Osteoarticular infections in young children: what has changed over the last years?
DOI: https://doi.org/10.4414/smw.2014.13971
Dimitri
Ceroni, Georgios
Kampouroglou, Raimonda
Valaikaite, Rebecca
Anderson Della Llana, Davide
Salvo
Summary
Osteoarticular infections remain a significant cause of morbidity worldwide in young children. They can have a devastating impact with a high rate of serious and long-lasting sequelae, especially on remaining growth. Depending on the localisation of infection, they manifest as osteomyelitis, septic arthritis, a combination of both (i.e., osteomyelitis with adjacent septic arthritis) or spondylodiscitis. Osteoarticular infections can be divided into three types according to the source of infection: haematogenous; secondary to contiguous infection; or secondary to direct inoculation. During the last few years, many principles regarding diagnostic assays and the microbiological causes of these infections have evolved in a significant manner. In the present current-opinion review, we discuss recent concepts regarding epidemiology, physiopathology, and the microbiology of bone and joint infections in young children, as well as clinical presentations, diagnosis, and treatment of these infections. Clinicians caring for children need to be especially well versed in these newer concepts as they can be used to guide evaluation and treatment.
Abbreviations
AHO acute haematogenous osteomyelitis
CA community-acquired
Cfu colony-forming units
HACEK Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella spp.)
Hib Haemophilus influenzaetype B
HIV human immunodeficiency virus
HO haematogenous osteomyelitis
MRSA methicillin-resistant Staphylococcus aureus
MSSA methicillin-sensitive S. aureus
OAI osteoarticular infections
PCR polymerase chain reaction
PVL+ Panton-Valentine leukocidin-positive
PSAHO primary sub-acute hematogenous osteomyelitis
SAPHO synovitis, acne, pustulosis, hyperostosis, and osteitis
WBC white blood cell
The respiratory tract: the breeding ground of pathogenic agents?
Direct inoculation into the bone or joint by bacteria via trauma, surgical reduction and internal fixation of fractures, spread from soft tissue infection, or nosocomial contamination are unusual occurrences in young children. Most osteoarticular infections (OAI) are primarily haematogenous in origin and result from symptomatic or asymptomatic bacteremia [1]. It is therefore important to recall the main portal of entry into the bloodstream for pathogens causing OAI in young children. Among all portals of entry, the respiratory tract is probably the most favourable to pathogens. Microorganisms can be found on droplets of moisture in the air and even in dust particles, and many diseases use this portal of entry.
The normal flora of the oropharynx contains a large population of common bacterial inhabitants. The most important group of microorganisms includes Streptococcus mitis, S. mutans, S. milleri,and S. salivarius. It is believed that these bacteria act as antagonists against invasion by potentially pathogenic microorganisms such as S. pneumoniae, S. pyogenes, Haemophilus influenzaetype b (Hib), Neisseria meningitidis, or even Staphylococcus aureus. In addition, cultures from this region also show the presence of large numbers of diphtheroids, Moraxella catarrhalis, Neisseriaspecies, and HACEK organisms (a group of gram-negative bacilli comprising Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella spp.)[2].
Invasive infections in young children are thus frequently caused by organisms carried asymptomatically in the respiratory tract [3, 4]. S. pneumoniae, Hib, N. meningitidis, S. aureus, and K. kingaemay reside in the mucosal surface and are able to penetrate the bloodstream, disseminate, and invade distant organs [3, 5, 6]. Therefore, colonisation of the respiratory tract by these organisms is a prerequisite for later invasion, and human populations with high rates of carriage of these pathogens are also at increased risk of acquiring disease [3, 5–7].
However, oropharyngeal carriage of pathogens does not imply the subsequent development of invasive OAI, but it suggests that other co-factors may play a role in the pathogenesis of invasive infections [8]. In addition, available evidence suggests that interactions occur with viral infections. Concomitant upper respiratory tract infection and stomatitis, including varicella-induced oral ulcers, are frequently present in affected patients, especially for K. kingae [2]. It appears that microorganisms colonising the oropharynx penetrate a mucosal layer previously damaged by a viral disease [9] and then progress throughout the airways, causing lower respiratory tract infection and/or invasion of the bloodstream [9]. Transient benign bacteremia may follow and the bacterium might be seeded in the joint space, bone, or intervertebral discs, resulting in a focal suppurative infection [9].
Pathogens responsible for OAI
The type of infecting pathogens for OAI depends on the age of the child and any associated medical problem. Apart from S. aureus, OAI with gram-negative organisms, Group B Streptococcus, and Candida are common in neonates. In children younger than 4 years, the reported number of cases of K. kingae-associated OAI has markedly increased since the 1980s. Indeed, several studies have demonstrated that K. kingae has been revealed to be the major bacterial cause of OAI in children aged between 6 and 48 months (30% to 93.8% of all culture-positive OAI) [8–14]. This now brings a coherent explanation to the fact that prior to the use of polymerase chain reaction (PCR) assays, 20 to 70% of OAI cases were culture-negative, despite the collection of blood, joint fluid, and bone for standard cultures [1, 15]. Although S. aureusis no longer considered as the most common cause of OAI in children aged less than 5 years, methicillin-sensitive S. aureus (MSSA) remains the most common pathogen responsible for OAI in older children. Since the use of penicillin in the early 1940s, OAI caused by methicillin-resistant S. aureus (MRSA) has become an increasingly common problem in the USA [15–18], even if MSSA is still the most common pathogenic organism. In addition, the emergence of Panton-Valentine leukocidin-positive (PVL+) community-acquired (CA-) MRSA and MSSA infections has been observed. OAI caused either by CA-MRSA or by MRSA/MSSA PVL+ have a more serious presentation, more complications, and require a more aggressive treatment than those due to MSSA [18–20].
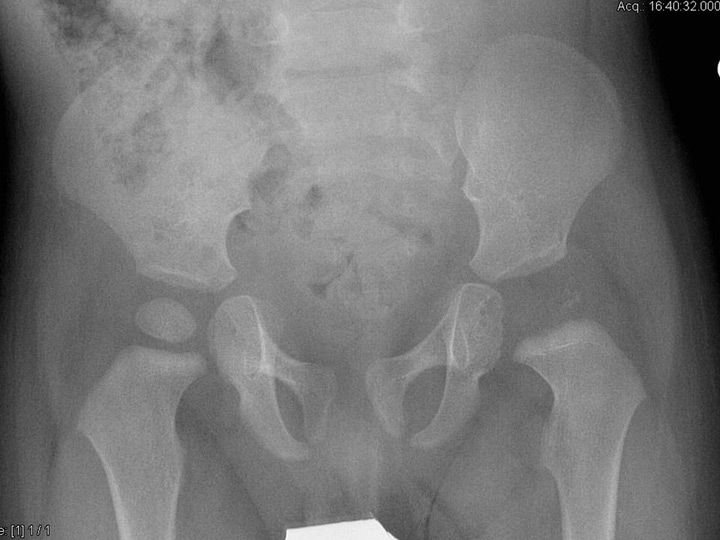
Figure 1
An 8-month-old boy sustained osteoarthritis of the left hip due to Streptococcus pneumoniae. Six months after the infection, the epiphyis had completely disappeared, confirming that children with osteoarticular infection due to S. pyogenesare more likely to have joint involvement, spread of infection into the epiphysis, and thus subsequent disturbed epiphysial growth.
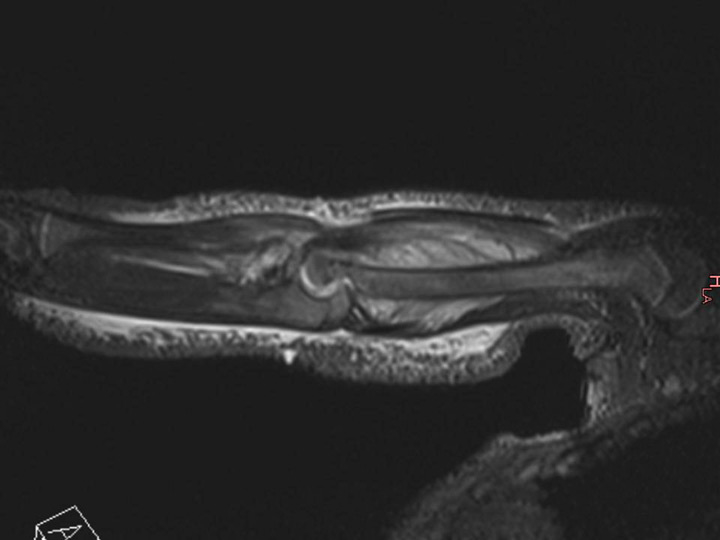
Figure 2
Acute pandiaphysitis of the right humerus with an important sub-periosteal abscess in a 16-month-old girl. The osteomyelitis was due to Haemophilus influenzaetype B despite the child being completely immunised.
Apart from K. kingae and S. aureus, other organisms causing OAI in young children include S. pyogenes and S. pneumoniae. Children with OAI due to S. pyogenes often have a previous history of varicella infection and usually present with a higher fever and white blood cell (WBC) count compared with those infected with S. aureus[1]. Children with OAI caused by S. pneumoniaeare younger than those infected with S. aureus and S. pyogenes [1, 21, 22]. They are more likely to have joint involvement, spread of infection into the epiphysis, and thus subsequent disturbed epiphysial growth (fig. 1) [1, 21, 22]. The incidence of Hib as a pathogen for OAI in young children has decreased noticeably as a result of an effective immunisation programme against this organism [1]. Hib invasive infections, such as OAI, are now rare in completely immunised children, but the onset of OAI due to Hib is not exceptional (fig. 2), considering on the other side that other serotypes are reported to cause bone and joint infections [1]. Young children with sickle cell disease have been reported to be particularly susceptible to OAI [1]. Causative organisms include Salmonella, S. aureus and, less commonly, Escherichia coli, Shigella, and S. pneumoniae.
Osteomyelitis
Osteomyelitis is an inflammation of the bone caused by infection involving bone and/or bone marrow with bacterial or fungal organisms. Osteomyelitis may take diverse forms and several classification systems have been developed to describe this condition. One of these focuses on the source of infection and distinguishes between osteomyelitis arising from haematogenous seeding and osteomyelitis secondary to spread of a contiguous focus of infection or vascular insufficiency [23]. However, osteomyelitis caused by contiguous spread of infection is rare in young children and those secondary to vascular insufficiency remain exceptional. A second system distinguishes between acute, sub-acute, and chronic osteomyelitis based on the elapsed time between the onset of symptoms and diagnosis, irrespective of the underlying source of the offending pathogen [23, 24].
Figure 3
Magnetic resonance imaging (MRI) of the distal femur of a 17-month-old boy, which showed a normal appearance on plain radiographs. MRI demonstrated a lytic lesion of the distal femoral epiphysis with an important abscess of the soft surrounding tissues. Specific cultures confirmed tuberculosis.
Acute haematogenous osteomyelitis
Acute haematogenous osteomyelitis (AHO) is defined as an infection diagnosed within two weeks of the onset of symptoms [23]. The incidence of AHO in children is 0.2–1.6/1000 children/year [25]. Approximately 50% of cases of AHO occur in the first 5 years of life [1]. Osteomyelitis in young children is generally of haematogenous origin and acute in most cases. Boys are more likely than girls to be affected and acute haematogenous osteomyelitis typically arises in the metaphysis of long tubular bones, with approximately two-thirds of all cases involving the femur, tibia, or humerus [1, 23, 26]. A seasonal variation can be observed with the hospital admission rate for osteomyelitis peaking in late summer and autumn. Clinically, trauma to the affected part can be observed in up to 50% of children who have AHO [27] and injury probably has an effect on decreasing resistance to infection.
Dysfunction of the immune system is another factor that has been observed to be associated with AHO. This is well illustrated by the increased susceptibility to infection in children with diseases characterised by deficient or altered immune function, in the neonate with an immature immune function, or during specific situations that may cause temporary and transient depression of the immune function (e.g.. intercurrent viral illness, surgery. or malnutrition). Due to its rich vascular supply in young children, the metaphysis of the bone is most often involved. Two vascular characteristics contribute to the translocation of germs through the capillaries at this location. First, vessels beneath the physeal plate are small arterial loops that empty into venous sinusoids, resulting in a turbulence of the blood flow [28]. In addition, the endothelium wall of the metaphyseal capillaries has gaps that allow the passage of bacteria. Translocated pathogens then find locally favourable conditions to proliferate as they are not phagocytised, due to the absence of phagocytic cells in this region of the bone. Once on site, the microorganism replicates and causes suppurative inflammation. Various inflammatory factors, bacterial toxins, and leucocytes themselves, contribute to tissue necrosis and the destruction of bone trabeculae and bone matrix [23]. Vascular channels are compressed and obliterated by the inflammatory process and the resulting ischemia also contributes to bone necrosis [23]. As a result, antibiotics and inflammatory cells cannot reach this avascular area; for this reason, surgical incision and drainage should be considered, especially when an abscess is present in the bone, sub-periosteally, or in the soft tissue [26]. Surgical intervention may enhance treatment as it may halt the phenomenon responsible for bone necrosis. It permits removal of devitalised bone and debridement of affected soft tissue, whereas surgical irrigation probably decreases the bacterial load. Thus, a surgical procedure should be considered not only when a child does not respond to empiric antibiotic therapy, but each time when pyogenic pathogens are thought to be responsible for AHO.
Sub-acute haematogenous osteomyelitis
Primary sub-acute haematogenous osteomyelitis (PSAHO) is an infectious process characterised by insidious onset, moderate localised bony pain, mild or no systemic manifestations, non-contributory laboratory results, negative blood cultures, and positive radiologic findings [29–39]. According to King and Mayo, any osseous infectious process of more than two weeks duration without acute symptomatology can be referred to as sub-acute osteomyelitis [36].
PSAHO is most likely due to an atypical host-pathogen relationship that may comprise any combination of increased host resistance, decreased virulence of the causative organism, and/or prior antibiotic exposure [31, 32, 38, 40, 41]. The primary form of sub-acute HO, which occurs mainly in children, must be distinguished from osteomyelitis modified by inadequate or partial treatment with antibiotics and from other forms of the condition, such as chronic recurrent multifocal osteomyelitis and the SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis) [42]. In many cases, cultures fail to identify the causative organism, especially when fine-needle aspiration is performed. Surgical drainage may yield positive cultures in 40% to 75% of patients. Some reports have suggested an increasing incidence of this form of osteomyelitis [35] and a higher prevalence in certain countries [34].
PSAHO can be divided into two main clinical forms according to the age of the child and its bacteriological aetiology. The first form, the infantile form, affects children aged between 6 months to 4 years. Approximately 90% of all PSAHO affect patients in this age group with K. kingae as the main observed microorganism (personal data to be published). In these young children, the clinical course of PSAHO is most likely explained by the natural low virulence of K. kingae. K. kingae osteoarticular infection is characterised by a mild-to-moderate clinical and biologic inflammatory response to infection with few (if any) criteria evocative of OAI. Many children in this age group are usually recognised late as having an osteoarticular infection, and an accurate diagnosis is generally delayed until after a bony lytic lesion has occurred (fig. 3). The second form, the juvenile form, affects children older than 4 years and S. aureus appears as the main bacteriological aetiology. In this situation, PSAHO is most likely the result of an increased host resistance and it can be hypothesised that the children who develop this resistance against S. aureus become able to contain the bone infection. Indeed, colonisation is recognised to be more frequent among children [43]. Remarkably, 20% of individuals are persistently colonised by S. aureus in the nares and 30% are transiently colonised [44]. Although colonisation predisposes an individual to S. aureus infection, colonised individuals may have less severe S. aureus disease compared with non-colonised individuals [45]. This raises the question as to whether colonisation could induce low level, adaptive immunity and subsequent milder infections [44].
PSAHO in children follows a benign course and the recommended treatment for sub-acute osteomyelitis with radiographic evidence of lucent lesions or nidus is currently curettage, biopsy, and culture followed by antibiotics [9–11, 46]. Many authors even suggest that antibiotics alone may be adequate and that surgery should be considered only for “aggressive lesions”, as well as those that do not respond to antibiotics [6, 41]. However, it is generally agreed that treatment should not be initiated until proper drainage and bacteriological samples have been obtained [9–11, 46]. In children less than 4 years, antibiotherapy should be directed above all against K. kingae, whereas S. aureus is the bacteria most often associated with PSAHO in older children.
Chronic osteomyelitis
Osteomyelitis is considered chronic if the duration of the illness has been more than 3 months [26]. Chronic osteomyelitis is defined by the presence of residual foci of infection, which give rise to recurrent episodes of clinical infection. Chronic osteomyelitis is therefore a persistent infection of bone and bone marrow due to the presence of intracellular bacteria [47], which allows the pathogens to escape to the immune system and invade adjacent bone cells [48]. In developing countries, the disease usually results from untreated acute HO. In developed countries, chronic osteomyelitis remains fortunately a rare condition generally encountered after open traumatic injuries or as a complication of surgical procedures, such as open reduction and internal fixation of fractures. Chronic osteomyelitis may also result from specific medical disorders, such as immunodeficiency or sickle cell disease. Finally, chronic osteomyelitis may be part of the characteristics of other forms of the condition, such as chronic recurrent multifocal osteomyelitis and the SAPHO syndrome. Eradication of the infection is often very difficult and complications associated with both the infection and treatment are frequent.
Septic arthritis
Septic arthritis is an infection of the joint space by means of haematogenous dissemination of bacteria into the vascular synovium. Rates of septic arthritis are estimated to be between 5.5 and 12 cases per 100,000 children with a peak incidence in the early years of the first decade [1]. However, most cases of septic arthritis occur in children 3 years old or younger. Again, boys are affected twice as much as girls as they are probably more likely to be involved in activities leading to repetitive minor joint trauma. The hip and knee are the most common sites of septic arthritis and symptoms include acute onset of joint pain, fever, irritability and limp.
An acute inflammatory response follows bacterial spreading of the joint, resulting in the migration of polymorphonuclear WBCs, production of proteolytic enzymes, and cytokine secretion by chondrocytes. If the infection is not quickly cleared by the host, the potent activation of the immune response, in association with high levels of cytokines and reactive oxygen species, increase the release of host matrix metalloproteinases and other collagen-degrading enzymes, which in conjunction with bacterial toxins lead to joint destruction [49]. The polymorphonuclear response with subsequent release of these proteolytic enzymes can initiate degradation of articular cartilage 8 h after the onset of infection and lead to permanent destruction of the intra-articular cartilage and sub-chondral bone loss in as little as three days [49]. In addition, metalloproteinases and the antigen-induced inflammatory response may persist and continue to damage the joint architecture even after the infection has been cleared [50, 51]. To ensure a good prognosis, treatment of septic arthritis not only requires prompt recognition and rapid and aggressive antimicrobial therapy, but primarily surgical irrigation of the joint in order to clear the factors responsible for the potent activation of the immune response.
Septic osteoarthritis
There are a few situations in which an infectious process affecting the metaphysis can spread into the joint and result in osteoarthritis. In certain anatomical sites, the bony metaphysis is intracapsular and any bone infection may potentially lead to osteoarthritis, with concomitant osteomyelitis and septic arthritis. Osteomyelitis of locations, such as the upper end of the femur, proximal humerus, proximal tibia, and distal fibula, are more prone to spread sub-periosteally into the joint space. By contrast, the epiphyses of children younger than 18 months are vascularised by transphyseal vessels [52]. As these vessels enter the epiphysis and thus potentially the joint space, young children are more prone to have a higher risk of joint space infection complicating osteomyelitis [1]. Clinicians caring for children less than 18 months should keep in mind these anatomic characteristics as they must be used to guide evaluation and treatment. As an example, a very young child with an AHO of the proximal femur must be suspected to have a spread of the infection into the joint space. In this case, surgical treatment must not only focus on the bone treatment – irrigation of the joint is probably the most important surgical procedure as it will decrease the potent activation of the immune response responsible for the joint damage. By contrast, AHO must be considered when facing a situation of septic arthritis as treating only the septic arthritis will expose these very young children to recurrence of the infection.
Spondylodiscitis
Childhood spondylodiscitis remains an uncommon and often missed ailment in young children from 6 to 48 months of age. The diagnosis should be considered in toddlers who present with refusal to walk, gait disturbances, back pain, or even abdominal pain [53–55]. The pathophysiology remains controversial. Some authors regard it as an infective process of the intervertebral disc or endplates [56–59], whereas others consider it as a self-limiting inflammatory condition [60–65]. Childhood spondylodiscitis represents a continuum of spinal infections ranging from discitis to vertebral osteomyelitis, with occasional associated soft tissue abscess [54]. These entities likely fall within a broad spectrum of manifestations of a single disease with varying severity. A few studies highlighted a triphasic age distribution with varying signs and symptoms according to age. Thus, spondylodiscitis in childhood should be classified according to three separate age groups, namely neonates, infants, and older children [56, 57, 60, 61, 63, 66, 67]. The form affecting neonates (less than 6 months) is the most serious manifestation of the disease and is often associated with septicemia and multiple infectious foci. The vertebrae are classically severely damaged and sometimes entirely destroyed, leading to major kyphosis, especially when the thoracic spine is involved [66–68]. Neurologic findings are most likely to occur in this age group. Approximately 80% of spondylodiscitis in neonates are due to S. aureus [66, 67]. The second infantile form affects children from 6 months (end of maternally-derived immunity) to 4 years of age. This age group represents 60% of childhood spondylodiscitis [53, 56–60, 62, 63, 65, 67, 69–75]. When performed, the vast majority of biopsies come back sterile [57, 60, 64, 72, 74] or positive for K. kingae[53, 67]. Finally, in the third form affecting children older than 4 years, patients are more prone to sustain vertebral osteomyelitis [57, 59, 70]. This age group is also likely to be febrile and ill-appearing, and S. aureus is the predominant pathogen. Laboratory findings in spondylodiscitis, such as WBC count, C-reactive protein, and erythrocyte sedimentation rate, often provide non-specific information [54, 70, 73]. Blood cultures are usually the only means available to direct antimicrobial therapy, but unfortunately yield a high percentage of negative results [54, 60, 70, 73, 75]. The indication for more invasive procedures, such as biopsy or needle aspiration, is currently not established, especially in young children [53]. The literature reports success rates for the identification of the causative organism ranging from 0% to 63% for needle aspiration and open biopsy, respectively, [64, 67, 75–77]. However, these interventions are still not regarded as standard diagnostic procedures for most authors due to surgical and anaesthetic risks.
Osteoarticular tubercular infections
The incidence and prevalence of paediatric tuberculosis worldwide varies significantly according to the burden of the disease in different countries. Europe and North America are traditionally considered as low burden regions, and paediatric incidence rates vary from 1 to 15/100,000/year [78]. Tuberculosis of bones or joints occurs in around 5% in cases of paediatric extrapulmonary tuberculosis and, classically, tubercular OAIs occur one to three years after pulmonary infection. Vertebral lesions (thoracic>lumbar>cervical) are probably the most common involvement and 80% of these affect more than one vertebrae. In decreasing order, other common sites are: hips; knee; ankle and foot; hand and wrist; elbow; shoulder; bursal sheaths; and other bones [79]. Tubercular OAI is rare, but not exceptional in Switzerland (fig. 4) and it must be considered when a very young child presents a clinical picture of sub-acute osteomyelitis with a mild-to-moderate clinical and biologic inflammatory response to infection. In general, contamination is intra-familial due to close proximity with elderly individuals from regions where the prevalence of tuberculosis is high (e.g., Balkans, Africa, India). Finally, immunocompromised children and those seropositive for human immunodeficiency virus (HIV) are supposed to be at high risk of exposure, as well as manifesting tubercular disease. The synergy of HIV and tuberculosis and the emergence of multidrug-resistant Mycobacterium tuberculosis have further complicated the issue.
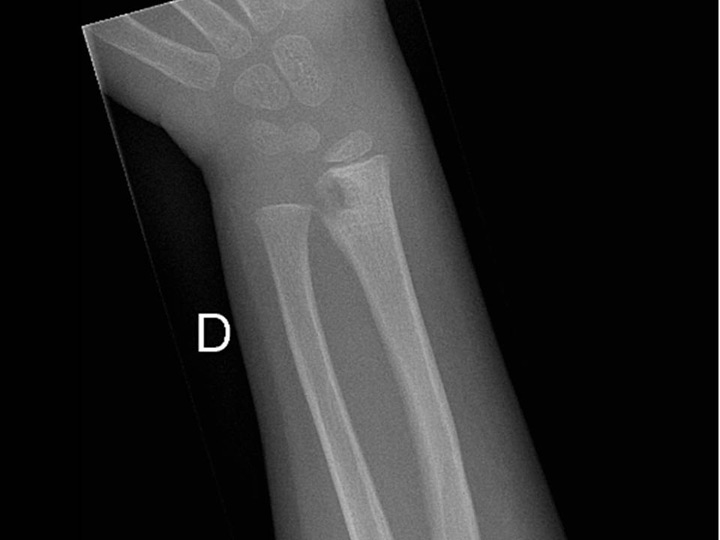
Figure 4
Sub-acute transphyseal osteomyelitis of the distal radius due to K. kingae in a 5-year-old boy who suffered from a chickenpox (varicella) infection three weeks before bone infection. This viral infection was considered as a predisposing factor for the development of osteomyelitis due to K. kingae resulting from a modulation of the immune system function.
Culture detection of pathogens
The yield of cultures has been significantly improved by inoculating clinical specimens into aerobic blood culture vials [80, 81] from a variety of automated or manual blood culture systems, such as BACTEC (Becton Dickinson, Cockeysville, MD, USA), BacT/Alert (Organon Teknika Corporation, Durham, NC, USA), Isolator 1.5 Microbial Tube (Wampole Laboratories, Cranbury, NJ, USA), or Hemoline DUO (bioMérieux Lyon, France). However, no controlled study has been performed to identify the best blood culture system for this purpose [9]. The primary isolation of any fastidious pathogens such as K. kingaefrom joint, bone, or blood samples appears strongly dependent on the methodology used [46]. Indeed, the recovery of K. kingae from purulent specimens seeded onto solid culture media is suboptimal and mostly results in a frustrating proportion of negative cultures [8–11, 46].
Detection of pathogens by PCR assay
PCR is a biochemical technology in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence (Wikipedia). Recognition of pathogens responsible for OAI by PCR assays has now become a common procedure, especially for fastidious microorganisms. There are currently two different nucleic acid amplification approaches. Broad-range 16S rRNA gene assay involves extracting DNA from clinical samples, incubating the DNA with broad-range oligonucleotide primers that anneal to constant regions of the 16S ribosomal RNA gene, and amplification of the intervening sequence, which varies according to the bacterial species [82]. The resulting amplification products are either sequenced and compared with sequences in the GenBank database or hybridised with organism-specific probes [83]. The use of the broad-range 16S rRNA gene assay offers the tremendous advantage of not requiring any a priori knowledge of the causative bacteria. However, this method is hampered by an insufficient sensitivity to detect all agents directly from clinical samples as the analytical sensitivity of the broad range 16S rRNA gene PCR is only 300 colony-forming units (cfu) [84]. In recent years, real-time PCR assays that amplify specific targets for most osteoarticular pathogens have been developed. Currently, real-time PCR assays that amplify K. kingae-specific targets, such as cpn60 or RTX toxin genes, have been developed and are associated with high reliability [8, 10–13, 84]. Real-time PCR assays specific to K. kingae targets, such as the RTX toxin, are 10–fold more sensitive than the broad-range 16S rRNA gene PCR (30 cfu vs 300 cfu) [84].
Antibiotic selection and treatment’s modalities
Successful treatment of osteoarticular infections depends on the appropriate selection and administration of antibiotic therapy and surgical procedure as needed [1]. Empiric therapy is usually selected to cover the most likely pathogens, which are determined above all by the age of the child, by local prevalence of specific infectious agents, and early laboratory results such as stain if available. When the culture’s results are then available, the antimicrobial therapy is modified depending on the organism and the susceptibility pattern. Absorption and penetration into the bony tissue, the joint, or the intervertebral disk should be satisfactory, and time-dependant antibiotics with a short circulating half-life are more likely to require frequent dosing [85]. Infants less than 6 months old with osteoarticular infections should be treated with antibiotics that have excellent coverage against S. aureus, S. agalactiae, and enteric gram-negative bacteria. In children aged between 6 months and 4 years, most of OAI are due to K. kingae.In Switzerland, as in Europe and Israel, there are very few beta-lactamase-producing clones of K. kingae and thus, beta-lactams are the drugs of choice for OAI due to this microorganism, as well for those due to S. aureus, S. pyogenes or S. pneumoniae. Finally, most of OAI in children are still exceptionally due to MRSA in our country and thus, the decision to cover empirically for MRSA is currently controversial due to concerns over developing resistances, costs, and potential complications with use of vancomycin. For spondylodiscitis, the situation is some confusing since there is no agreement in the literature regarding the antibiotics’ ability to enter discs in an active form. In fact, the antibiotic’s ability to spread through all parts of the disc is not only influenced by the vascular supply and structure of the disc (size and health), but also by the properties of the drug (size, solubility, binding and charge) [86]. The antibiotic’s charge in particular has been discussed in the literature, since the nucleus pulposus is rich in glycosaminoglycans and has a high density of negative charge [86]. Thus, it has been postulated that positively charged antibiotics (gentamicin or vancomycin) can enter the IVD, whereas negatively charged antibiotics (penicillin and cephalosporins) have limited [87–89] or poor penetration [90] because of repellent charges.
The length and route of treatment depend above all on the pathogen’s virulence, as well as the clinical and laboratory response to treatment (decreases of pain, fever, CRP, and ESR). Most of OAI in children could be converted to oral antibiotics between 3 or 5 days, and there are currently probably few advantages with antibiotic courses that are prolonged for more than 3 weeks. In countries such as the United States, where MRSA is a common pathogen and when the clinical setting is suggestive for an OAI due to MSSA PVL+, a more cautious conservative approach is probably well founded.
How to recognise K. kingae OAI
If K. kingae is currently considered as the most common cause of OAI in young children, most of these infections still remain unrecognised. Diagnosis requires a high index of suspicion since the presentation of K. kingae OAI is often characterised by a mild-to-moderate clinical and biologic inflammatory response to infection, with the consequence that these children present few, if any, criteria evocative of OAI [2]. Improving recognition of infection due to K kingaeis thus the next problem to resolve and there is a need for new diagnostic tests to improve their diagnosis. For example, a simple technique to detect K. kingae RTX toxin genes in the oropharynx might provide strong evidence that this microorganism is responsible for the OAI [91]. The positive predictive value of PCR detection in a pharyngeal sample is around 90%; however, the negative predictive value of this test is very high and failure to detect RTX gene sequences in the pharynx practically excludes the bacterium as the aetiology of the OAI [91, 92]. Such a non-invasive approach to diagnosis improves patient safety and comfort and reduces healthcare costs by reducing the need for invasive diagnostic procedures. A recent paper has also demonstrated that magnetic resonance imaging was useful in differentiating OAI due to K. kingae from those due to gram-positive cocci. In this study, epiphyseal cartilaginous involvement and modest soft tissues and bone reactions were suggestive for AHO due to K. kingae. There is also the need to have a criterion for distinguishing quickly OAI due to K. kingaethan those due to pyogenic pathogens. A model to allow the differentiation of K. kingaeOAI from those due to typical pathogens in children aged less than 4 years, has been described and consists of the following four parameters: T° at admission <38°; C-reactive protein <55 mg/L; WBC count <14,000 leucocytes/mm3, and band shift <150 forms/mm3 [10]. This model is a subject of controversy, but it underlines the need for prospective studies to better define the clinical presentation according to the children’s age and causative organisms.
Conclusion
During these last years, the use of PCR assays has completely changed the microbiological ecology for OAI in young children. More than 50% of cases of OAI occur in the first 5 years of life and K. kingae has become the major bacterial cause of infection in this age group. The clinical presentation of K.
kingae OAI is often subtle and may be associated with normal acute-phase reactants. Treatment of OAI is usually instituted empirically before the causative agent and its resistance pattern is known. The timing of surgery depends on the suspected pathogen and on the extent of the OAI. Trepanating an infected bone, draining an abscess, or washing the joint space might speed up the healing process. Aggressive debridement should be considered in difficult-to-treat cases of MRSA or when MSSA/MRSA is producing PVL. Any less virulent microorganisms, such as K. kingae, usually do not require a surgical procedure. There is also the need to have criteria for distinguishing quickly OAI due to K. kingaethan those due to pyogenic pathogens.
References
1 Gutierrez K. Bone and joint infection. In: Long SS PL, Prober CG, eds Principles and Practice of Pediatric Infectious Disease 2nd ed Philadelphia, PA: Churchill Livingstone 2003:467–474.
2 Ceroni D, Dubois-Ferriere V, Cherkaoui A, Lamah L, Renzi G, Lascombes P, et al. 30 years of study of Kingella kingae: post tenebras, lux. Future microbiology. 2013;8(2):233–45.
3 Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24(5):860–6.
4 Yagupsky P, Peled N, Katz O. Epidemiological features of invasive Kingella kingae infections and respiratory carriage of the organism. J Clin Microbiol. 2002;40(11):4180–4.
5 Aniansson G, Alm B, Andersson B, Larsson P, Nylen O, Peterson H, et al. Nasopharyngeal colonization during the first year of life. J Infect Dis. 1992;165(Suppl 1):S38–42.
6 Gray BM, Converse GM, 3rd, Dillon HC, Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142(6):923–33.
7 Riordan T, Cartwright K, Andrews N, Stuart J, Burris A, Fox A, et al. Acquisition and carriage of meningococci in marine commando recruits. Epidemiol Infect. 1998;121(3):495–505.
8 Ceroni D, Dubois-Ferriere V, Anderson R, Combescure C, Lamah L, Cherkaoui A, et al. Small Risk of Osteoarticular Infections in Children with Asymptomatic Oropharyngeal Carriage of Kingella kingae. The Pediatric infectious disease journal 2012.
9 Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis. 2004;4(6):358–67.
10 Ceroni D, Cherkaoui A, Combescure C, Francois P, Kaelin A, Schrenzel J. Differentiating osteoarticular infections caused by Kingella kingae from those due to typical pathogens in young children. Pediatr Infect Dis J. 2011;30(10):906–9.
11 Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop. 2010;30(3):301–4.
12 Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Berard J, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007;26(5):377–81.
13 Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Liguori S, et al. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol. 2009;47(6):1837–41.
14 Rosey AL, Abachin E, Quesnes G, Cadilhac C, Pejin Z, Glorion C, et al. Development of a broad-range 16S rDNA real-time PCR for the diagnosis of septic arthritis in children. J Microbiol Methods. 2007;68(1):88–93.
15 Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26(6):703–8.
16 Kaplan SL. Implications of methicillin-resistant Staphylococcus aureus as a community-acquired pathogen in pediatric patients. Infect Dis Clin North Am. 2005;19(3):747–57.
17 Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17(3):113–9.
18 Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, Hammerman W, Mason EO, Jr., Kaplan SL. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23(8):701–6.
19 Dohin B, Gillet Y, Kohler R, Lina G, Vandenesch F, Vanhems P, et al. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J. 2007;26(11):1042–8.
20 Moumile K, Cadilhac C, Lina G, Berche P, Glorion C, Ferroni A. Severe osteoarticular infection associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Diag Microbiol Infect Dis. 2006;56(1):95–7.
21 Bradley JS, Kaplan SL, Tan TQ, Barson WJ, Arditi M, Schutze GE, et al. Pediatric pneumococcal bone and joint infections. The Pediatric Multicenter Pneumococcal Surveillance Study Group (PMPSSG). Pediatrics. 1998;102(6):1376–82.
22 Tan TQ, Mason EO, Jr., Barson WJ, Wald ER, Schutze GE, Bradley JS, et al: Clinical characteristics and outcome of children with pneumonia attributable to penicillin-susceptible and penicillin-nonsusceptible Streptococcus pneumoniae. Pediatrics. 1998;102(6):1369–75.
23 Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–79.
24 Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175–81.
25 Gavilan MG, Lopez JB, Artola BS. Peculiarities of osteo-articular infections in children. Bailliere’s best practice & research. 1999;13(1):77–94.
26 Krogstad P SA. Osteomyelitis and septic arthritis. In: Feigin RD, Cherry JD eds In Textbook of Pediatric Infectious Diseases 4th ed Philadelphia, PA: WB Saunders, 1998:683–704.
27 Winters JL, Cahen I. Acute hematogenous osteomyelitis. A review of sixty-six cases. J Bone Joint Surg. 1960;42–A:691–704.
28 Schenk RK, Wiener J, Spiro D. Fine structural aspects of vascular invasion of the tibial epiphyseal plate of growing rats. Acta anatomica. 1968;69(1):1–17.
29 Ezra E, Cohen N, Segev E, Hayek S, Lokiec F, Keret D, Wientroub S. Primary subacute epiphyseal osteomyelitis: role of conservative treatment. J Pediatr Orthop. 2002;22(3):333–7.
30 Ezra E, Wientroub S. Primary subacute haematogenous osteomyelitis of the tarsal bones in children. J Bone Joint Surg Br. 1997;79(6):983–6.
31 Gillespie WJ, Moore TE, Mayo KM. Subacute pyogenic osteomyelitis. Orthopedics. 1986;9(11):1565–70.
32 Gledhill RB. Subacute osteomyelitis in children. Clin Orthop Relat Res. 1973(96):57–69.
33 Hamdy RC, Lawton L, Carey T, Wiley J, Marton D. Subacute hematogenous osteomyelitis: are biopsy and surgery always indicated? J Pediatr Orthop. 1996;16(2):220–3.
34 Harris NH, Kirkaldy-Willis WH. Primary Subacute Pyogenic Osteomyelitis. J Bone Joint Surg Br. 1965;47:526–32.
35 Jones NS, Anderson DJ, Stiles PJ. Osteomyelitis in a general hospital. A five-year study showing an increase in subacute osteomyelitis. J Bone Joint Surg Br. 1987;69(5):779–83.
36 King DM, Mayo KM. Subacute haematogenous osteomyelitis. J Bone Joint Surg Br. 1969;51(3):458–63.
37 Kozlowski K. Brodie’s abscess in the first decade of life. Report of eleven cases. Pediatr Radiol. 1980;10(1):33–7.
38 Roberts JM, Drummond DS, Breed AL, Chesney J. Subacute hematogenous osteomyelitis in children: a retrospective study. J Pediatr Orthop. 1982;2(3):249–54.
39 Season EH, Miller PR. Multifocal subacute pyogenic osteomyelitis in a child. A case report. Clin Orthop Relat Res. 1976(116):76–9.
40 Dormans JP, Drummond DS. Pediatric Hematogenous Osteomyelitis: New Trends in Presentation, Diagnosis, and Treatment. J Am Acad Orthop Surg. 1994;2(6):333–41.
41 Green NE, Beauchamp RD, Griffin PP. Primary subacute epiphyseal osteomyelitis. J Bone Joint Surg. 1981;63(1):107–14.
42 Rasool MN. Primary subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br. 2001;83(1):93–8.
43 Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. The Lancet infectious diseases. 2005;5(12):751–62.
44 Liu Y, Cui J, Wang R, Wang X, Drlica K, Zhao X. Selection of rifampicin-resistant Staphylococcus aureus during tuberculosis therapy: concurrent bacterial eradication and acquisition of resistance. J Antimicrob Chemother. 2005;56(6):1172–5.
45 Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364(9435):703–5.
46 Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol. 1992;30(5):1278–81.
47 Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26(6):317–23.
48 Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, et al. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br. 2003;85(6):918–21.
49 Roy S, Bhawan J. Ultrastructure of articular cartilage in pyogenic arthritis. Archives of pathology. 1975;99(1):44–7.
50 Smith RL, Schurman DJ. Bacterial arthritis. A staphylococcal proteoglycan-releasing factor. Arthritis and rheumatism. 1986;29(11):1378–86.
51 Smith RL, Schurman DJ, Kajiyama G, Mell M, Gilkerson E. The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J Bone Joint Surg. 1987;69(7):1063–8.
52 Trueta J, Morgan JD. The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br. 1960;42–B:97–109.
53 Ceroni D, Cherkaoui A, Kaelin A, Schrenzel J. Kingella kingae spondylodiscitis in young children: toward a new approach for bacteriological investigations? A preliminary report. J Child Orthop. 4(2):173–5.
54 Early SD, Kay RM, Tolo VT. Childhood diskitis. J Am Acad Orthop Surg. 2003;11(6):413–20.
55 Rocco HD, Eyring EJ. Intervertebral disk infections in children. Am J Dis Child. (1960) 1972;123(5):448–51.
56 Bonfiglio M, Lange TA, Kim YM. The Classic: Pyogenic vertebral osteomyelitis: disk space infections. 1973. Clin Orthop Relat Res. 2006;444:4–8.
57 Crawford AH, Kucharzyk DW, Ruda R, Smitherman HC, Jr. Diskitis in children. Clin Orthop Relat Res. 1991(266):70–9.
58 Jansen BR, Hart W, Schreuder O. Discitis in childhood. 12–35–year follow-up of 35 patients. Acta Orthop Scand. 1993;64(1):33–6.
59 Wenger DR, Bobechko WP, Gilday DL. The spectrum of intervertebral disc-space infection in children. J Bone Joint Surg. 1978;60(1):100–8.
60 Brown R, Hussain M, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg. 2001;83(1):106–11.
61 Cushing AH. Diskitis in children. Clin Infect Dis. 1993;17(1):1–6.
62 Hensey OJ, Coad N, Carty HM, Sills JM. Juvenile discitis. Arch Dis Child. 1983;58(12):983–7.
63 Menelaus MB. Discitis. an Inflammation Affecting the Intervertebral Discs in Children. J Bone Joint Surg. 1964;46:16–23.
64 Ryoppy S, Jaaskelainen J, Rapola J, Alberty A. Nonspecific diskitis in children. A nonmicrobial disease? Clin Orthop Relat Res. 1993(297):95–9.
65 Spiegel PG, Kengla KW, Isaacson AS, Wilson JC, Jr. Intervertebral disc-space inflammation in children. J Bone Joint Surg. 1972;54(2):284–96.
66 Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Vertebral osteomyelitis in infants. J Bone Joint Surg. 1982;64(1):32–5.
67 Garron E, Viehweger E, Launay F, Guillaume JM, Jouve JL, Bollini G. Nontuberculous spondylodiscitis in children. J Pediatr Orthop. 2002;22(3):321–8.
68 Tsirikos AI, Tome-Bermejo F. Spondylodiscitis in infancy: a potentially fatal condition that can lead to major spinal complications. J Bone Joint Surg. 94(10):1399–402.
69 Chandrasenan J, Klezl Z, Bommireddy R, Calthorpe D. Spondylodiscitis in children: a retrospective series. J Bone Joint Surg. 93(8):1122–5.
70 Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18–year review. Pediatrics. 2000;105(6):1299–304.
71 Kayser R, Mahlfeld K, Greulich M, Grasshoff H. Spondylodiscitis in childhood: results of a long-term study. Spine. 2005;30(3):318–23.
72 Rubio Gribble B, Calvo Rey C, Garcia-Consuegra J, Ciria Calabria L, Navarro Gomez ML, Ramos Amador JT. Spondylodiscitis in the autonomus community of Madrid (Spain). An Pediatr. (Barc) 2005;62(2):147–52.
73 Spencer SJ, Wilson NI. Childhood discitis in a regional children’s hospital. J Pediatr Orthop B. 21(3):264–8.
74 Tapia Moreno R, Espinosa Fernandez MG, Martinez Leon MI, Gonzalez Gomez JM, Moreno Pascual P. Spondylodiscitis: Diagnosis and medium-long term follow up of 18 cases. An Pediatr. (Barc) 2009;71(5):391–9.
75 Ventura N, Gonzalez E, Terricabras L, Salvador A, Cabrera M. Intervertebral discitis in children: a review of 12 cases. Int Orthop. 1996;20(1):32–4.
76 Enoch DA, Cargill JS, Laing R, Herbert S, Corrah TW, Brown NM. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clinical Pathol. 2008;61(6):750–3.
77 Sehn JK, Gilula LA. Percutaneous needle biopsy in diagnosis and identification of causative organisms in cases of suspected vertebral osteomyelitis. Eur J Radiol. 81(5):940–6.
78 Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. The Lancet infectious diseases. 2008;8(8):498–510.
79 Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg. 1996;78(2):288–98.
80 Host B, Schumacher H, Prag J, Arpi M. Isolation of Kingella kingae from synovial fluids using four commercial blood culture bottles. Eur J Clin Microbiol Infect Dis. 2000;19(8):608–11.
81 Yagupsky P. Diagnosis of Kingella kingae arthritis by polymerase chain reaction analysis. Clin Infect Dis. 1999;29(3):704–5.
82 Fenollar F, Roux V, Stein A, Drancourt M, Raoult D. Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J Clin Microbiol. 2006;44(3):1018–28.
83 Yagupsky P, Porsch E, St Geme JW, 3rd. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011;127(3):557–65.
84 Cherkaoui A, Ceroni D, Emonet S, Lefevre Y, Schrenzel J. Molecular diagnosis of Kingella kingae osteoarticular infections by specific real-time PCR assay. J Med Microbiol. 2009;58(Pt 1):65–8.
85 Peltola H, Paakkonen M. Acute osteomyelitis in children. N Engl J Med. 2014;370(4):352–60.
86 Walters R. Lumbar Intervertebral Disc Infection: Pathology, Prevention and Treatment. Adelaide: University of Adelaide; 2006.
87 Boscardin JB, Ringus JC, Feingold DJ, Ruda SC. Human intradiscal levels with cefazolin. Spine. 1992;17(6 Suppl):S145–8.
88 Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14(9):1025–32.
89 Rhoten RL, Murphy MA, Kalfas IH, Hahn JF, Washington JA. Antibiotic penetration into cervical discs. Neurosurgery. 1995;37(3):418–21.
90 Riley LH, 3rd, Banovac K, Martinez OV, Eismont FJ. Tissue distribution of antibiotics in the intervertebral disc. Spine. 1994;19(23):2619–25.
91 Ceroni D, Dubois-Ferrière V, Cherkaoui A, et al. Detection of Kingella kingae Osteoarticular Infections in Children by Oropharyngeal Swab PCR. Pediatrics. 2013;131(January):1–6.
92 Ceroni D, Cherkaoui A, Kaelin A, Schrenzel J. Kingella kingae spondylodiscitis in young children: toward a new approach for bacteriological investigations? A preliminary report. J Child Orthop. 2010;4(2):173–5.



