Bisphosphonate induced hypocalcaemia – report of six cases and review of the literature
DOI: https://doi.org/10.4414/smw.2014.13979
Veronika
Kreutle, Claudine
Blum, Christian
Meier, Miriam
Past, Beat
Müller, Philipp
Schütz, Katrin
Borm
Summary
Intravenous bisphosphonates are widely used to treat osteoporosis and bone metastasis in cancer patients
The risk of hypocalcaemia is a rare but underestimated side effect of anti-resorptive treatment. Clinically apparent hypocalcaemia is mostly related to high-dose treatment with zoledronate and denosumab in cancer patients
Particular caution is mandatory in all malnourished patients and patients with renal failure who are treated for either bone metastases or osteoporosis.
To avoid serious hypocalcaemia, pre-treatment calcium and vitamin D status should be assessed and corrected if appropriate.
Introduction
Anti-resorptive drugs (i.e., bisphosphonates and denosumab) are frequently used in patients with advanced cancer and osteoporosis to reduce the incidence of skeletal-related events.
Until now anti-resorptive drugs were the mainstay first line therapy for reducing fracture risk in patients with osteoporosis [1]. Prolonged administration of these bone modifying agents in patients with advanced malignancy is generally well tolerated. Intravenous bisphosphonates (such as zoledronate) and denosumab are characterised by rapid and effective suppression of osteoclastic bone resorption [2]. Due to sustained anti-resorptive effect [3] resulting in a shift of calcium back into the skeleton in patients with high bone turnover (such as in osteolytic bone metastases) patients may be at risk of developing severe hypocalcaemia [4]. Also the use of denosumab is associated with significantly increased risk of developing high-grade hypocalcaemia in comparison to the controls. The incidence of hypocalcaemia after high dose denosumab varies between 5.2% and 13% [5–7].
Especially malnourished patients with renal failure are at a higher risk for clinically symptomatic side effects due to insufficient mechanisms to compensate for a state of relative hypocalcaemia, such as vitamin D deficiency or hypomagnesaemia [8, 9]. Herein, we describe six patient cases admitted to our inpatient medical wards with severe symptomatic hypocalcaemia, and present an overview of the literature and recommendations for appropriate treatment.
|
Table 1: Serum analyte concentrations in patients given bisphosphonates. |
|
|
Adjusted
Calcium
(mmol/l)
|
Calcium
(mmol/l)
|
Magnesium
(mmol/l)
|
PO4(mmol/l)
|
Parathyroid
Hormone
(ng/l)
|
25-Vitamin D (nmol/l)
|
Albumin
(g/l)
|
Nutritional
Risk
Score
|
Days until
normalisation of calcium
|
|
Reference range
|
2.15–2.55 |
2.15–2.55 |
0.74–0.99 |
0.81–1.62 |
10–73 |
>75 |
34–50 |
|
|
|
Case 1
|
|
|
|
|
|
|
|
5 |
21 |
| before starting
bisphosphonate |
|
2.11 |
|
|
|
30.8 |
|
|
|
| day of admission |
<1.57 |
<1.25 |
0.63 |
1.42 |
257 |
|
27.2 |
|
|
| 3–5 days after |
1.69 |
|
0.74 |
|
|
|
22.7 |
|
|
| 7–9 days after |
1.84 |
|
0.74 |
|
|
|
22.8 |
|
|
| day of discharge
13 days after |
1.85 |
|
|
|
|
|
|
|
|
|
Case 2
|
|
|
|
|
|
|
|
4 |
4 |
| before starting
bisphosphonate |
|
2.2 |
|
|
408 |
32 |
|
|
|
| day of admission |
1.85 |
1.62 |
0.85 |
|
|
|
30.7 |
|
|
| 3–5 days after |
1.98 |
|
|
0.67 |
|
|
29 |
|
|
| 7–9 days after |
2.18 |
1.81 |
|
|
|
|
25 |
|
|
| day of discharge
15 days after |
2.26 |
|
|
|
|
|
|
|
|
|
Case 3
|
|
|
|
|
|
|
|
2 |
42 |
| before starting
bisphosphonate |
|
2.49 |
|
|
|
|
|
|
|
| day of admission |
1.74 |
1.44 |
|
|
|
|
28.1 |
|
|
| 3–5 days after |
|
|
|
|
383 |
<10 |
|
|
|
| 7–9 days after |
|
1.86 |
|
|
|
|
|
|
|
| day of discharge
9 days after |
|
1.86 |
|
|
|
|
|
|
|
|
Case 4
|
|
|
|
|
|
|
|
2 |
2 |
| before starting
bisphosphonate |
|
|
|
|
|
|
|
|
|
| day of admission |
1.84 |
1.59 |
0.78 |
0.93 |
1441 |
10.7 |
30.1 |
|
|
| 3–5 days after |
2.25 |
1.86 |
0.79 |
|
|
|
25.1 |
|
|
| 7–9 days after |
2.31 |
|
0.92 |
|
|
|
21.9 |
|
|
| day of discharge
12 days after |
|
2.01 |
|
|
|
|
|
|
|
|
Case 5
|
|
|
|
|
|
|
|
|
2 |
| before starting
bisphosphonate |
|
2.36 |
|
|
|
77.2 |
|
|
|
| day of admission |
1.72 |
1.65 |
|
|
|
|
37.3 |
|
|
| 3–5 days after |
2.36 |
|
0.8 |
0.95 |
|
|
32.5 |
|
|
| 7–9 days after |
2.67 |
2.51 |
0.75 |
1.67 |
|
|
33.5 |
|
|
| day of discharge
22 days after |
2.48 |
|
|
|
|
|
|
|
|
|
Case 6
|
|
|
|
|
|
|
|
5 |
>30 |
| before starting
bisphosphonate |
2.33 |
2.25 |
|
|
|
91 |
|
|
|
| day of admission |
1.77 |
|
0.86 |
0.52 |
|
|
25 |
|
|
| 3–5 days after |
|
|
0.86 |
0.7 |
|
|
21 |
|
|
| 7–9 days after |
|
|
0.61 |
0.93 |
|
|
20 |
|
|
| day of discharge
30 days after |
|
|
|
|
|
|
|
|
|
Case reports
Case 1
An 82–year-old man with adenocarcinoma of the prostate and osteoblastic metastases presented with confusion, tiredness, dizziness with recurrent falls and occasional mild paraesthesia in arms and legs as well as muscle weakness. The patient had received the most recent bisphosphonate infusion (IV zolendronate, 4 mg) 11 days prior to admission. The cancer treatment consisted of an anti-androgen therapy with leuprorelin every six months with the last administration three months prior to admission. On admission, the albumin-corrected plasma calcium (Cacorr) was not measurable (below 1.57 mmol/l; norm, 2.15–2.55 mmol/l), magnesium (Mg) was 0.63 mmol/l (norm, 0.74–0.99 mmol/l), intact PTH (iPTH) was 257 ng/l (norm, 10–73 ng/l), 25–OH-Vitamin D3 (VitD3) was 30.8 nmol/l (norm, 50–75 nmol/l). The nutrional risk score (NRS) was 5 points. The treatment was started with two vials of calcium gluconate 1.375 mg as well as a supplementation of MgSO4 (2 g) and 300,000 units of vitamin D3. In the following days, the patient received calcium (both IV and orally) and calcitriol. The patient was discharged asymptomatic, with a Cacorrof 1.85 mmol/l and an ionised calcium level of 0.83 mmol/l. One month later, Cacorr had returned to normal (2.20 mmol/l).
Case 2
An 86–year-old man with adenocarcinoma of the prostate and diffusely spread osteoblastic metastases presented with acute increasing paraparesis progressing to paraplegia of the lower extremities. Isolated muscle testing showed M0 in all muscles, and the Babinski reflex was bilaterally positive. The patient had received bisphosphonate treatment (IV zolendronate, 4 mg) 6 days prior to admission; the cancer treatment consisted of an anti-androgen therapy with bicalutamide and leuprorelin subcutaneously. He had received leuprorelin two days prior to admission. On admission, Cacorr was 1.85 mmol/l, Mg 0.85 mmol/l, iPTH 408 ng/l, VitD3 32 nmol/l, and albumin was 32 g/l. NRS was 4 points. Plasma calcium level increased slowly despite a highly dosed calcium substitution and calcitriol administration. The patient was discharged asymptomatic with a serum Cacorrof 2.26 mmol/l.
Case 3
A 40–year-old man suffered from gastric cancer with pericardial and osteoblastic bone metastases. He presented with joint pain in feet and knees and paraesthesia in both hands and feet. Eight days prior to admission he had received a bisphosphonate therapy (IV zolendronate, 4 mg), chemotherapy with EOX (epirubicin, oxaliplatin, capecetabine) had been stopped three weeks earlier. On admission, Cacorr was 1.74 mmol/l, iPTH 383 ng/l, VitD3 <10 nmol/l, and albumin was 28.1 g/l. NRS was 2 points. He received calcium supplements and calcitriol. The patient responded well to treatment and was discharged with a plasma calcium of 1.86 mmol/l.
Case 4
A 61–year-old man with prostate adenocarcinoma and diffuse osteoblastic metastases was admitted to the hospital with leg weakness and dizziness. Muscle tendon reflexes were diminished, the Babinski reflex was positive. Cancer treatment consisted of anti-androgen therapy with leuprorelin and cabazitaxel. The bisphosphonate infusion, which he had received monthly for the past 17 months, had been stopped 2 months prior to admission; Calcium was 1.59 mmol/l, Cacorr 1.84 mmol/l, iPTH 1441 ng/l. Severe vitamin D deficiency (10.7 nmol/l) was discovered. Mg was 0.78 mmol/l, albumin 30 g/l. Nutritional Risk Score (NRS) was 2 points. Vitamin D3 was substituted with 300,000 U. Treatment consisted of calcium supplementation with 1 g calcium carbonate three times a day and 3 μg calcitriol per day. Mg supplements were given from day four on. Hypocalcaemia resolved within two days, and the patient was released without any symptoms with Cacorrof 2.44 mmol/l.
Case 5
A 77–year-old patient with osteoporosis presented with a fine bilateral tremor and restlessness. Bisphosphonate therapy (IV zolendronate, 4 mg) consisted of one infusion six days prior to admittance. On admission, plasma calcium was 1.65 mmol/l, Cacorr 1.72 mmol/l, Mg 0.87 mmol/l, iPTH was not measured, VitD3 was 77.2 nmol/, and albumin was 37.3 g/l. NRS was 0 points. Hypocalcaemia treatment consisted of one dose of cholecalciferol 800 U and calcium carbonate 2.5 g. One day after admission, 1 g calcium carbonate three times per day, 0.5 μg Calcitriol per day and 2 sachets of Mg (12.4 mmol) per day were started. This supplementation was stopped on day 6. At that point, Cacorr was 2.91 mmol/l and ionised plasma calcium was 1.41 mmol/l (not shown in table 1). Hypercalcaemia resolved within three days and was stable until discharge.
Case 6
A 55–year old patient with osteoporosis was admitted to the hospital unable to intake food orally because of vomiting and severe abdominal pain. She had had laparoscopic gastric bypass surgery one year before with multiple complications requiring several surgical revisions. Bisphosphonate treatment (IV zolendronate, 5 mg) had been given once four days before admission. She also suffered from a small bowel obstruction and needed surgery three days after admission. Over 8 days she had parenteral nutrition. On admission, Cacorr was 1.77 mmol/l, Mg 0.86 mmol/l, iPTH was not measured, VitD3 was 99.1 nmol/l, P04 was 0.52 mmol/l. NRS was 5 points. Treatment consisted of intravenous supplementation of calcium and magnesium. The patient was discharged without a supplementation of calcium. It is not clear when serum calcium returned to normal range, but three months later, a normal Cacorrof 2.34 mmol/l was measured while hypomagnesaemia persisted.
|
Table 2: Emergency treatment of hypocalcemia (calcium ionized <0.8 mmol/l), albumin-corrected plasma calcium <2.0 mmol/l). |
|
Symptoms
|
Treatment
|
Control of calcium
|
|
mild
|
Calcium p.o. 2×1 g/d
Calcitriol 2×0.25 μg/d |
1–2×/d |
|
severe
(e.g. tetany, spasm)
|
Calcium i.v. 1–2 mg/kg/h
Calcitriol 0.25 μg 2×/d**
Magnesium i.v.
2 g Mg2+ sulphate over 10–20 minutes in (20 ml 10% Mg2>+ sulphate), followed 1 g sulphate/h/100 ml of fluid. |
every 4 h |
|
Life-threatening (e.g. stridor, laryngospasm)
|
Calcium i.v.
1–2 vials calcium i.v. (Calcium-Sandoz®)
à 90 mg over 10 minutes (10 ml 10% calciumglubionate = 2.25 mmol/l Ca2+)
maintenance dose 1–2 mg/kg/h in 5% dextrose solution
Calcitriol0.25 μg 2×/d**
Magnesium i.v.
2 g Mg2+ sulphate over 10–20 minutes
in (20 ml 10% Mg2+ sulphate), followed 1 g Mg2+ sulphate/h/100 ml of fluid. |
every 4 h |
| ** as soon as p.o. medication is possible |
|
Table 3: Risk factors contributing to BP-induced hypocalcemia. |
| Metastatic cancer |
| Malnutrition |
| Malabsorption |
| Renal insufficiency/failure |
| 25 (OH) vitamin D3 deficiency |
| Hypomagnesaemia |
|
Table 4: Check-up before and during BP treatment. |
| Assessment of calcium homeostasis before BP therapy |
| Correct plasma calcium, magnesium, 25 (OH) vitamin D3
|
| Control of nutritional risk score (NRS) |
| Control of renal function |
| Regular and adequate calcium and vitamin D supplementation |
| Check adherence to treatment |
| Check plasma calcium in the first week post-treatment and further if necessary |
Discussion
Symptoms of hypocalcaemia can substantially impair well-being of patients and can be even life-threatening. These six cases illustrate the clinically relevant consequences and therapeutic challenges of hypocalcaemia in patients receiving IV bisphosphonates. Obviously, in our clinical routine, not enough emphasis is given to avoid this preventable problem despite this side effect being described in the directions of use and in numerous case reports, both in osteoporosis and in patients with metastatic bone disease [4, 10–14]. Furthermore, none of these patients has been informed about the possible risks and clinical symptoms of impending hypocalcaemia. Hypocalcaemia after bisphosphonate administration is usually mild and transient, but in severe cases hospitalisation and therapeutic interventions are required.
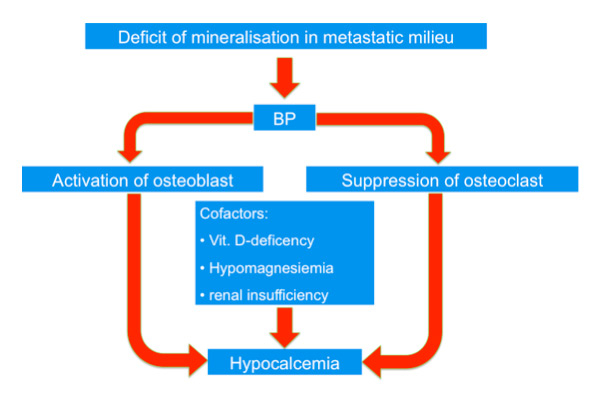
Figure 1
Increased deposition of calcium and phosphate in bone through osteoblastic metastases is postulated, which leads to decreased calcium and phosphate which stimulates PTH secretion and leads to secondary hyperparathyroidism. The application of bisphosphonates leads to further lowering of Ca and thus stimulates secondary hyperparathyroidism.
The risk of symptomatic hypocalcaemia in patients with osteoporosis receiving bisphosphonates is small.
The true incidence of symptomatic hypocalcaemia is difficult to obtain because underreporting of cases must be assumed. The risk of symptomatic hypocalcaemia is higher in cancer patients with bone metastases receiving higher dosage of bisphosphonates and denosumab [15] as we have also illustrated in our cases.
In a retrospective study of breast cancer patients, Ding et al. found a high incidence rate of hypocalcaemia (16%), which they attribute to an insufficient calcium supplementation, as only 54.2% of the patients were taking oral calcium and vitamin D [3]. Zuradelli et al. even found higher incidence rates of up to 38.8% during treatment [9]. Vitamin D deficiency is frequent in elderly persons, especially in cancer patients and patients with osteoporosis that are treated with bisphosphonates; therefore, vitamin D deficiency will very likely aggravate bisphosphonate-induced hypocalcaemia. However, often neither measurement of vitamin D status nor supplementation before starting IV bisphosphonate treatment has been done [16–20]. Calcium and vitamin D supplementation in patients receiving intravenous bisphosphonates has not been included in standard care protocols in oncology departments for a long time but is now recommended at our institution and elsewhere [21, 22].
Bisphosphonates interact with bone remodeling in several ways. Bisphosphonates bind to hydroxylapatite in the bone, which is taken up by the osteoclasts and in this way hinder them in their attachment to bone. They affect the osteoclast mediated bone resorption in a variety of ways that include effects on osteoclasts recruitment, differentiation and resorptive activity, life span and may also affect osteoclast morphology and cause osteoclast apoptosis in vitro [23]. Osteoclasts are indirectly inhibited through reduction of the osteoclast stimulating activity of osteoblasts [24]. It is well-known that bisphosphonate-induced inhibition of osteoclasts diminishes calcium mobilisation, which leads to hypocalcaemia, but it is also assumed that bisphosphonates can cause a shift of calcium into the bone if large populations of osteoblasts exist, as in osteoblastic metastases [4, 15].
Osteoblastic metastases (such as in prostate cancer) are known to undergo avid mineralisation and calcium uptake resulting in hypocalcaemia. The effect of bisphosphonates on osteoblasts is not completely clarified. Laboratory studies demonstrate that bisphosphonates increase osteoblast and osteoblastic metastases maturation, activity and bone mineralisation. This could lead to a syndrome similar to hungry bone. This interesting hypothesis needs to be confirmed by further studies. It is also inconclusive whether the number and nature/type of metastases are predictors of bisphosphonate-induced hypocalcaemia [4, 15].
Increased deposition of calcium and phosphate in bone through osteoblastic metastases is postulated, which leads to decreased calcium and phosphate which stimulates PTH secretion and leads to secondary hyperparathyroidism. The application of bisphosphonates leads to further lowering of Ca and thus stimulates secondary hyperparathyroidism (fig. 1).
Mild secondary hyperparathyroidism after infusion of zoledronic acid is often observed, possibly as a compensatory mechanism to maintain normocalcaemia [25, 26], and most patients do not become overtly hypocalcaemic. This increase in parathyroid hormone prevents hypocalcaemia by renal reabsorption of calcium, increased vitamin D production, and stimulation of osteoclasts to resorb bone [24]. For patients taking bisphosphonates, the effect of PTH on bone resorption by osteoclasts is blocked especially in patients with co-existing magnesium or vitamin D deficiency, respectively [27].
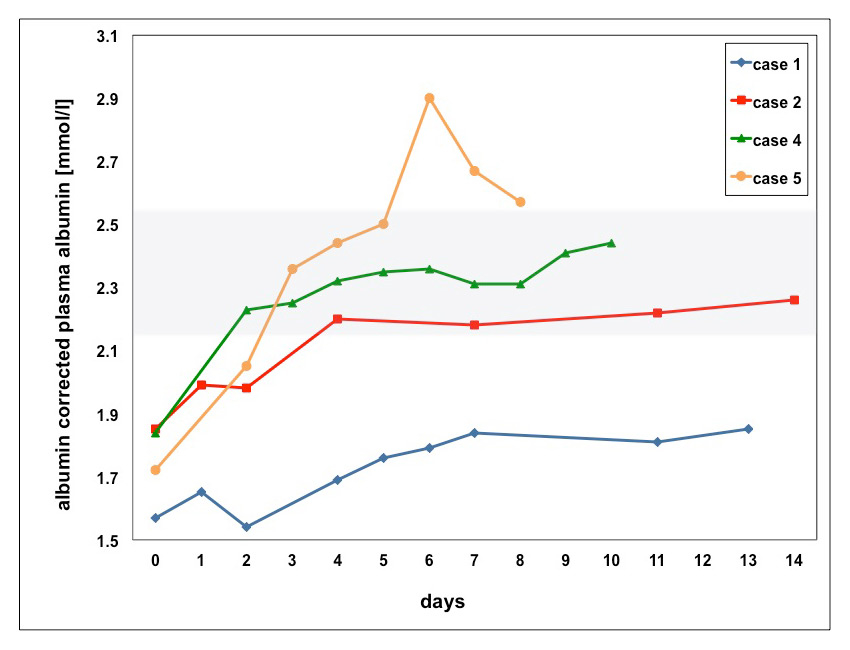
Black et al. found that in osteoporotic patients, hypocalcaemia occured 9–11 days after the first infusion of once-yearly zoledronic acid (5 mg). All reported events were transient and asymptomatic [28]. In our cases (most of them were cancer patients), hypocalcaemia occured slightly earlier between the 4th and 11th day after the most recent bisphosphonate dose. Time for correction of hypocalcaemia varied, ranging from 2–4 days (case 2, 4 and 5) to 21 days (case 1) and 42 days (case 3). In case 6, calcium could not be corrected during hospitalisation (fig. 2).
In cases 1–4, severe hypocalcaemia was attributed to bisphosphonate therapy in patients with osteoblastic metastases. In the fourth case, however, hypocalcaemia resolved faster because the infusion of bisphosphonate two months earlier had been combined with an excellent PTH response without severe magnesium deficiency. Hypomagnesaemia, malnutrition, and in case 1 impaired renal function, promoted hypocalcaemia by impaired physiological correcting mechanisms.
With the exception of case 5 and 6, all of our patients had secondary hyperparathyroidism that was most likely due to vitamin D deficiency.
As we illustrated in case 5, hypocalcaemia can develop despite a prophylactic supplementation with calcium and vitamin D and dose adjusted bisphosphonate treatment [26]. This was also shown in a study by Chennuru et al. that included 120 patients with multiple myeloma and other tumours. In total, 42 patients (35%) had hypocalcaemia with ten patients (8%) showing symptoms [8]. In our case five had neither malnutrition nor vitamin D deficiency nor hypomagnesaemia, which may explain why therapeutic measures to correct hypocalcaemia lead to hypercalcaemia after six days.
Taken together, we document that patients with bone metastases, especially osteoblastic metastases, osteoporosis and after bariatric surgery are at risk for life-threatening hypocalcaemia, especially if bisphosphonates are applied in high dosages and if co-factors, such as renal insufficiency, malnutrition, vitamin D or magnesium deficiency, are present [18]. As illustrated above, all of our patients had underlying conditions that impaired the homeostatic response to bisphosphonates and contributed to hypocalcaemia.
Symptomatic patients and asymptomatic patients with a corrected serum calcium below 2.0 mmol/l should initially be treated with calcium gluconate IV (table 2). Oral calcium supplements should also be given and calcitriol and magnesium should be added if needed [29].
According to the recommendations given in the summary of product characteristics and based on these severe clinical observations, it is prudent to measure vitamin D3 and calcium before starting treatment with bisphosphonates in patients with a high-risk of developing symptomatic hypocalcaemia. Furthermore, high-risk patients should be monitored closely after initiation of bisphosphonate treatment to detect and correct bisphosphonate-induced hypocalcaemia.
Contributing factors and comorbidities augmenting the risk should be assessed carefully before initiating bisphosphonates treatment and corrected appropriately (table 3).
This is mandatory in all patients with metastatic cancer receiving higher dosage of bisphosphonates and denosumab.
There is no evidence-based recommendation on time intervals for monitoring electrolyte imbalances during bisphosphonate treatment [30, 31]. Berenson et al. recommend checking calcium, sodium, potassium, phosphate, and magnesium at least every three months, and if patients are symptomatic and renal insufficiency is present, monthly [32]. In our experience, the first check should be done earlier, i.e., one week after bisphosphonate therapy, since our patients became hypocalcaemic usually after seven days.
Regular calcium and vitamin D supplementation before and during bisphosphonate treatment should be assured, if no contraindications are present [8, 33, 34]. Patients should have minimum enteral doses of 1'200 mg of calcium and 800 IU of vitamin D or corresponding supplements [35]. Furthermore, we recommend monitoring the nutritional status (e.g., by calculating the NRS) and to adequately correct malnutrition, since special attention with bisphosphonate treatment should be taken in malnourished patients, as it has been illustrated in case 1, 2 and 6 (table 4).
Although the problem of hypocalcaemia is allegedly known, the high frequency of severe electrolyte imbalance presenting in a short time period to our clinic was worrisome. It is tempting to speculate that this might be similar at other institutions. The clinical risk of bisphosphonate use in regard to hypocalcaemia seems to be underestimated by physicians but will likely become increasingly important since it is widely used due to the increasing rate of advanced malignancy. The risk of hypocalcaemia is mentioned in the section warning and precautions in the prescribing information of bisphosphonates and denosumab. Under consideration of the increasing use of bisphosphonates and denosumab and the published case reports in the literature it must be given more attention. A more careful monitoring of these patients may lead to the prevention of long and expensive hospitalisations and preservation of quality of life in these patients with only limited expectancy of life.
References
1 Lippuner K. The future of osteoporosis treatment – a research update. Swiss Med Wkly. 2012;142:w13624.
2 Meier C, Kraenzlin ME. Curriculum Osteoporose: Therapie-Update 2013, Teil 2. Medikamente heute und morgen. Swiss Med Forum. 2013;13(42):835–40.
3 Ding X, Fan Y, Ma F, Li Q, Wang J, Zhang P, et al. Prolonged administration of bisphosphonates is well-tolerated and effective for skeletal-related events in Chinese breast cancer patients with bone metastasis. Breast. 2012;21(4):544–9.
4 Ho JW, Sundar S. Prolonged hypocalcemia after zoledronic acid in a patient with metastatic prostate carcinoma: did zoledronic acid trigger osteoblastic activity and avid calcium uptake? Clin Genitourin Cancer. 2012;10(1):50–3.
5 Qi WX, Lin F, He AN, Tang LN, Shen Z, Yao Y. Incidence and risk of denosumab-related hypocalcemia in cancer patients: a systematic review and pooled analysis of randomized controlled studies. Curr Med Res Opin. 2013;29(9):1067–73.
6 Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32.
7 Milat F, Goh S, Gani LU, Suriadi C, Gillespie MT, Fuller PJ, et al. Prolonged hypocalcemia following denosumab therapy in metastatic hormone refractory prostate cancer. Bone. 2013;55(2):305–8.
8 Chennuru S, Koduri J, Baumann MA. Risk factors for symptomatic hypocalcaemia complicating treatment with zoledronic acid. Intern Med J. 2008;38(8):635–7.
9 Zuradelli M, Masci G, Biancofiore G, Gullo G, Scorsetti M, Navarria P, et al. High incidence of hypocalcemia and serum creatinine increase in patients with bone metastases treated with zoledronic acid. Oncologist. 2009;14(5):548–56.
10 Maalouf NM, Heller HJ, Odvina CV, Kim PJ, Sakhaee K. Bisphosphonate-induced hypocalcemia: report of 3 cases and review of literature. Endocr Pract. 2006;12(1):48–53.
11 Joshi A, Price E, Collins D, Williamson L. Comment on: “Hypovitaminosis D among rheumatology outpatients in clinical practice”. Rheumatology (Oxford). 2009;48(2):203–4; author reply 4.
12 Sims EC, Rogers PB, Besser GM, Plowman PN. Severe prolonged hypocalcaemia following pamidronate for malignant hypercalcaemia. Clin Oncol (R Coll Radiol). 1998;10(6):407–9.
13 Tsukasa K, Fujimoto C, Ariyama H, Esaki T, Murakawa M, Syoji T, et al. A case of bone marrow carcinosis from gastric cancer that presented hypocalcemia caused by zoledronic acid during the treatment of methotrexate/5–fluorouracil sequential therapy. Gan To Kagaku Ryoho. 2009;36(3):489–92.
14 Tsourdi E, Rachner TD, Gruber M, Hamann C, Ziemssen T, Hofbauer LC. Seizures associated with zoledronic acid for osteoporosis. J Clin Endocrinol Metab. 2011;96(7):1955–9.
15 Ho JW. Bisphosphonate stimulation of osteoblasts and osteoblastic metastasis as a mechanism of hypocalcaemia. Med Hypotheses. 2012;78(3):377–9.
16 Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE, et al. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20(2):239–44.
17 Mouyis M, Ostor AJ, Crisp AJ, Ginawi A, Halsall DJ, Shenker N, et al. Hypovitaminosis D among rheumatology outpatients in clinical practice. Rheumatology (Oxford). 2008;47(9):1348–51.
18 Segal E, Felder S, Haim N, Yoffe-Sheinman H, Peer A, Wollner M, et al. Vitamin D deficiency in oncology patients – an ignored condition: impact on hypocalcemia and quality of life. Isr Med Assoc J. 2012;14(10):607–12.
19 Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19(3):420–32.
20 Guessous I, Dudler V, Glatz N, Theler JM, Zoller O, Paccaud F, et al. Vitamin D levels and associated factors: a population-based study in Switzerland. Swiss Med Wkly. 2012;142:0.
21 Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24(5):1141–62.
22 Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897–907.
23 Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–57.
24 Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19(1):80–100.
25 Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–68.
26 Polyzos SA, Anastasilakis AD, Litsas I, Efstathiadou Z, Kita M, Arsos G, et al. Profound hypocalcemia following effective response to zoledronic acid treatment in a patient with juvenile Paget’s disease. J Bone Miner Metab. 2010;28(6):706–12.
27 Peter R, Mishra V, Fraser WD. Severe hypocalcaemia after being given intravenous bisphosphonate. BMJ. 2004;328(7435):335–6.
28 Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22.
29 Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336(7656):1298–302.
30 Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29(9):1221–7.
31 Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–72.
32 Berenson JR, Stopeck AT. Risks of therapy with bone modifying agents in patients with advanced malignancy http://www.uptodate.com : uptodate.
33 Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs. 2011;71(6):791–814.
34 Fallah-Rad N, Morton AR. Managing hypercalcaemia and hypocalcaemia in cancer patients. Curr Opin Support Palliat Care. 2013;7(3):265–71.
35 Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66.