Vascular dysfunction in children conceived by assisted reproductive technologies: underlying mechanisms and future implications
DOI: https://doi.org/10.4414/smw.2014.13973
Stefano F
Rimoldi, Claudio
Sartori, Emrush
Rexhaj, David
Cerny, Robert
Von Arx, Rodrigo
Soria, Marc
Germond, Yves
Allemann, Urs
Scherrer
Summary
Epidemiological studies in humans have demonstrated a relationship between pathological events during fetal development and increased cardiovascular risk later in life and have led to the so called “Fetal programming of cardiovascular disease hypothesis”. The recent observation of generalised vascular dysfunction in young apparently healthy children conceived by assisted reproductive technologies (ART) provides a novel and potentially very important example of this hypothesis. This review summarises recent data in ART children demonstrating premature subclinical atherosclerosis in the systemic circulation and pulmonary vascular dysfunction predisposing to exaggerated hypoxia-induced pulmonary hypertension. These problems appear to be related to the ART procedure per se. Studies in ART mice demonstrating premature vascular aging and arterial hypertension further demonstrate the potential of ART to increase cardiovascular risk and have allowed to unravel epigenetic alterations of the eNOS gene as an underpinning mechanism. The roughly 25% shortening of the life span in ART mice challenged with a western style high-fat-diet demonstrates the potential importance of these alterations for the long-term outcome. Given the young age of the ART population, data on cardiovascular endpoints will not be available before 20 to 30 years from now. However, already now cohort studies of the ART population are needed to early detect cardiovascular alterations with the aim to prevent or at least optimally treat cardiovascular complications. Finally, a debate needs to be engaged on the future of ART and the consequences of its exponential growth for public health.
Background
There is abundant evidence from epidemiological studies that adverse events during early life are associated with increased prevalence of cardiovascular disease later in life; 20 years ago Barker coined the term “Fetal programming of cardiovascular diseases” to describe this phenomenon [1–3]. While these earlier studies conducted in adults focused on clinical cardiovascular endpoints, more recent work focused on alterations of the circulation that occur many years before the clinical event and are already detectable during childhood (fig. 1) [4, 5]. This is of importance, because the detection of subpopulations at risk at an early stage may allow the prevention of future development of cardiovascular diseases.
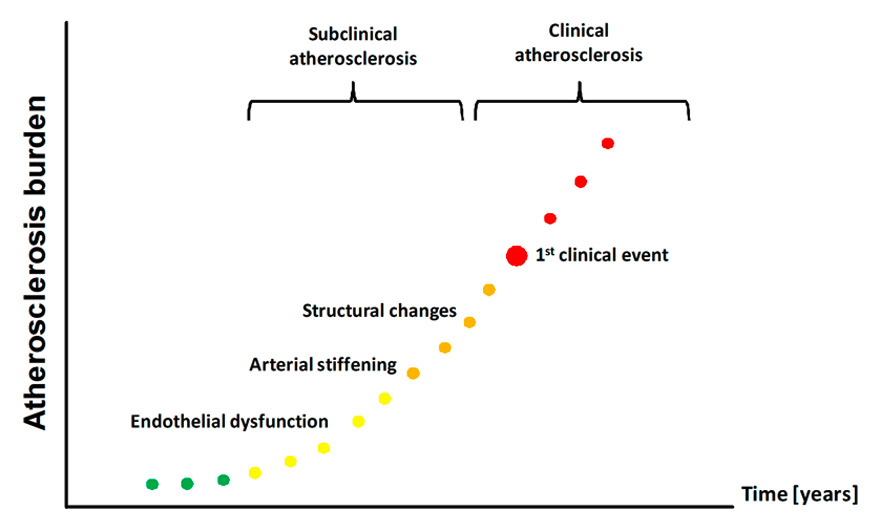
Figure 1
Atherosclerosis burden starts early in life. Endothelial dysfunction, arterial stiffening and structural alterations of the arterial wall represent early steps in the development of atherosclerosis that are already detectable in children known to be at increased cardiovascular risk later in life.
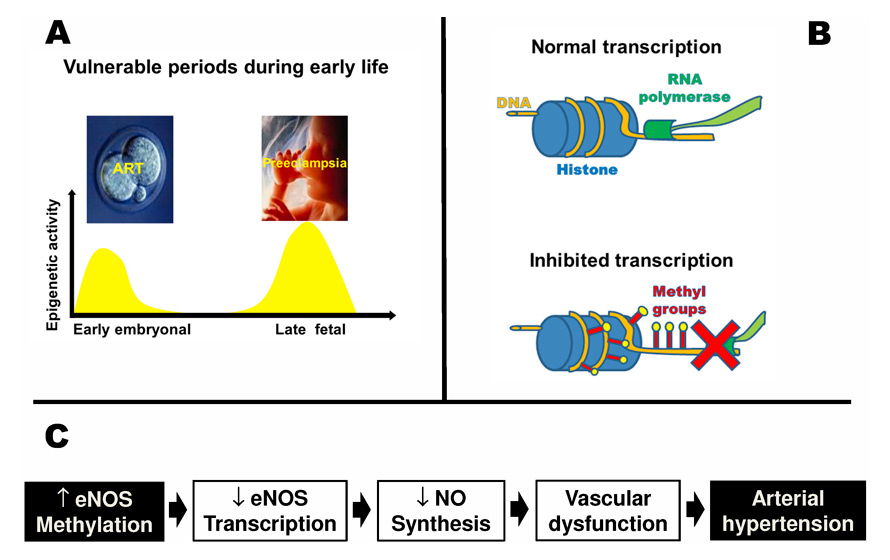
Figure 2
Role of epigenetic alterations in the fetal programming of vascular dysfunction. Vulnerable periods during early life known to be associated with fetal programming of cardiovascular disease coincide with increased epigenetic activity (Panel A). Increased DNA methylation inhibits transcription (Panel B). In mice conceived by assisted reproductive technologies, DNA-methylation of the eNOS gene was altered resulting in decreased eNOS transcription in the vasculature and impaired vascular nitric oxide synthesis. These problems translated into vascular dysfunction and arterial hypertension (Panel C).
Recent data in young apparently healthy offspring of mothers suffering from preeclampsia provide an example of this shift of focus [6]. Epidemiological data show that preeclampsia is associated with an increased risk of cardiovascular morbidity in the adult offspring [7, 8]. Based on these data, we speculated that early predictors of future cardiovascular risk may already be detectable in young apparently healthy offspring of mothers with preeclampsia. To this end, we assessed flow mediated dilation and vascular stiffness in the systemic circulation, two established independent predictors of future cardiovascular risk [9, 10] in offspring of preeclampsia and controls. Moreover, we also assessed the pulmonary artery pressure response to hypoxia in these two groups. In line with our hypothesis, we found that young (14 years old) offspring of mothers with preeclampsia display systemic and pulmonary vascular dysfunction. This vascular dysfunction was related to preeclampsia itself, because siblings of offspring of mothers with preeclampsia who were born after an uncomplicated pregnancy showed normal vascular function [6]. Since underpinning mechanisms are difficult to be investigated in healthy young subjects, we next examined pulmonary vascular function in offspring of restrictive diet pregnancy (RDP) mice, a mouse model mimicking many aspects of preeclampsia in humans. We found that pulmonary vascular function was defective and hypoxic pulmonary hypertension exaggerated in offspring of RDP mice [11]. Epigenetic activity is increased towards the end of fetal development (fig. 2, Panel A) and may be particularly vulnerable to pathological insults during this critical period [12]. In line with this hypothesis, we found that pulmonary vascular dysfunction in RDP mice was related to an epigenetic mechanism [11].
Epigenetic mechanisms are involved in fetal programming of vascular dysfunction
The term “epigenetic” refers to mechanisms that regulate gene expression without affecting the DNA sequence itself. The most relevant epigenetic mechanisms include DNA methylation and histone modification [13]. Increased DNA methylation is associated with inhibition of transcription (fig. 2, Panel B). In RDP mice, pulmonary vascular dysfunction and exaggerated hypoxic pulmonary hypertension was associated with increased pulmonary DNA methylation [11]. These findings provided the first direct evidence for an epigenetic mechanism underpinning pulmonary vascular dysfunction induced by a pathological insult during late fetal life. It appears possible that a similar mechanism may be operational in preeclampsia-induced vascular dysfunction in the offspring in humans [12].
Figure 2 (Panel A) shows that in addition to the late fetal phase, the early embryonic phase is also characterised by increased epigenetic activity. Assisted reproductive technologies (ART) allowed millions of infertile couples to have children who now make up 2% to 4% of births in industrialised countries [14, 15]. ART implies multiple manipulations of the embryo during this critical period where it may be particularly susceptible to environmental alterations that may induce epigenetic modifications [16]. In line with this speculation, ART induces epigenetic alterations in the embryo [17, 18] and is associated with an increased frequency of rare diseases known to be related to an epigenetic mechanism [16, 19]. Based on these observations, we speculated that ART induces vascular dysfunction in the offspring that is related to an epigenetic mechanism.
In the following we will briefly review the data consistent with this hypothesis.
ART induces vascular dysfunction in the offspring
Due to the young age of the ART population in humans (the first ART child was born in 1978) it is not known yet whether ART is associated with increased risk for clinical cardiovascular endpoints. However, there is abundant evidence that in populations at risk, atherosclerosis already starts in childhood many years before the first clinical event occurs (fig. 1) [4, 5]. In humans several non-invasive techniques allow detection of subclinical atherosclerosis.
To test our hypothesis, we have used the following three methods to assess systemic vascular function and structure in ART and matched control children.
Systemic endothelial dysfunction represents the first step in the development of atherosclerosis and is already detectable in apparently healthy children at increased cardiovascular risk [4, 5, 9]. Systemic conduit artery endothelial function can be assessed by determining the increase of the brachial artery diameter evoked by reactive hyperemia (flow-mediated vasodilation, FMD) using high-resolution ultrasound and automatic wall tracking software [20]. Figure 3A shows that young children conceived by ART display systemic endothelial dysfunction [21]. Interestingly, this impairment of endothelial function in ART children was of similar magnitude as the one we previously observed in offspring of mothers with preeclampsia [6].
In addition to endothelial dysfunction, stiffening of the vasculature also takes place during the early development of atherosclerosis and represents an independent predictor of future cardiovascular events in populations at risk [10, 22]. Exaggerated stiffening of the vasculature has been invariably found in children suffering from conditions known to predispose to premature cardiovascular morbidity and mortality such as diabetes, dyslipidemia and hypertension [23–25]. Carotid-femoral pulse wave velocity (PWV) is considered the gold standard to measure arterial stiffness in humans [10]. We found that PWV was significantly faster in ART children than in controls (7.8 ± 2.4 vs 6.5 ± 1.3 m/s; P <0.001) [21].
Finally, atherosclerosis goes along with structural changes of the vasculature. Measurement of carotid intima-media thickness (IMT) by ultrasound is the most widely used non-invasive method to assess structural changes of the systemic circulation. IMT is considered a surrogate marker of subclinical atherosclerosis and increased IMT has been reported in children with familial hypercholesterinemia, arterial hypertension and type 1 or 2 diabetes mellitus [26–31]. We found that carotid IMT was significantly greater in ART than in control children [21]. In line with this finding, Gratacos and colleagues recently reported increased aortic IMT in utero and early after birth in ART children [32].
To test whether vascular dysfunction in ART children was not limited to the systemic circulation but generalised, we assessed pulmonary artery pressure responses to high-altitude exposure, a maneuver designed to facilitate the detection of pulmonary vascular dysfunction [33, 34]. Figure 3 (Panel B) shows that in ART children vascular dysfunction also affects the pulmonary circulation, as evidenced by exaggerated hypoxic pulmonary hypertension at high altitude [21]. Hypoxic pulmonary hypertension in ART children was even more pronounced than in offspring of mothers with preeclampsia, a condition known to be associated with hypoxic pulmonary hypertension [6, 34]. We don’t know yet how this pulmonary vascular dysfunction will evolve, but it appears possible that the ART population may be at increased risk for sustained pulmonary hypertension in disease states associated with chronic hypoxemia.
Taken together these findings demonstrate systemic and pulmonary vascular dysfunction in ART children that is at least of similar severity as the one observed in offspring of mothers with preeclampsia, a population known to be at increased cardiovascular risk later in life [7, 8].
Mechanisms underpinning ART-induced vascular dysfunction
Evidence from studies in humans
Parent-related factors such as infertility, older age of the mother necessitating to resort to ART and/or hormonal stimulation of the ovulation in the mother could contribute to vascular dysfunction in ART children. For example, sterility could be associated with vascular dysfunction in humans, a problem that could then be transmitted to the offspring by ART. To test for this possibility, we assessed systemic endothelial function (fig. 3, Panel C) and arterial stiffness (fig. 3, Panel D) in sterile and fertile parents and found that it was comparable in the two groups. In line with this concept, vascular function was normal in siblings of ART children who were conceived naturally [21].
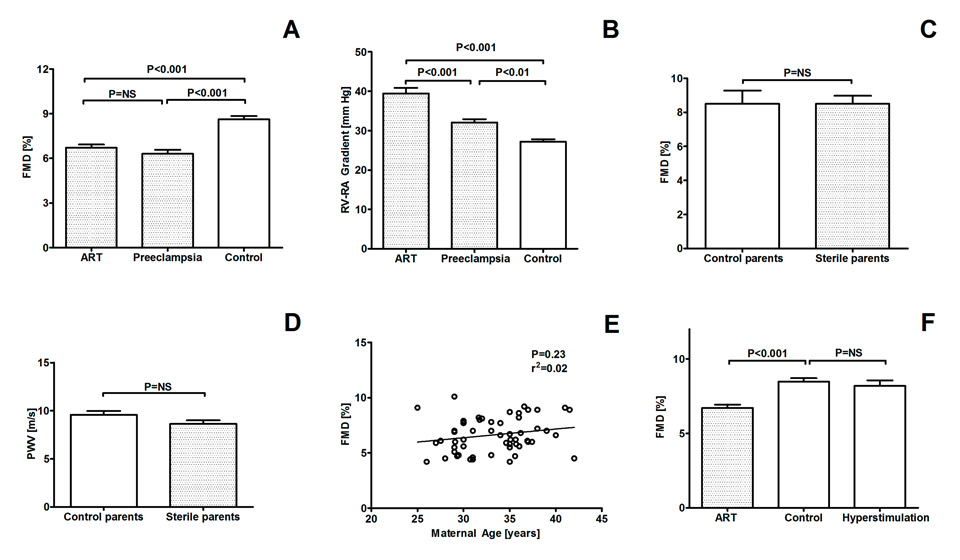
Figure 3
Flow-mediated vasodilation (FMD) of the brachial artery and right ventricular to right atrial (RV-RA) pressure gradient in children conceived by assisted reproductive technologies (ART), in offspring of mothers suffering from preeclampsia and in age- and gender-matched healthy control children (Panel A and B). FMD and pulse wave velocity (PWV) in fertile parents of control children and of sterile parents ART children (Panel C and D). Relationship between maternal age and FMD in ART children (Panel E). FMD in control children, ART children and children naturally conceived during stimulation of the ovulation in the mother (Panel F). Data are expressed as mean ± SEM. ANOVA and Bonferroni post-hoc test (for Panel A, B and F).
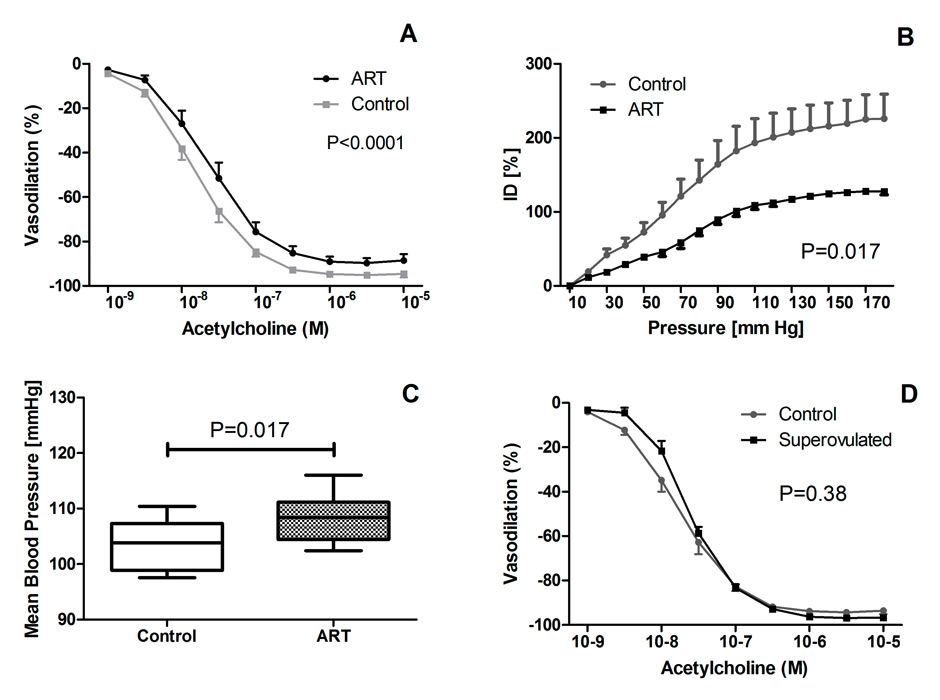
Figure 4
Vascular function and arterial blood pressure in mice generated by ART and control mice. Panel A shows acetylcholine-induced mesenteric artery vasodilation, Panel B the carotid artery pressure-diameter relationship, a proxy of vascular stiffness, Panel C shows mean arterial blood pressure obtained by telemetry. Panel D shows acetylcholine-induced mesenteric artery vasodilation in mice conceived during stimulation of the ovulation in the mother and control mice.
Alternatively, the age of the parents could be a determinant of vascular function in the offspring. This possibility is unlikely, since in our study we found no relationship between maternal age and vascular function of the progeny (fig. 3, Panel E). Finally, stimulation of the ovulationdid not appear to play a major role, since vascular function was normal in children conceived naturally during hormonal stimulation of the ovulation in the mother (fig. 3, Panel F).
Taken together, these data in humans suggest that ART per se is the main culprit and that parent-related factors do not contribute significantly to vascular dysfunction in ART children. However, the underlying mechanisms are unknown and difficult to investigate in healthy children.
To this end we turned our attention to ART mice.
Evidence from studies in ART mice
Vascular dysfunction, arterial hypertension and shortened life span in ART mice
We found that in ART mice endothelium-dependent mesenteric artery dilation was defective and carotid artery stiffness increased (fig. 4, panel A+B) [35]. In ART mice, this defective vascular function in vitro translated into marked arterial hypertension in vivo. (Figure 4, Panel C). These data in mice further demonstrate the potential of ART to cause premature vascular dysfunction and aging and arterial hypertension. Moreover, and of potential importance for the long-term outcome of the ART population in humans, we observed a dramatic, roughly 25% shortening of the life span in ART mice challenged with a western style high-fat diet [35].
In addition, ART mice provided unique insight into mechanisms underpinning ART-induced vascular dysfunction and premature mortality.
Underlying mechanisms
In humans it is difficult to completely exclude the possibility that parental factors contribute to vascular dysfunction in ART children (see above). The finding that in normal mice, ART induces premature vascular aging and arterial hypertension, however, provides strong additional evidence for the concept that ART per se is the main culprit. The findings in mice also strengthen the concept that hormonal stimulation of the ovulation in the mother per se is not an important determinant of ART-induced vascular dysfunction, because endothelium-dependent vasodilation of the mesenteric artery was normal in offspring of super-ovulated mice (fig. 4, Panel D).
In offspring of RDP in mice, pulmonary vascular dysfunction is associated with altered lung DNA methylation suggesting that epigenetic mechanism may be involved in the fetal programming of the vascular system [11]. Moreover, it is well established that epigenetic alterations can be transmitted to the next generation [36, 37]. We, therefore, assessed vascular function and blood pressure in offspring of male ART mice mated with normal females. We found vascular dysfunction and arterial hypertension in the progeny that was comparable to the one observed in their fathers [35]. In a next step, we then investigated the methylation of genes known to be important in the regulation of systemic vascular function. We found that the methylation of the promoter of the gene coding for endothelial nitric oxide synthase (eNOS) was altered in the aorta of ART mice. This dysmethylation had important consequences, as evidence by decreased eNOS and eNOS RNA expression in the vasculature and impaired vascular nitric oxide synthesis in ART compared with control mice. Of note, dysmethylation of the eNOS gene was not generalised, as evidenced by normal methylation in the liver [35].
Taken together these findings indicate that in ART mice the entire chain of events starting from the methylation of the promoter of the eNOS gene and ending up in premature vascular aging and arterial hypertension is altered (fig. 2, Panel C).
To further test for the importance of this epigenetic mechanism we then assessed the effects of histone deacetylase-inhibitor administration to ART mice. The rationale was that histone deacetylase inhibitors (i.e. butyrate) are known to reverse altered methylation and phenotypic changes induced by pathological events during the fetal period [38]. In line with this concept, we found that butyrate administration to male ART mice normalised methylation of the eNOS gene and vascular function and prevented transmission of these problems to the progeny [35]. Collectively, these data in mice demonstrate that ART-induced cardiovascular alterations are related to an epigenetic mechanism.
Finally, these studies in ART mice also provided insight into the mechanisms underpinning these epigenetic and vascular alterations. Culture media currently used for ART are suboptimal, as evidenced by lower pregnancy rates and impaired growth of cultured embryos [39]. This problem may be related to physical and chemical features of culture media that are different from those of human oviductal fluid. Our data in mice indicate that shortening of the culture time does not appear to be beneficial, since vascular dysfunction was comparable in embryos implanted at the 2–cell stage and as blastocysts [35]. In line with this observation, altered methylation patterns and gene expression were already detectable in 2-cell stage embryos [40]. Moreover, there is evidence that suboptimal culture conditions in vitro compromise the ability of the embryo to maintain genetic imprinting [39]. Taken together, these findings suggest that suboptimal culture conditions contribute to ART-induced cardiovascular dysfunction. In line with this speculation, preliminary data suggest that chemical modification of the culture media may attenuate epigenetic alterations and vascular dysfunction in ART mice [41].
Conclusion and future perspectives
The data presented in this review suggest that ART has lost its innocence and represents a major novel cardiovascular risk factor expected to be present in 2% to 4% of the population in industrialised countries and with the potential to be transmitted to future generations (fig. 5). A major problem at this time is that given the young age of the ART population we do not know yet, and will not know before 20 to 30 years from now, how the consequences of ART-induced premature atherosclerosis on cardiovascular endpoints (arterial hypertension, stroke, myocardial infarction, hypoxic pulmonary hypertension and related morbidity and mortality) will be. Extrapolation from young populations with similar risk profile and known prevalence of cardiovascular morbidity and mortality later in life suggest that they may be important.
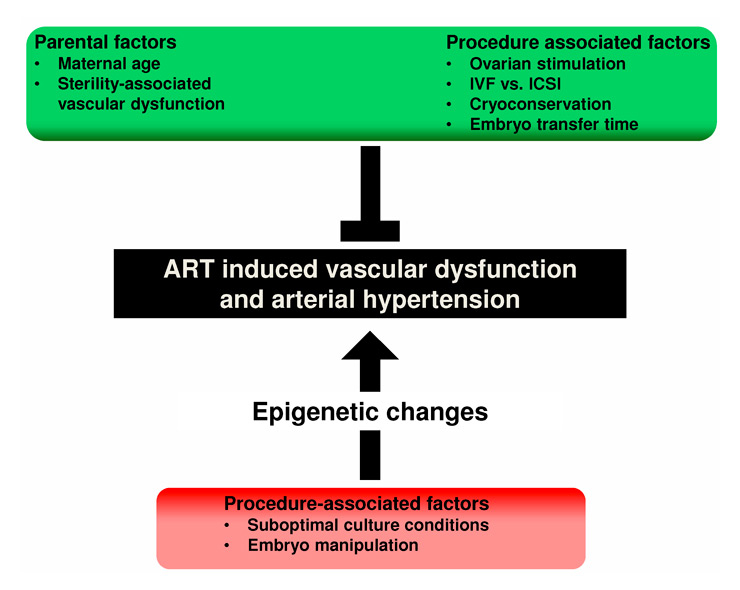
Figure 5
Potential factors involved in the development of ART-induced vascular alterations. Parental and procedure-associated factors that seem not to be major determinants of vascular changes are marked in green, procedure-associated factors that may play a major role in red.
What can be done? First, for the already existing ART population there is a need for optimal medical care in order to early detect cardiovascular alterations with the aim to prevent, post-pone or at least optimally treat cardiovascular complications.
(Note added in proof: Recent data indicate that antioxidant administration to ART children improved NO bioavailability and vascular responsiveness suggesting that ART-induced vascular dysfunction is subject to redox regulation and reversible [42].)
In this regard, cohort studies of ART populations would be very important. Second, there is an urgent need for a better mechanistic understanding of ART-induced cardiovascular alterations in order to find ways to prevent them in the future. Third, society and politics need to engage a debate now on the future of ART and the consequences of its exponential growth for public health. The impetus for starting this debate is unlikely to come from the medico-industrial ART complex; wait and see for another 20 or 30 years does not appear to represent a valid option here.
References
1 Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25(7):457–63.
2 Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl 1):3–6.
3 Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7.
4 Charakida M, Deanfield JE, Halcox JP. Childhood origins of arterial disease. Curr Opin Pediatr. 2007;19(5):538–45.
5 Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919–50.
6 Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122(5):488–94.
7 Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61.
8 Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80.
9 Charakida M, Masi S, Luscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. Eur Heart J. 2010;31(23):2854–61.
10 Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605.
11 Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, Mathieu C, et al. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011;301(1):H247–52.
12 Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–9.
13 Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–63.
14 De Geyter C. Assisted reproductive medicine in Switzerland. Swiss Med Wkly. 2012;142:w13569.
15 Nyboe Andersen A, Erb K. Register data on Assisted Reproductive Technology (ART) in Europe including a detailed description of ART in Denmark. Int J Androl. 2006;29(1):12–6.
16 De Rycke M, Liebaers I, Van Steirteghem A. Epigenetic risks related to assisted reproductive technologies: risk analysis and epigenetic inheritance. Hum Reprod. 2002;17(10):2487–94.
17 Chen SL, Shi XY, Zheng HY, Wu FR, Luo C. Aberrant DNA methylation of imprinted H19 gene in human preimplantation embryos. Fertil Steril. 2010;94(6):2356–8.
18 Santos F, Hyslop L, Stojkovic P, Leary C, Murdoch A, Reik W, et al. Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum Reprod. 2010;25(9):2387–95.
19 Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18(12):2508–11.
20 Ghiadoni L, Versari D, Giannarelli C, Faita F, Taddei S. Non-invasive diagnostic tools for investigating endothelial dysfunction. Curr Pharm Des. 2008;14(35):3715–22.
21 Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125(15):1890–6.
22 Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27.
23 Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27(12):2911–7.
24 Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56(1):159–65.
25 Leeson CP, Whincup PH, Cook DG, Mullen MJ, Donald AE, Seymour CA, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101(13):1533–8.
26 Ayer JG, Harmer JA, Nakhla S, Xuan W, Ng MK, Raitakari OT, et al. HDL-cholesterol, blood pressure, and asymmetric dimethylarginine are significantly associated with arterial wall thickness in children. Arterioscler Thromb Vasc Biol. 2009;29(6):943–9.
27 Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104(24):2943–7.
28 Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109(14):1750–5.
29 Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48(1):40–4.
30 Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41(4):661–5.
31 Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119(22):2913–9.
32 Valenzuela-Alcaraz B, Crispi F, Bijnens B, Cruz-Lemini M, Creus M, Sitges M, et al. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013;128(13):1442–50.
33 Allemann Y, Stuber T, de Marchi SF, Rexhaj E, Sartori C, Scherrer U, et al. Pulmonary artery pressure and cardiac function in children and adolescents after rapid ascent to 3,450 m. Am J Physiol Heart Circ Physiol. 2012;302(12):H2646–53.
34 Scherrer U, Allemann Y, Rexhaj E, Rimoldi SF, Sartori C. Mechanisms and drug therapy of pulmonary hypertension at high altitude. High Alt Med Biol. 2013;14(2):126–33.
35 Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013;123(12):5052–60.
36 Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. BioEssays. 2007;29(2):145–54.
37 Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Current Opinion in Genetics & Development. 2008;18(3):273–9.
38 Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–8.
39 Market-Velker BA, Fernandes AD, Mann MR. Side-by-side comparison of five commercial media systems in a mouse model: suboptimal in vitro culture interferes with imprint maintenance. Biol Reprod. 2010;83(6):938–50.
40 Shi W, Haaf T. Aberrant methylation patterns at the two-cell stage as an indicator of early developmental failure. Mol Reprod Dev. 2002;63(3):329–34.
41 Rexhaj E, Rimoldi SF, Paoloni-Giacobino A, Sartori C, Allemann Y, Scherrer U. Epigenetically-induced systemic vascular dysfunction and hypertension by in vitro fertilization: prevention by addition of melatonin to the culture media. FASEB J. 2012;26:874.5.
42 Rimoldi SF, Sartori C, Rexhaj E, Bailey DM, de Marchi SF, McEneny J, et al. Antioxidants improve vascular function in children conceived by assisted reproductive technologies: A randomized double-blind placebo-controlled trial. Eur J Prev Cardiol. 2014 (in press).




