. In addition, the study was performed at one institution with the identical medical investigators over the entire study period, applying accepted diagnostic cut off values, and using comparably strict inclusion criteria for patients with suspicion of central AI (e.g., time period between interventions and testing), as well as in those with exogenous glucocorticoids (e.g., withholding hydrocortisone for a defined period). Finally, we used a HDT protocol and no statements can be made on the validity of either cortisol measurement after the application of lower doses of ACTH since this was not the focus of the present study.
In conclusion, the present results suggest that a cortisol measurement taken 30 minutes after stimulation with a high dose of ACTH does not offer additional diagnostic value in the diagnosis of AI as compared to a single measurement after 60 minutes. As a consequence, if HDT is chosen to evaluate a specific patient with potential AI in clinical practise, the present results suggest that the HDT protocol may be simplified to a single cortisol measurement 60 minutes after ACTH stimulation, thereby reducing labour and costs.
Acknowledgments:We are grateful to the study nurses performing the tests as well as all involved patients.
References
1 Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–93.
2 Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91:43–7.
3 Agwu JC, Spoudeas H, Hindmarsh PC, Pringle PJ, Brook CG. Tests of adrenal insufficiency. Arch Dis Child. 1999;80:330–3.
4 Endert E, Ouwehand A, Fliers E, Prummel MF, Wiersinga WM. Establishment of reference values for endocrine tests. Part IV: Adrenal insufficiency. Neth J Med. 2005;63:435–43.
5 Fischli S, Jenni S, Allemann S, Zwahlen M, Diem P, Christ ER, Stettler C. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2008;93:539–42.
6 Reimondo G, Bovio S, Allasino B, Terzolo M, Angeli A. Secondary hypoadrenalism. Pituitary. 2008;11:147–54.
7 Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur J Endocrinol. 2009;160:9–16.
8 Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:1558–62.
9 Suliman AM, Smith TP, Labib M, Fiad TM, McKenna TJ. The low-dose ACTH test does not provide a useful assessment of the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Clin Endocrinol (Oxf). 2002;56:533–9.
10 Nye EJ, Grice JE, Hockings GI, Strakosch CR, Crosbie GV, Walters MM, Torpy DJ, Jackson RV. Adrenocorticotropin stimulation tests in patients with hypothalamic-pituitary disease: low dose, standard high dose and 8–h infusion tests. Clin Endocrinol (Oxf). 2001;55:625–33.
11 Soule S, Van Zyl Smit C, Parolis G, Attenborough S, Peter D, Kinvig S, Kinvig T, Coetzer E. The low dose ACTH stimulation test is less sensitive than the overnight metyrapone test for the diagnosis of secondary hypoadrenalism. Clin Endocrinol (Oxf). 2000;53:221–7.
12 Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204.
13 Gleeson HK, Walker BR, Seckl JR, Padfield PL. Ten years on: Safety of short synacthen tests in assessing adrenocorticotropin deficiency in clinical practice. J Clin Endocrinol Metab. 2003;88:2106–11.
14 Maghnie M, Uga E, Temporini F, Di Iorgi N, Secco A, Tinelli C, Papalia A, Casini MR, Loche S. Evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic-pituitary disorders: comparison between insulin-induced hypoglycemia, low-dose ACTH, standard ACTH and CRH stimulation tests. Eur J Endocrinol. 2005;152:735–41.
15 Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26:53–9.
16 Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79:14–9.
17 Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48:519–23.
18 Gonzalbez J, Villabona C, Ramon J, Navarro MA, Gimenez O, Ricart W, Soler J. Establishment of reference values for standard dose short synacthen test (250 microgram), low dose short synacthen test (1 microgram) and insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in normal subjects. Clin Endocrinol (Oxf). 2000;53:199–204.
19 Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159:275–80.
20 Longui CA, Vottero A, Harris AG, Chrousos GP. Plasma cortisol responses after intramuscular corticotropin 1–24 in healthy men. Metabolism. 1998;47:1419–22.
21 Talwar V, Lodha S, Dash RJ. Assessing the hypothalamo-pituitary-adrenocortical axis using physiological doses of adrenocorticotropic hormone. QJM. 1998;91:285–90.
22 Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44:141–6.
23 Kane KF, Emery P, Sheppard MC, Stewart PM. Assessing the hypothalamo-pituitary-adrenal axis in patients on long-term glucocorticoid therapy: the short synacthen versus the insulin tolerance test. QJM. 1995;88:263–7.
24 Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet. 1988;1:1208–10.
25 Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol (Oxf). 1998;49:287–92.
26 Sacre K, Dehoux M, Chauveheid MP, Chauchard M, Lidove O, Roussel R, Papo T. Pituitary-adrenal function after prolonged glucocorticoid therapy for systemic inflammatory disorders: an observational study. J Clin Endocrinol Metab. 2013;98:3199–205.