Arterial age as a substitute for chronological age in the AGLA risk function could improve coronary risk prediction
DOI: https://doi.org/10.4414/smw.2014.13967
Michel
Romanens, Franz
Ackermann, Isabella
Sudano, Thomas
Szucs, J. David
Spence
Summary
PRINCIPLES: As a result of the relatively low sensitivity of coronary risk charts, such as the Swiss coronary risk calculator (Arbeitsgruppe Lipide und Atherosklerose, AGLA), for detecting subjects with future myocardial infarction, the performance of arterial age (aa) as a surrogate marker for chronological age (ca) was tested.
METHODS: In a practice based sample, burden of carotid plaque was obtained with ultrasound, using total plaque area (TPA). In this derivation cohort, sex-specific 5-year groups of mean TPA were calculated in subjects aged between 35 and 79 years. The arterial age formula was found by fitting an exponential function on these data. AGLAca and AGLAaa were tested externally for their ability to detect 13 myocardial infarctions in 684 subjects (validation cohort).
RESULTS: The derivation cohort included 1,500 subjects (mean age 59 ± 9 years, mean TPA 54 ± 52 mm2, 5% diabetics, 43% women). Arterial age was found to be y = 5.4175e0.0426x in men and y = 4.1942e0.0392x in women. Mean 10-year AGLAca coronary risk was comparable to AGLAaa (8% ± 9% vs 9% ± 15%). Receiver operating characteristic (ROC) analysis of AGLAca and AGLAaa results showed areas under the curve of 0.65 (p = 0.041) and 0.78 (p <0.0001), respectively, (p = 0.041 for the difference = 0.13). This finding was also confirmed by a Cox proportional hazards regression model on patients' event-free survival (p = not significant for AGLAca, p = 0.0003 for AGLAaa).
CONCLUSIONS: Arterial age derived from TPA could be used instead of chronological age in the AGLA coronary risk function. Further studies on the external validity and cost effectiveness of the additional ultrasound imaging study are necessary.
Abbreviations
aa arterial age
AGLA Arbeitsgruppe Lipide und Atherosklerose (Swiss coronary risk calculator)
AUC area under the curve
ca chronological age
HDL high-density lipoprotein
LDL low-density lipoprotein
IMT intima media thickness
NRI net reclassification improvement
TPA total plaque area of the carotid arteries
ROC receiver operator characteristic
Introduction
Arterial age is considered to be a marker for biological age and Grundy probably described it best when he paraphrased William Osler, who first observed that by transforming age as a risk factor, patients are as old as their arteries [1]. Since increasing age can be dominant over other major independent cardiovascular risk factors in coronary risk functions, it has become appropriate to determine the vascular/arterial age of a subject. The heart age in the Framingham Heart Study is calculated as the age of a person at the predicted risk but with all other risk factor levels in normal ranges [2]. It has been suggested that arterial age can be used instead of the chronological age in risk functions since chronological age is only a surrogate of the atherosclerotic burden on the basis of carotid intima-media thickness [3]. An arterial age using coronary artery calcium score has been derived from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, where replacing observed age with arterial age performed significantly better in the area under the receiver operating curve [4].
Use of the Framingham vascular age and risk functions [2] to calculate arterial age has led to the realisation that, although it would be helpful in communicating risk to patients, there was no underlying determination of atherosclerotic burden [5]. Therefore, use of noninvasive, radiation-free methods for determining the atherosclerotic burden of subjects is most desirable. Determining the arterial age may be more convincing for both patients and doctors [6].
In order to compensate for the shortcomings of not knowing the atherosclerotic burden of a subject, determination of carotid atherosclerosis as a validated marker of coronary and stroke risk can be used to define arterial age. This is achieved by measuring the area of carotid plaques (total plaque area, TPA), which makes it possible to quantify and track atherosclerosis burden within the whole carotid tree [7–12]. Carotid TPA has been shown to be a stronger predictor of cardiovascular risk than carotid intima-media thickness [8, 9, 11] and is useful in patient management [7, 8].
The intent was to derive and validate an arterial age formula based on TPA, which may replace chronological age in coronary risk prediction.
Methods
Subject selection
Subjects were selected from two single-centre databases that were prospectively collected during the last 10 years. (A) The CORDICARE database of the Vascular Risk Foundation VARIFO, consisting of consecutive self-referred subjects from a study approved by the local ethics committee (n = 900), who were alerted to participate in the study by mass media (radio broadcasting and newspaper advertisements). Subjects were prospectively recruited and provided written informed consent. (B) The KARDIOLAB database consisting of consecutive physician referred patients (n = 600). These healthy patients were referred to KARDIOLAB in order to further stratify coronary risk using carotid plaque imaging. The selection criteria were absence of cardiovascular disease derived from the history of subjects (CORDICARE) or from the patients' history including a cardiological work-up (KARDIOLAB), where appropriate, and a complete survey of the presence and extent of the major independent cardiovascular risk factors at the time of the carotid examination. The clinical characteristics of this study population (derivation cohort) have previously been extensively described [15]. In brief, coronary risk assessment was performed by determining a coronary risk factor including medical history, blood pressure, measurement of total, low-density lipoprotein(LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides, blood glucose levels and quantifying TPA with an ultrasound examination as described below.
Total plaque area measurements
Presence of plaque was determined by a transverse scan applied to the left and right carotid artery with a high resolution ultrasound linear transducer using a 7.5–12.0 MHz probe, which identified all plaques defined by intimal thickening ≥1.0 mm. The complete length of the common carotid artery, the visible parts of the internal and external carotid arteries and the bulb were scanned. Once the largest extent of a plaque was found by panning around the artery, the frozen longitudinal images were used to trace the plaque directly on the screen. The longitudinal area of the plaque was then automatically displayed and the areas of all such plaques were summed up to compute the value for TPA in mm2. The imaging procedure was completed with the subject in the supine position, with the head retroflexed at 10°–20° and tilted at 35°–45° away from the sonographer, who was standing in front of the subject as previously described [16]. All TPA measurements were made by a single investigator and the reproducibility of this test has been reported elsewhere [9].
Calculation of arterial age
The mean values of TPA derived from 5-year intervals for men and women aged 35 to 79 years were plotted against the chronological age. An exponential function was added, which connected these 5-year intervals, and the equation of the line was displayed along with the 95% confidence intervals (CIs). These two exponential equations describing TPA (y) as a function of age (x) were solved for x in order to determine the age at which such an amount of TPA is generally found in the population, i.e. the arterial age, for men and women separately.
Integration of arterial age into the coronary risk function
Swiss 2012 guidelines for coronary risk assessment (AGLA) were used [17]. These guidelines incorporate the German PROCAM risk function with a calibration factor of 0.7 for Switzerland in order to determine the 10-year risk for incident myocardial infarction. The calculations for the individual 10-year coronary risks were based on gender, blood pressure, smoking status, HDL and LDL cholesterol profile, triglyceride levels, presence or absence of diabetes mellitus, premature coronary artery disease in the parents of the subjects and either chronological or arterial age.
External validation of arterial age in the AGLA coronary risk function
Arterial age was confirmed externally using a validation cohort provided by the Robarts Research Institute in London, Canada [9]. Of the original 1,686 patients, 150 were excluded because of diabetes mellitus, 356 were excluded because of a previous transient ischaemic attack, 152 were excluded because of a previous stroke, 156 were excluded because of a previous myocardial infarction and 188 were excluded because of missing laboratory values needed to calculate AGLA risk. Therefore, 684 primary care subjects from the original London cohort remained, in which 13 myocardial infarctions occurred during a follow-up of 3.3 years. For the calculation of arterial age, individual TPA of these 684 subjects was measured locally and introduced into our arterial age formula in order to determine arterial age.
Statistics
Datasets for each patient were entered into an Excel spread sheet (Microsoft, Richmond, USA) where all basic calculations such sa population characteristics were calculated. Statistical analysis such as receiver operator characteristic (ROC) analysis was performed using the MedCalc software [18], using the DeLong-DeLong method in order to compare the ability of AGLAca and AGLAaa to predict incident myocardial infarctions in the validation cohort [19]. For the net reclassification improvement (NRI) the standard method originally described by Pencina et al. [20] was employed as follows: NRI = [correct reclassification of events minus incorrect reclassification of events] x [1/all events] + [correct reclassification of nonevents minus incorrect reclassification of non-events] x [1/all non-events]. Pearson's Chi-squared statistic was used to calculate the level of significance. Cox regression analysis was calculated for AGLAca und AGLAaa separately and in combination and was performed with the level of statistical significance set at p <0.05.
|
Table 1:Patient characteristics from which arterial age was derived.
|
|
n = 1,500
|
n
|
%
|
| Females |
642 |
43 |
| Age (years) mean ± SD |
59 ± 9 |
|
| Family history for CAD |
250 |
17 |
| Diabetes mellitus type II |
78 |
5 |
| Current smoker, no. % |
270 |
18 |
| Blood pressure systolic (mm Hg) mean ± SD |
131 ± 17 |
|
| TPA (mm2) mean ± SD |
54 ± 52 |
|
| Individuals with zero TPA |
109 |
7 |
| Total cholesterol (mmol/l) mean ± SD |
5.8 ± 1.1 |
|
| HDL cholesterol (mmol/l) mean ± SD |
1.5 ± 0.5 |
|
| LDL cholesterol (mmol/l) mean ± SD |
3.6 ± 1.0 |
|
| Triglycerides (mmol/l) mean ± SD |
1.5 ± 1.0 |
|
| AGLAca, mean ± SD |
8 ± 9 |
|
| AGLAaa, mean ± SD |
9 ± 15 |
|
| aa = arterial age; AGLA = Arbeitsgruppe Lipide und Atherosklerose (Swiss coronary risk calculator); ca = chronological age; CAD = coronary artery disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation; TPA = total plaque area of the carotid arteries |
|
Table 2:Mean, standard deviation (SD) and number (n) of patients imaged to derive age- and sex-specific values for 5-year intervals. |
| |
|
Age group (years)
|
| |
|
35–39
|
40–44
|
45–49
|
50–54
|
55–59
|
60–64
|
65–69
|
70–74
|
75–79
|
| Male |
n |
7 |
30 |
107 |
170 |
165 |
179 |
121 |
53 |
19 |
| |
Mean |
21 |
32 |
38 |
50 |
57 |
71 |
87 |
104 |
125 |
| |
SD |
26 |
31 |
45 |
48 |
51 |
48 |
61 |
76 |
69 |
| Female |
n |
5 |
12 |
70 |
117 |
127 |
149 |
88 |
44 |
24 |
| |
Mean |
21 |
20 |
25 |
22 |
28 |
43 |
61 |
70 |
86 |
| |
SD |
24 |
25 |
44 |
23 |
25 |
33 |
45 |
55 |
53 |
Results
The derivation cohort contained 1,500 subjects (mean age 59 ± 9 years, mean TPA was 54 ± 52 mm2, 5% were diabetics, 43% were women; table 1). The number of subjects without carotid plaque was 109 and the exponential nature of carotid total plaque area (TPA), grouped into 5-year sex-specific TPA amounts is outlined in table 2 and figure 1. The best fit correlation was an exponential function (Appendix):
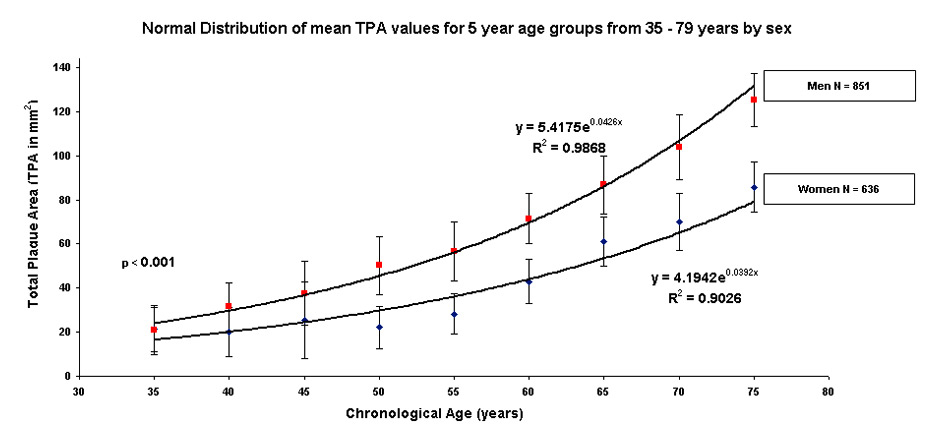
Figure 1
Distribution of mean (± standard deviation) TPA values by age group in 851 men and 636 women.
Exponential curve of total plaque area (TPA) for 851 male and 636 female patients aged between 35 and 79 years. The formulae shown above the male curve in red and below the female curve in blue form the basis of the arterial age calculations.
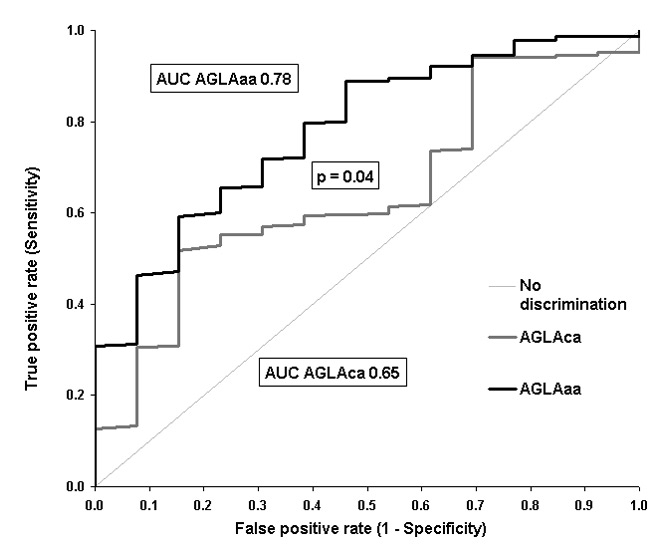
Figure 2
ROC Curve for AGLAca and AGLAaa for the detection of coronary events in the validation cohort [12].
Ability of AGLAca (black line, area = 0.65 (95% CI = 0.61–0.68, p = 0.041) and AGLAaa (grey line, area = 0.78, AGLAaa (95% CI = 0.75–0.81, p <0.0001) to detect 13 incident myocardial infarctions (p = 0.041).
aa = arterial age; AGLA = Arbeitsgruppe Lipide und Atherosklerose (Swiss coronary risk calculator); AUC = area under the curve; ca = chronological age; CI = confidence interval; ROC = receiver operator characteristic
1 TPA = 5.4175e0.0426age in men
2 TPA = 4.1942e0.0392age in women.
The final formulas for arterial age read as follows:
7 arterial age = [ln (TPA / 5.4175)] / (0.0426) in men
8 arterial age = [ln (TPA / 4.1942)] / (0.0392) in women
The mean arterial age of the derivation cohort was 49 ± 21 years. AGLAca 10-year coronary risk was 8% ± 9% and AGLAaa was 9% ± 15%. The validation cohort consisted of 684 healthy subjects with a follow-up time of 3.3 ± 1.8 years, and has been described elsewhere [12]; mean chronological age was 50 ± 13 years and mean arterial age was 42 ± 25 years.
The 10-year coronary risk in the derivation cohort was 5% ± 8% for AGLAca and 6% ± 13% for AGLAaa. In ROC analysis the area under the curve (AUC) was 0.65 for AGLAca (95% CI = 0.61–0.68, p = 0.041) and was 0.78 (95% CI = 0.75–0.81, p <0.0001) for AGLAaa. This improvement of 0.13 was statistically significant (p = 0.041) (fig. 2). The Cox proportional hazard regression model was not statistically significant for AGLAca (p = 0.2519), but was highly significant for AGLAaa (p = 0.0003). The reclassification calculations for the NRI in subjects without events was –22/671 = –3% and was 3/13 = 23% in subjects with an event, giving to a total NRI of 20% (p <0.0001).
Discussion
The concept of substituting chronological age by arterial age in the AGLA risk function in order to improve coronary risk prediction was tested. The distribution of average sex-specific 5-year aggregated TPA values in this relatively large single-centre, single observer, group of healthy subjects (n = 1,500) served to derive an arterial age formula (see fig. 1). Use of the inverse of these functions allowed us to calculate the arterial age of any given patient based on TPA.
The prognostic impact of this arterial age function implemented into AGLAaa was validated externally in a validation cohort previously published by our group [12]. ROC analysis, the net reclassification improvement (NRI) and a Cox survival regression model showed that the arterial age risk model (AGLAaa) performed significantly better than the chronological age model AGLAca (increase in AUC by 0.13 from 0.65 to 0.78, p = 0.041; NRI of + 20%; Cox regression AGLAca p = not significant, AGLAaa p <0.0003).
Performing reclassification using arterial age instead of chronological age, average coronary risk did not significantly increase in the derivation cohort, since average AGLA risk was comparable for AGLAca and AGLAaa (8% vs 9%, p = not significant).
Arterial age is an appealing surrogate for chronological age, since it is obtained rapidly, may be cost effective (<75 CHF per examination), and may increase the real risk perception of an individual with respect to coronary risk. It may therefore become an additional nonscreening tool in selected primary care subjects in order to increase the accuracy of preventive medicine in cases where the physician is left with uncertainty, for example, with respect to prescribing preventive medication. Further, a higher adherence to risk-lowering therapies may ensue [6] and arterial age may even be used to assess the effect of risk-lowering activities in a follow up-coronary risk assessment, where increases over time in atherosclerotic burden have been shown to increase coronary risk both using TPA [9] and coronary calcifications [21]. It has been shown that preventive therapy is effective in halting or even reversing the formation of carotid plaque, thereby halting the process of vascular aging in long-term observations [5, 18]. Such observations were possible in plaque-imaging techniques, such as for carotid plaque using 3D magnetic resonance, but not when using ultrasound derived intima-media thickness (IMT) in patients treated with statins for 6 months [23].
The concept of arterial or vascular age has been developed by others, especially using carotid IMT [24]. However, IMT measurements rely on submillimetre risk assessments and require much more expertise, which may hinder the widespread use of this method, as was shown from arterial age calculations using the Atherosclerosis Risk in Communities (ARIC) database, in which a 0.1 millimetre increase of IMT (from 0.70 to 0.80 mm) increases arterial age from 60 to 70 years in a 40-year-old white man [25]. Moreover, the clinical utility of IMT measures, especially when excluding plaque measures from the assessment, has been questioned in several studies [6, 8, 20–22]. Vascular age can also be calculated using the Systematic Coronary Risk Evaluation (SCORE) risk charts; however, such calculations do not rely on imaging of atherosclerosis [29].
There are some limitations of our approach. Because arterial age was validated externally in a rather small cohort with only 13 coronary events during follow up [12], our validation can furnish only preliminary results and there is a need for further validation of our arterial age function. However, although arterial age was derived from the Swiss derivation cohort, its external application to TPA values in the Canadian validation cohort using ALGAaa preserved its prognostic strength. Further, our carotid imaging tool-derived formulae are from a single centre and single observer setting. These results need to be validated externally. Another limitation lies in the fact that the AGLA calculator allows calculating coronary risk only up to an age of 65 years. This age limitation was increased to 80 in our risk calculations for both chronologically and arterially determined AGLA risk. Given the high prognostic improvement when applied to the validation cohort, such an approach can be justified. However, as an alternative, the clinician may just rely on arterial age as an indicator for coronary risk and prefer to calculate post-test risk using the Bayes formula, as published previously by our group [12]. Finally, our results stem from a relatively low risk-population and may be less valid in higher risk patients; however, from a preventive point of view, early risk intervention may be more effective.
In conclusion, our arterial age coronary risk function outperformed the AGLA risk function when chronological age was substituted by arterial age. One can expect that the knowledge of arterial age is a better motivator for patients to adhere to a preventive lifestyle and medications. Indeed, Spence has used this approach explicitly in his clinic for more than 10 years, and has been objectively shown to have the highest medication compliance rates among patients participating in the Insulin Resistance Intervention after Stroke trial, a large multicentre randomised trial funded by the US National Institutes of Health [30]. Therefore, ultrasound-based measures of carotid plaques, a low cost, rapid and nonirradiating specific marker of atherosclerosis, is a new concept for refined coronary risk assessments in selected subjects, for example, at intermediate coronary risk. It permits assistance in preventive strategies in primary care. Further practice based studies are needed to confirm our findings externally, prognostically and with respect to cost-effectiveness.
References
1 Grundy SM. Age as a risk factor: you are as old as your arteries. Am J Cardiol. 1999;83:1455–7.
2 D'Agostino RB, Vasan RS, Pencina MJ, Wolf P a, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53.
3 Stein JH, Fraizer MC, Aeschlimann SE, Nelson-Worel J, McBride PE, Douglas PS. Vascular age: integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27(7):388–92.
4 McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial Age as a Function of Coronary Artery Calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2009;103:59–63.
5 Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–90.
6 Rodondi N, Collet T-H, Nanchen D, Locatelli I, Depairon M, Aujesky D, et al. Impact of carotid plaque screening on smoking cessation and other cardiovascular risk factors: a randomized controlled trial. Arch Intern Med. 2012;172:344–52.
7 Spence D, Hackam D. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 2010;41:1193–9.
8 Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., S. Atherosclerosis. 2012;220:34–5.
9 Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid Plaque Area: A Tool for Targeting and Evaluating Vascular Preventive Therapy. Stroke. 2002;33:2916–22.
10 Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen M-L, Njølstad I. Carotid Plaque Area and Intima-Media Thickness in Prediction of First-Ever Ischemic Stroke: A 10–Year Follow-Up of 6584 Men and Women: The Tromso Study. Stroke. 2011;42:972–8.
11 Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Løchen M-L, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6–year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–80.
12 Romanens M, Ackermann F, Schwenkglenks M, Szucs T, Spence JD. Posterior probabilities in sequential testing improve cardiovascular risk prediction using carotid total plaque area. Kardiovaskuläre Medizin. 2011;14:53–7.
13 Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–33.
14 Spence JD. Technology Insight: ultrasound measurement of carotid plaque-patient management, genetic research, and therapy evaluation. Nat Clin Pr. Neuro. 2006;2:611–9.
15 Romanens M, Ackermann F, Sudano I, Szucs T, Riesen WF, Darioli R, et al. LDL-cholesterol and the potential for coronary risk improvement. Kardiovaskuläre Medizin. 2011;14:345–50.
16 Romanens M, Ackermann F, Riesen W, Spence JD, Darioli R. Imaging as a cardiovascular risk modifier in primary care patients using predictor models of the European and inter- national atherosclerosis societies. Kardiovaskuläre Medizin. 2007;10:139–50.
17 Eckardstein A. AGLA Guidelines. 2012. Available at: www.agla.ch.
18 MedCalc Software bvba, Ostend B. MedCalc Statistical Software version. 2013. Available at: http://www.medcalc.org.
19 Delong E, Delong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
20 Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
21 Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, et al. Progression of Coronary Calcium and Incident Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013;61:1231–9.
22 Herder M, Arntzen KA, Johnsen SH, Eggen AE, Mathiesen EB. Long-term use of lipid-lowering drugs slows progression of carotid atherosclerosis: the Tromso study 1994 to 2008. Arterioscler Thromb Vasc Biol. 2013;33(4):858–62.
23 Migrino RQ, Bowers M, Harmann L, Prost R, Ladisa JF. Carotid plaque regression following 6–month statin therapy assessed by 3T cardiovascular magnetic resonance: comparison with ultrasound intima media thickness. J Cardiovasc Magn Reson 2011;13:37.
24 Stein JH. Carotid intima-media thickness and vascular age: you are only as old as your arteries look. J Am Soc Echocardiogr. 2004;17:686–9.
25 DeGoma E. CIMT Arterial Age Calculator. Available at: www.emildegomamd.com/home_htm_files/CIMT Calculator EdG v1.3.xls.
26 Folsom AR, Kronmal R a, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch intern Med. 2008;168:1333–9.
27 Bots ML, Baldassarre D, Simon A, de Groot E, O'Leary DH, Riley W, et al. Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Hear. J 2007;28:398–406.
28 Den Ruijter HM, Peters S a E, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803.
29 Cuende J, Cuende N, Calaveras-Lagartos J. How to calculate vascular age with the SCORE project scales: a new method of cardiovascular risk evaluation. Eur Hear. J 2010;31:2351–8.
30 Anon. Insulin Resistance Intervention after Stroke Trial. A Coop. Progr. funded by Natl. Inst. Neurol. Disord. Stroke (A Div. Natl. Institutes Heal. Available at: http://www.iristrial.org/generalinfo.html. Accessed May 23, 2013.

