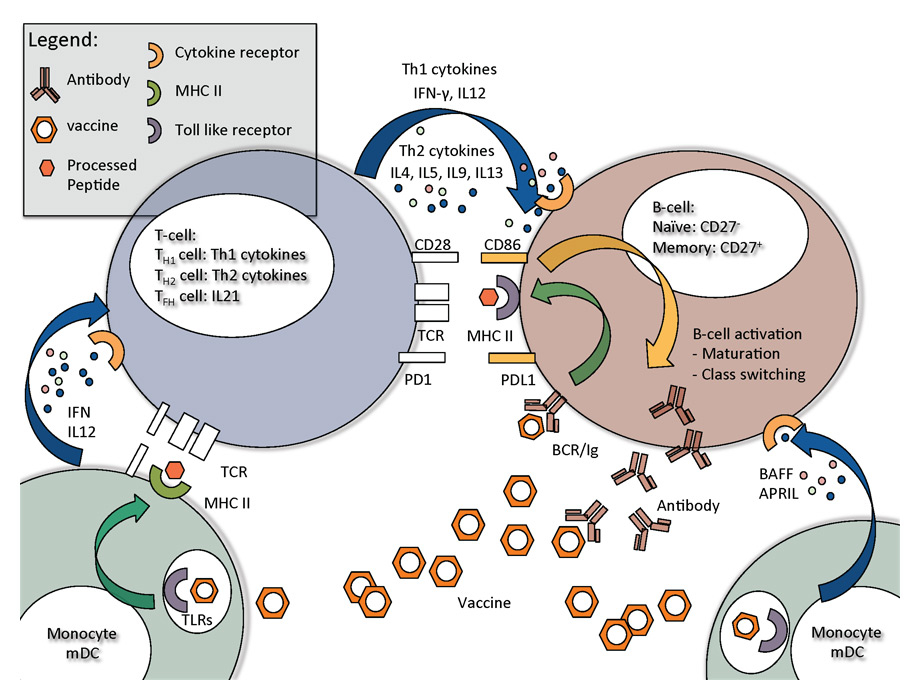
Figure 1
Key steps in B-cell activation and B-cell interaction with T-helper cells and monocytes, and monocyte derived macrophages and dendritic cells.
DOI: https://doi.org/10.4414/smw.2014.13940
In 1796, Edward Jenner’s first observations of protective effects after inoculation with inactivated cowpox, manifest by reduced disease burden following pathogen challenge, heralded the era of vaccination [1]. A century later, Emil von Behring and Shibasaburo Kitasato discovered that sera from animals immune to diphtheria contained an antitoxin activity, later called antibodies [2]. These key findings of an inducible immune response resulted in the development of vaccines at the beginning of the last century. Contemporaneously, the immunomodulatory potential of aluminium was discovered. In 1926, aluminium was the first commercially used adjuvant to improve the immunogenicity of diphtheria vaccine [3]. For several decades thereafter, oil-in-water emulsions were the only compounds added to the list of adjuvants (table 1). In recent years, many potential new classes of adjuvants have been discovered, most notably toll-like receptor (TLR) agonists, and these have undergone testing for efficacy and safety in humans.
| Table 1: List of adjuvants. | ||||||
| Adjuvant | Class | Component | Company | Mechanism of action | Vaccines | References |
| Licensed adjuvants | ||||||
| Alum | Aluminium mineral salts | - Potassium aluminium sulphate - Often wrongly classified | - Necrosis causing urate crystals - Induction of inflammasome - IL-1 secretion | Multiple | [162–166] | |
| MF59 | Oil-in-water emulsion | - Squalene - Polysorbate 80 - Sorbitan trileate | Novartis | - Slow release of antigen - Nonspecific immune stimulation | Fluad (seasonal influenza) Focetria (pandemic influenza) Aflunov (prepandemic influenza) | [98, 103, 167–171] |
| Virosomes | Liposomes | - Lipids - Haemagglutinin | Berna Biotech | - Slow release of antigen | Infexal (seasonal influenza) Epaxal (hepatitis A) | [172–174] |
| AS04 | Alum-absorbed TLR4 agonist | - Aluminium hydroxide - MPL | Glaxo SmithKline | - induction of Th1 response | Fendrix (hepatitis B) Cervarix (human papilloma virus) | [175–178] |
| AS03 | Oil-in-water emulsion | - Squalene - Tween 80 - α-Tocopherol | Glaxo SmithKline | - Slow release of antigen - Nonspecific immune stimulation | Pandremix (pandemic influenza) Prepandrix (prepandemic influenza) | [179, 180] |
| Unlicensed adjuvants | ||||||
| Pam3Cys | TLR2 agonist | - Lipopeptide | - | - Induction of Th1 response | - | [181] |
| Poly I:C | TLR3 agonist | - ds-RNA analogues | Hemispherx Biopharma | - Induction of Th1 response | - | [182, 183] |
| Flagellin | TLR5 agonist | - Bacterial protein linked to antigen | Moffitt Cancer Center | - Induction of Th1 response | - | [184–186] |
| Imidazoquinolines | TLR7 and TLR8 agonist | - Small molecules | Cancer Research Technology | - Induction of Th1 response - Direct activation of B cells | - | [187, 188] |
| CpG | TLR9 agonist | - CpG oligonucleotides ±alum/emulsion | Chiron | - Induction of Th1 response - Direct activation of B cells | - | [189, 190] |
| AS01 | Combination | - Liposome - MPL - Saponin | Glaxo SmithKline | - Slow release of antigen - Induction of Th1 response | - | [191] |
| AS02 | Combination | - Oil-in-water emulsion - MPL - Saponin | Glaxo SmithKline | - Slow release of antigen - Induction of Th1 response | - | [192] |
| AF03 | Oil-in-water emulsion | - Squalene - Montane 80 - Eumulgin B1PH | Sanofi Pasteur | - Slow release of antigen - Nonspecific immune induction | - | [193] |
| CAF01 | Combination | - Liposome - DDA - TDB | Statens Serum Institute | - Slow release of antigen | - | [194] |
| IC31 | Combination | - Oligonucleotide - Cationic peptides | Novartis | - Induction of Th1 response | - | [195] |
| Iscomatrix | Combination | -Saponin - Cholesterol - Dipalmitoyl-phosphatidylcholine | CSL Behring | - Slow release of antigen | - | [196–198] |
| CAF = cationic adjuvant formulation; DDA = dimethyldioctadecylammonium; IL = interleukin; MPL = monophosphoryl lipid; poly I:C = polyinosinic:polycytidylic acid; RNA = ribonucleic acid; TDB = trehalose-6,6-dibehenate Th = T helper cell; TLR = toll-like receptor | ||||||
In this review article, we will address vaccine adjuvant themes using influenza as a model of acute viral infection. Influenza is a common acute viral infection and the currently available vaccines strive to generate neutralising antibodies. Influenza infection is associated with increased morbidity and mortality in elderly, obese and immunosuppressed patients (e.g., after transplantation or during chemotherapy) and newborns, and during pregnancy [4–7]. Influenza virus replication is controlled by a complex interaction of the innate and adaptive immune response. Neutralising antibodies prevent infection and CD8 cytotoxic T-cell responses (CTL) help to clear the infection [8–14]. Annual vaccination against influenza is recommended (http://www.who.int/influenza/vaccines/virus/en/).
The purpose of vaccination is to prime a naïve immune system and establish a pathogen-specific protective immunological memory. Essentially, vaccination induces two important immune phenotypes: (i.) a virus-specific B-cell response with production of neutralising antibodies [15] with the help of a T helper cell type 2 (Th2) memory and/or (ii.) a virus-specific CTL and T helper cell type 1 (Th1) memory. Both are capable of providing protective memory, but one phenotype can predominate at the expense of the other. The relative importance for protection is likely dependent upon the particular pathogen. The type of vaccine-induced immune response is highly dependent on the antigen and adjuvant composition. Important factors to consider in vaccine design are: pathogen transmissibility, replication dynamics, tropism of the pathogen and the natural immune response. Currently, most commercially available vaccines target the induction of neutralising antibodies.
Antigen-presenting cells (APCs: B cells, dendritic cells [DCs] or macrophages) play a central role in inducing vaccine responses. Antigens such as viral structural proteins are processed by the proteosome complex (CD8+ CTL) or lysosomal enzymes (CD4+ T helper cells), cleaved into peptides, and then presented in a human leucocyte antigen- (HLA-) dependent manner via the major histocompatibility complex class I (MHC-I) and MHC-II to CD8 and CD4 T cells, respectively [16] (signal 1). Simultaneously, APCs are activated via pattern recognition receptors (PRRs), such as TLRs [17] and cytokines such as interferons (IFNs). PRRs which sense ribonucleic acid (RNA) contained in inactivated whole influenza vaccines are endosomal TLR3 and TLR7/8, as well as cytoplasmic retinoic acid-inducible gene-1- (RIG-I-) like receptors [18–20]. However, in subunit vaccines, only protein is present and stimulation of TLRs may need specific adjuvants. Activation of PRRs results in the up-regulation of costimulatory ligands on the cell surface of APCs. Costimulatory ligands on APCs modulate the activation and downstream signalling cascade of the T cell (signal 2). Important T-cell activating costimulatory ligands are CD80, CD86 and CD40; important T-cell inactivating costimulatory ligands are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death ligand (PD-L)1/2 [21–23].
Successful induction of a CTL response involves the initial stimulation of DCs by influenza virus. DCs then preferentially secrete IFN-α [24] and interleukin-12 (IL-12), and up-regulate MHC-I and MHC-II [25]. Thus unprimed naïve T helper cell (Th) differentiation is skewed towards a Th1 direction, with IFN-γ, IL-12 and IL-6 production further amplifying the response [26]. Th1 cells, which produce a significant amount of IFN-γ, are essential for the induction of an optimal CTL killing response including release of perforin and granzyme from cytotoxic granules. In addition, IFN-γ generated from Th1 CD4+ T cells can affect isotype switching in B cells [27]. IFN-γ can promote the induction of HA-specific neutralising antibodies and may in fact help with broadening responses to heterologous influenza viruses [28, 29]. This effect could be due to broadening the spectra of available epitopes after proteasome cleavage [30].
With respect to B-cell responses, adequate priming of a Th2 immune response (IL-4, IL-5, IL-9 and IL-13) is important [31–33]. The Th1 response is known to suppress Th2 responses [34, 35] – via suppressor of cytokine signal (SOCS) proteins [36] – culminating in lower antibody titres [24]. In addition, a new class of T cells, T follicular helper cells (TFH), predominantly producing IL-21, also have a key role in B-cell maturation [37, 38]. For example, patients infected with human immunodeficiency virus (HIV) who had been successfully vaccinated showed significantly increased IL-21 serum levels and frequency and mean fluorescence intensity of IL-21R-expressing B cells, which correlated with H1N1 antibody titres [39, 40]. Other important growth factors for B cells, such as B-cell activating factor (BAFF) or a proliferation-inducing ligand (APRIL), are released by activated monocytes, DCs and macrophages in the lymph nodes [41]. Figure 1 highlights the most important interaction steps between monocytes/DCs, T cells and B cells during vaccination. The interaction between B cells and Th2cells leads to B-cell activation. The priming steps of a naïve B cell precede a maturation phase characterised by immunoglobulin class switching and affinity maturation. In turn this triggers the production of more specialised antibodies such as Immunoglobulin G (IgG) subclasses, IgA and IgE [37]. Seroconversion, which is commonly defined as a 4-fold antibody titre increase from baseline, serves as an important surrogate marker suggesting successful vaccination.
Changing population demographics, in particular the increasing number of immunosuppressed hosts and elderly persons, significantly impacts vaccine-related outcomes. Overcoming the immunosuppressive effects of the aging immune system will become a key challenge for the vaccines of the 21st century [42–45]. Immunosenescence affects DCs, and T and B cells at various levels [46]. Nonadjuvanted influenza vaccines show low effectiveness, with seroconversion rates of only 30% in healthy adults above 65 years old [47–51], which is comparable to the weak response seen in transplant recipients taking immunosuppressive drugs [52]. Nonetheless, vaccination remains highly recommended for these groups in whom the prevalence of influenza-associated morbidity and mortality is high [53].

Figure 1
Key steps in B-cell activation and B-cell interaction with T-helper cells and monocytes, and monocyte derived macrophages and dendritic cells.
Adjuvants offer a strategy to improve vaccine outcomes via natural, synthetic or endogenous molecules that function to modulate and/or increase the immune effect. This may result in an enhanced, accelerated and prolonged pathogen-specific immune response. The immune response can be preferentially skewed in a certain direction, such as with respect to immunoglobulin classes or the induction of cytotoxic or T helper cell responses (Th1 versus Th2). Thereby, the immunogenicity of particular antigens can be improved and the nature of the immune response modified, whilst the amount of required antigen is also reduced. Adjuvants thus have the potential to boost immune responses in elderly and immunocompromised hosts. Table 1 provides a list of adjuvants and their proposed mechanisms of action.
As antigens vary in immunogenic and biological characteristics, a particular adjuvant has to be optimised for a specific antigen. Adjuvants should be added on the basis of the type of immune response desired; for influenza vaccines, for example, cytotoxic and Th1 responses against haemagglutinin might be of less clinical importance to prevent infection. More important is a robust Th2response to induce haemagglutinin-specific neutralising antibodies.
A large amount of data regarding adjuvanted vaccines concerns their safety and tolerability. Prevention of infection compared with nonadjuvanted vaccines is rarely examined and often compared with historical controls or literature. An important debate concerns the best immune biomarker indicative of protection and prevention of infection. Most research has considered antibody titres, which certainly are easy to measure, as a surrogate marker for protection. However, T-cell responses, especially Th2 cytokine release; and measuring B-cell activation directly might correlate better with protection [54–57]. An increase in antibody levels does not necessarily correlate with protection; nevertheless, as mentioned, seroconversion is the most commonly used surrogate marker of protection. It has been shown that seroprotection increases to a greater extent in patients with low or zero baseline titres, compared with patients with high baseline titres, and that the relative increase in vaccine recipients with prevaccination titres >1:40 is significantly lower [58, 59]. This could be due to a higher “activation threshold”, which needs to be reached.
Differential cytokine responses are observed following administration of different adjuvants. In general, when used with pure proteins, oil-in-water (O/W) emulsions up-regulate Th2 responses. Addition of TLR agonists to the emulsions skews the response to Th1. Much of this work has been done with TLR4 agonists, including monophosphoryl lipid A (MPL1), and glucopyranosyl lipid A (GLA) oil-in-water formulations [60, 61].
The adjuvant potential of aluminium salts was discovered in 1926 [62], but their mechanism of action is certainly complex and still not fully understood. Aluminium salts exist in various forms with various chemical specifications, however, and aluminium salts are often described as “alum”. “True” alum should be reserved for hydrated potassium aluminium sulphate. The misleading term might explain the variability reported in the literature, as different aluminium salts induce distinct effects on the immune system. Aluminium is cytotoxic via the rupture of endolysosomes and the induced release of uric acid, which act as damage-associated molecular patterns (DAMP) [63]. This leads to an activation of the Nod-like receptor family protein-3 (NLRP3) inflammasome and caspase-1, and release of IL-1, IL-18 and IL-33 [64–67]. This promotes antigen uptake and presentation by human macrophages and their recruitment to sites of inflammation [64, 68]. In addition, HLA-DR, CD40 and CD86 are up-regulated on DCs [69] in a MyD88-dependent manner (MyD88 is a critical adaptor protein for most TLRs). Other studies have suggested that “alum” may not act through TLRs [70, 71]. The recruited DCs prime a naïve CD4+ T-cell response, in particular Th2 [64, 72, 73]. Th2 cytokines are crucial for the differentiation of B cells and the maturation processes leading to IgG1 production.
Although aluminium salts induce a favourable Th2 response, not many licensed influenza vaccines are adjuvanted in this way – and no licensed influenza vaccine is available (table 1). In the last decade, new data on the mechanism of action have emerged, but most studies on vaccine efficacy have been performed in mice. The potential to reduce antigen amounts has been recently summarised in a meta-analysis [74]. Table 2 summarises the published studies with aluminium salts in influenza vaccine during the last 10 years.
| Table 2: Studies of alum-adjuvanted influenza vaccines in humans. | |||||
| Type of influenza | Study cohort | Treatment groups | Major outcomes | Type of aluminium salt | Reference |
| Whole virion inactivated Influenza A/California/7/2009 pandemic H1N1 | Double-blind randomised Phase I (n = 50, 18–50 y) Phase II/III (n = 330, >3 y) | Phase I: 10 μg vs 15 μg, i.m. injection Phase II/III: 10 μg vs 15 μg Three age groups: 3–17 y, 18–49 y, >50 y | Phase I: In 20% mild side effects during 42-day follow-up. No adverse effects Phase II: same. Seroconversion in 18–49 y: 10 μg 90.4%, 15 μg 90.4% in >50 y: 10 μg 87%, 15 μg 76% | Aluminium hydroxide | Kulkarni et al. [199] |
| Whole virus inactivated Influenza A/Vietnam/1203/2004 H5N1 | Randomised dose-escalation Phase I and II (n = 275) | 18–45 y Adjuvanted: 3.75, 7.5, 15, and 30 μg Nonadjuvanted: 7.5, 15 μg. | Higher seroconversion in nonadjuvanted group Higher seroconversion in lower antigen group (7.5 vs 15 μg); Phase I; Phase II | “Alum” adjuvant | Ehrlich et al. [162] |
| Whole viron inactivated Influenza A/Vietnam/1194/2004–A/PR/8/34 H5N1 | Randomised placebo-controlled double-blind study (n = 120) | 18–60 y, i.m. injected 1.25, 2.5, 5, or 10 μg in two doses vs placebo | Seroconversion: 1.25 μg: 23.5%; 2.5 μg: 18.8% 5 μg: 78.6%; 10 μg: 80% 2nd booster dose increased seroconversion. | “Alum” adjuvant | Lin et al. [200] |
| Inactivated-split Influenza A/Vietnam/1194/2004 | Prospective, randomised, observational, multicentre trial, n = 400 in each trial | Phase I, n = 400, 18–45 y Adjuvanted: 7.5, 15 μg Nonadjuvanted:7.5, 15 μg Phase II, n = 400, 18–64 y Adjuvanted: 30, 45 μg two doses | Phase I, d21, seroconversion: 7.5 µg: 21% 7.5 μg + Al: 14% 15 µg: 28% 15 μg + Al: 17% Phase II, d21, seroconversion: 30 μg + Al: 31% 45 μg + Al: 30% double seroconversion after 2nd dosage | Aluminium phosphate + thiomersal | Nolan et al. [201] |
| Inactivated Influenza A/Hong Kong/1073/99 H9N2 | Randomised dose-comparison study, n = 353 | 1.7, 5, 15, 45 μg i.m. 5, 15 μg i.d. Whole virus vs virasomal >18 y, two doses | Seroprotection, d21 and d42, <40 y Dose-dependent increase Increase with alum Virosomal unit and intradermal injection minimal increased response | Aluminium phosphate | Nicholson et al. [202] |
| Inactivated-split Influenza A/California/7/2009, H1N1 | Randomised double-blind, placebo-controlled study, n = 2,200 | Age groups: 3–11, 12–17, 18–60, >61 y 7.5, 15, 30 μg 2nd dose vs placebo | 18–60 y: 15 μg 97.1%; 30 μg 92.6%; Pl: 10.7% >61 y: 15 μg 79.1%; 30 μg 84.1% | “Alum” adjuvant | Zhu et al. [203] |
| Whole viron, inactivated Influenza A/Vietnam/1194/2004 | Randomised study | Phase II/III trial Adult, n = 337; 20–59 y; i.m. vs s.c. 15 μg Children, n = 374; 3 m – 19 y; 3, 7.5 μg | Seroconversion Adult: i.m. 82.8%, s.c. 71.4% Children: no clear information | “Alum” adjuvant | Nakayama et al. [204] |
| Whole viron inactivated Influenza A/Vietnam/1194/2004, H5N1 and A/PR(8/34 H1N1 | Randomised study (n = 120) | Phase I, healthy Japanese men 20–40 y s.c.: 1.7, 5, 15 μg i.m.: 1.7, 5, 15 μg 2nd dose | Seroconversion s.c. 15 μg 42.1%, vs i.m. 15 μg 65% Seroconversion after 2nd dose s.c. 15 μg. 68.4% vs 75% | Aluminium hydroxide | Ikeno et al. [205] |
MF59 and AS03 are squalene-based oil-in-water emulsions. MF59 is composed of 0.5% Tween-80 as a water-soluble surfactant, 0.5% Span85 as an oil-soluble surfactant, 4.3% squalene oil, and water. The emulsion droplet size is approximately 130 nm. Experiments with nanoparticle adjuvants suggest that the particle size may be a key factor for the activity: microspheres with diameters of <10 nm seem to activate APCs, whereas particles with diameters of 30–100 nm show a slow release of antigen, known as “depot effect” [75]. MF59 squalenes are internalised by DCs [76] and act independently of the NLRP3 inflammasome [77], but are dependent on MyD88, which might have an adaptor protein function for tumour necrosis factor receptor superfamily member 13B (also known as TACI) or the IL-1 receptor [78]. Studies have shown that pretreatment with MF59 prior to vaccine application resulted in a maintained “immunocompetent environment” within the muscle [79]. Interestingly, this effect does not occur if MF59 is injected later. The number of leucocytes isolated in the muscle increased seven-fold within 2 days, and a slow decay was observed thereafter. MF59 function is dependent on CC chemokine receptor type 2 (CCR2), the receptor for monocyte chemoattractant proteins 1–5 (MCP-1–MCP-5), and on intracellular adhesion molecule-1 (ICAM-1) [80, 81]. MF59 additionally induces monocyte-to-DC differentiation [82]. Of note, the individual components of MF59 are not as effective as the entire formulation [83].
AS03, containing an α-tocopherol (a form of vitamin E), modulates cytokine release and cell recruitment to regional lymph nodes, leading to enhanced antibody responses [84]. Slow release of antigen compounds seems not to be a primary mechanism of action. Squalene is rapidly degraded in tissues and studies with viral glycoproteins showed that antigens did not bind to the emulsion droplets, and that binding was not necessary to achieve a potent adjuvant effect [79, 85]. MF59 and AS03 showed higher immunostimulatory potential than aluminium salts in several clinical studies [74].
The immunogenicity of seasonal and pandemic MF59-adjuvanted influenza viruses has been widely evaluated in open and controlled studies involving a broad range of different patient groups – inclusive of elderly and immunosuppressed populations. To summarise, these studies showed that MF59-adjuvanted influenza vaccines induce a more potent immune response with higher rates of seroconversion compared with nonadjuvanted vaccines [86–91]. Local reactions – mainly mild reactions at the site of injection sites – were increased.
In addition, O/W emulsions carry the potential to generate cross-reactive antibody responses. MF59 increases the diversity of the epitope repertoire against haemagglutinin-1 [92]. Given the frequency with which antigenic drift occurs in influenza viruses, this seems to be the most important immune advantage arising from the use of MF59. In addition, the potency of vaccines against weakly antigenic pandemic vaccine strains could be significantly increased [93–95]. Reductions in the amount of antigen (lower than 15 μg of haemagglutinin) needed to generate a sufficient response is critical when the capacity to generate vaccines is limited, in particular during a pandemic [96–104]. Although the immunogenicity of MF59-adjuvanted vaccines has been demonstrated in terms of antibody responses, the increased protective effect has only been shown in two studies [105, 106].
The potency of AS03-adjuvanted influenza vaccines to increase antibody levels was shown for the avian A/H5N1 influenza virus [107–109] and the A/H1N1 pandemic virus [110–114]. Data from clinical trials involving the elderly, children of different ages and immunocompromised patients have shown enhanced antibody mediated immune responses to haemagglutinin. Again, only one study demonstrated the impact on infection prevention [115].
Protein interactions that modulate the immune response and have been identified in genome-wide association assays and gene hub analysis can also be used to design new adjuvants (see below). On the basis of these interactions, peptide libraries can be designed to block or stimulate the interaction. These peptides may be used as adjuvants by themselves [116, 117] or be used as lead compounds for in-silico screening to design small molecules.
Small molecule adjuvants (SMAs) include both natural products such as muramyl dipeptide, byrostatin-1, monophosporyl Lipid A (MPL), QS-21 and QuilA (saponin based), and PAM2CSK4 [118–123]; and fully-synthetic drug-like molecules such as the group of imidazoquinolines and bestatine [124–126]. Best described is the so-called family of imidazoquinolines [127, 128), including imiquimod, resiquimod and gardiquimod [127–129], which act as TLR7/8 agonists. Other examples of SMAs are synthetic CpG oligodeoxynucleotides which act as TLR9 agonists [130]. For CpG oligonucleotides, structure-activity relationship data to design similar compounds [131] has been used; in addition, quantitative structure-activity relationship (QSAR) technology with optimised activity, selectivity and toxicity was used to generate novel variations of compounds such as, CPG-1826 [132] and CPG-7909 [133]. Polyinosinic:polycytidylic acid (or poly I:C) is another analogous immunostimulant, and acts as a TLR3 agonist [134].
| Table 3: Summary of genome-wide association studies in vaccine cohorts. | ||||||
| Study | Vaccine | Sample size | Region | Mapped genes | SNP | p-value |
| Pajewski NM et al. 2012 | Anthrax | 726, European ancestry | 18q21.2 | SRSF10P1 – MEX3C | rs7230711–C | 1 x 10–6 |
| 1p36.22 | SPSB1 | rs11121382–C | 4 x 10–6 | |||
| 5q31.1 | LOC100996485 | rs634308–G | 4 x 10–6 | |||
| 6p21.32 | MTCO3P1 – HLA-DQA2 | rs3104402–A | 6 x 10–6 | |||
| 9q33.1 | ASTN2 | rs6478282–A | 6 x 10–6 | |||
| 9p21.1 | RPS11P4 – TMEM215 | rs10758161–G | 8 x 10–6 | |||
| 13q14.3 | PCDH8 – OLFM4 | rs732949–C | 8 x 10–6 | |||
| 4q24 | TET2 – PPA2 | rs2647264–G | 9 x 10–6 | |||
| Kennedy RB et al 2012 | Smallpox | 512, European ancestry 199, African American | 18q21.2 | MEX3C | rs8096445–A | 9 x 10–9 (AA) |
| 5q11.2 | PDE4D | rs17444059–G | 2 x 10–8 (AA) | |||
| 2p22.3 | LINC00486 | rs6728021–G | 4 x 10–8 (AA) | |||
| 3q28 | PYDC2 – FGF12 | rs1516489–C | 8 x 10–8 (AA) | |||
| 9p21.1 | NDUFB6 | rs17290760–G | 1 x 10–7 (AA) | |||
| 1p36.12 | LINC00339 – CDC42 | rs2501276–A | 2 x 10–7 (AA) | |||
| 6q22.33 | C6orf58 – THEMIS | rs17299841–C | 2 x 10–7 (AA) | |||
| 8p23.1 | BLK | rs2255327–A | 3 x 10–7 (AA) | |||
| 5q34 | TENM2 | rs2973662–A | 5 x 10–7 (AA) | |||
| Ovsyannikova IG et al 2012 | Smallpox | 217, African American ancestry 580, European ancestry 217, Hispanic ancestry | 10p12.1 | MKX | rs10508727–? | 1 x 10–10 (AA) |
| 10q21.1 | SNRPEP8 – PCDH15 | rs12256830–? | 2 x 10–10 (Hispanic) | |||
| 8p12 | VENTXP5 – RPL6P22 | rs10503951–? | 3 x 10–9 (AA) | |||
| 10p12.1 | GPR158 – GPN3P1 | rs12775535–? | 4 x 10–9 (AA) | |||
| 8q24.13 | ZHX2 | rs10108684–? | 1 x 10–8 (AA) | |||
| 18p11.21 | SPIRE1 | rs9959145–? | 3 x 10–8 (AA) | |||
| 10p14 | PRKCQ | rs4748153–? | 3 x 10–8 (Hispanic) | |||
| 1q31.1 | RPS3AP9 – FAM5C | rs10489759–? | 8 x 10–8 (EA) | |||
| 6p21.2 | KIF6 | rs9380880–? | 1 x 10–7 (Hispanic) | |||
| 1q43 | GREM2 | rs10495471–? | 2 x 10–7 (AA) | |||
| Png E et al. 2011 | Hepatitis B | 1683, Indonesian ancestry | 6p21.32 | BTNL2 – HLA-DRA | rs3135363–? | 7 x 10–22 |
| 6p21.33 | C2; ZBTB12 | rs9267665–? | 1 x 10–17 | |||
| 6p21.32 | HLA-DPB1 | rs9277535–? | 3 x 10–12 | |||
| Fellay J et al. 2011 | HIV | 831, all male mixed ancestry | 6p10 | HLA B | rs4713462 | 1.9x10–6 |
| 6p10 | HLA B | rs4713460 | 2.4x10–6 | |||
| AA = African American; EA = European ancestry; HIV = human immunodeficiency virus; SNP = single nucleotide polymorphism | ||||||
During the last decade tremendous progress has been achieved in understanding the complex interaction between the various key components of the immune system. Complex data detailing messenger RNA (mRNA) and protein expression profiles using systems biology approaches has helped us to understand many key steps involved in immune activation [135–138]. The data-gathering techniques for modelling and simulation of immunological processes, and the required tools and techniques to question vaccine responses have been reviewed recently [139]. Application of these approaches to vaccinology at the genome-wide, transcriptome, and proteome levels may identify new pathways and molecular targets for immune modulation.
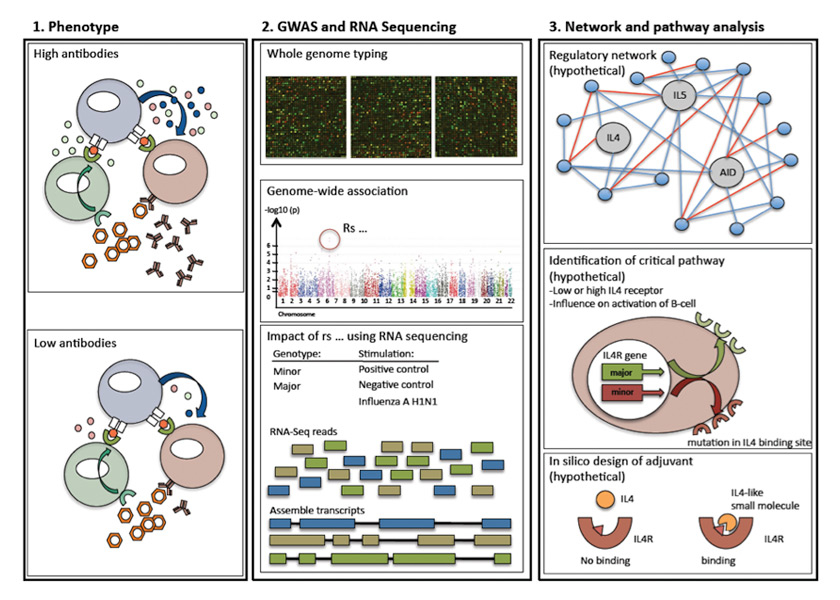
Figure 2
Possible work-flow to develop novel adjuvants. The following example is only used as an illustration and not based on “real” data – it should highlight critical steps, which could be used for different vaccines and pathways. First step: observation of distinguished phenotypes, such as antibody production different due to defective cytokine production. Second step: description of respective phenotype – identification of involved genes using a genome-wide association study. Next, RNA sequencing could be used to describe the pathophysiological impacts of the newly discovered SNP. Third step: generation of a genetic network to identify critical interaction points (so called gene hubs). In our “imaginary example” an important cytokine receptor would be affected – the interleukin 4 receptor. In-silico design of small molecules, which can modulate or compensate for the polymorphisms in the signalling cascade. In this example IL4 cannot bind sufficiently owing to a mutation within the IL4-receptor. A small molecule could be specifically designed to overcome this.
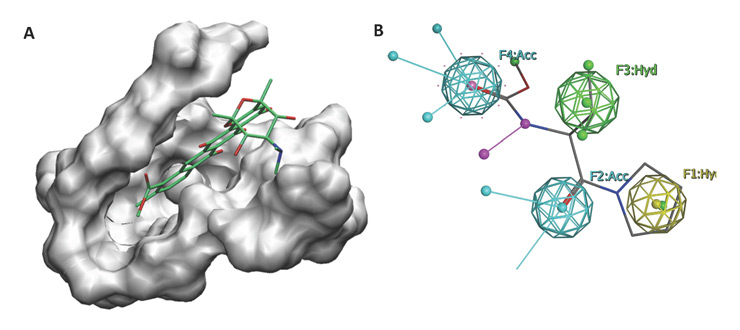
Figure 3
In-silico drug discovery strategies. (A) Virtual screening campaigns usually identify small molecules that fit within a particular pocket in a protein. (B) An illustration of a pharmacophore construction describing the distribution of important features for a drug that has three hydrophobic regions with two hydrogen bond acceptors. Based on these models virtual screens can be performed to focus only on a couple of interesting candidate compounds rather than screening a whole library.
Based on genome-wide association studies, several critical single nucleotide polymorphisms (SNPs) influencing vaccine-induced responses have been found. These genes reflect “gene-hubs”; critical interaction points in an inflammatory signalling cascade. SNPs with a high frequency are attractive targets for compensatory molecules specifically targeting the immune response of an individual (personalised vaccination). Figure 2 summarises such a screening approach and gives a hypothetical example using IL-4 as a key cytokine for B-cell activation.
Studies continue to suggest the promise of this approach. Umlauf and colleagues associated the measles-mumps-rubella (MMR) induced vaccine response with 307 common candidate SNPs from 12 antiviral genes / immune signalling cascade such as RIG-I, interferon-induced GTP-binding protein Mx1 (Mx1) 2’-5’-oligoadenylate synthetase 1 (OAS1), etc. Genetic variants within the DDX58/RIG-I and OAS1 gene were associated with measles-specific antibody variations. DDX58 and ADAR polymorphisms were associated with variations in both measles-specific IFN-γ and IL-2 secretion. After correction for false discovery rate, 15 single-SNP associations (11 SNPs in Caucasians and 4 SNPs in African-Americans) still remained significant at the q-value (minimal false discovery rate) <0.20 [140]. Larger studies have been performed using genome-wide association of SNPs (GWAS) with vaccine-induced immune responses against smallpox [141–143], anthrax [144], HIV [145], and hepatitis B virus [146]. Table 3 summarises the findings of all mentioned studies. One GWAS explored the association of SNPs with side effects from vaccines [147].
At the transcriptome level, several high-frequency sampling studies of vaccine responses to yellow fever [148] and influenza [12, 149] vaccination have identified transcriptome signatures of the unadjuvanted vaccine response. Application of this knowledge to adjuvant design has yet to be published.
One caveat to the “-omics” approach as applied to adjuvants is the need for better data at the site of the adjuvant effect (i.e., the site of injection and the lymph nodes), to better characterise in-situ molecular and cellular responses. Virtually all human studies have involved examining transcriptome and proteome profiles of peripheral blood. With current technology, muscle and lymph node sampling of inflammatory cells and parenchymal tissue is highly invasive and not an option for ethical reasons in human studies. Complicating matters is that murine models may differ in the fundamental biology of key immune responses. For example, there are significant differences in murine B-cell responses to some classes of TLR agonists [150].
A successful adjuvant should be specific and selective for a target receptor either in an agonistic or antagonistic fashion to modulate the immune response. This could significantly reduce any possible side effects and lead to a robust and controlled modulation of the immune response. These side effects signify a major problem in adjuvant development and are not always predictable, as in the likely association of narcolepsy with the AS03 adjuvanted influenza vaccine [151, 152]. Nevertheless, computer-modelling (or in-silico) techniques could be the new path of hope to develop novel adjuvants that are potent, selective and safe (Figures 3A and B).
For the last two decades, molecular modelling approaches have been used to develop new drug candidates (small molecules or short peptides) that fit within a binding site in a particular target (usually protein). The objective is to complement a binding pocket that would regulate the activity of the target in terms of shape, charge and other physiochemical properties. In order to reduce side effects and enhance the pharmacological properties, this regulator has to be potent for and selective toward the designated pocket, and to be suitable for further modifications and optimisations. In this regard, for an adjuvant to act as a modulator of the immune response, it must be specific for one of the receptors involved in this process and regulate its activity. Depending on the availability of a receptor structure or a number of predetermined potent regulators, one of many modelling strategies can be pursued.
If the target three-dimensional structure is available and the binding site that would regulate the activity of the target is known, one could use receptor-based virtual screening [153]. In this technique, a set of small molecules is docked to the surface of the identified pocket (Figure 3A). The ones that best fit within the pocket are then retained and their binding affinities are further calculated and used to rank them for experimental testing [154–156]. Only a few success stories reported in the literature followed this path to discover immune-related adjuvants. This small number of computer-developed adjuvants could be attributed to the limited number of protein structures involved in the immune response process. Among the few examples of success is the work done by Goel et. al. to modulate the chemokine receptor-4 (CCR4). They designed a set of small molecules specific for CCR4, a protein that is expressed on Th2 cells [157–161].
When only a set of potent regulators is available, ligand-based approaches are used. This may involve one of two strategies: pharmacophore construction or quantitative structure activity relationship (QSAR) modelling. In the former, the common chemical and physical features that exist in the training set are used to build a hypothetical structure that distributes these features in space (Figure 3B). These features include hydrogen-bond donors and acceptors, hydrophobic regions, aromatic rings and excluded volumes. QSAR usually uses two-dimensional properties of the ligands to construct a mathematical model that would predict the activity of a future ligand. These properties include, but are not limited to, the molecular weight, the hydrophobicity of the ligand, the number of hydrogen bonds and many other properties.
All of the above-mentioned methodologies hold the promise to develop novel classes of adjuvants. Combining information from “vaccine-ome” studies identifying crucial junctures in immune activation, together with powerful in-silico modelling tools will certainly become the future for adjuvant design.
Infectious diseases are a continuous threat. Without doubt the development of vaccination has saved millions of lives. However, immunosuppressed and elderly people remain vulnerable to vaccine preventable disease as a consequence of reduced vaccine responses. Despite profound advances in the understanding of the immunological processes involved in vaccine responses, we have failed to improve further vaccine responses. In the last 150 years only a few adjuvants have been discovered and applied clinically. However, the future is bright. New small molecules may help to significantly increase vaccine responses. Emerging technology such as in-silico modelling of adjuvant receptor interactions using super-computers; combined with RNA sequencing and microarray SNP discovery, uncovering critical steps in vaccine responses, will bring about the design of novel classes of adjuvants.
1 Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent). 2005;18(1):21–5. PubMed PMID: 16200144. Pubmed Central PMCID: 1200696. Epub 2005/10/04.
2 von Behring E, Kitasato S. [The mechanism of diphtheria immunity and tetanus immunity in animals. 1890]. Mol Immunol. 1991;28(12):1317, 9–20. PubMed PMID: 1749380. Epub 1991/12/01. Ueber das Zustandekommen der Diphtherie-Immunitat und der Tetanus-Immunitat bei Thieren. German.
3 Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–93. PubMed PMID: 19247370. Pubmed Central PMCID: 3147301. Epub 2009/02/28.
4 Medina RA, Garcia-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol. 2011;9(8):590–603. PubMed PMID: 21747392. Epub 2011/07/13.
5 Vilchez RA, McCurry K, Dauber J, Lacono A, Griffith B, Fung J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2(3):287–91. PubMed PMID: 12096793. Epub 2002/07/05.
6 Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348(9):867–8. PubMed PMID: 12606750. Epub 2003/02/28.
7 Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. PubMed PMID: 23974637.
8 Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, et al. Influenza vaccine immunology. Immunol Rev. 2011;239(1):167–77. PubMed PMID: 21198671. Epub 2011/01/05.
9 Garcia-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162(1–2):12–8. PubMed PMID: 22027189. Epub 2011/10/27.
10 Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79–87. PubMed PMID: 19139171. Pubmed Central PMCID: 2626661. Epub 2009/01/14.
11 Kreijtz JH, Fouchier RA, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011;162(1–2):19–30. PubMed PMID: 21963677. Epub 2011/10/04.
12 Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12(8):786–95. PubMed PMID: 21743478. Pubmed Central PMCID: 3140559. Epub 2011/07/12.
13 Chiu C, Wrammert J, Li GM, McCausland M, Wilson PC, Ahmed R. Cross-reactive humoral responses to influenza and their implications for a universal vaccine. Annals of the New York Academy of Sciences. 2013;1283:13–21. PubMed PMID: 23405860.
14 Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–42. PubMed PMID: 23330954.
15 Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–70. PubMed PMID: 9143688. Epub 1997/01/01.
16 Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–36. PubMed PMID: 22076556. Epub 2011/11/15.
17 Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010 Jan 15;327(5963):291–5. PubMed PMID: 20075244. Pubmed Central PMCID: 3645875. Epub 2010/01/16.
18 Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1(4):226–32. PubMed PMID: 22440781. Epub 2012/03/24.
19 Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7–mediated recognition of single-stranded RNA. Science. 2004 5;303(5663):1529–31. PubMed PMID: 14976261. Epub 2004/02/21.
20 Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science. 2006;314(5801):997–1001. PubMed PMID: 17038589. Epub 2006/10/14.
21 Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. PubMed PMID: 23470321. Epub 2013/03/09.
22 Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108(4):431–9. PubMed PMID: 12667204. Pubmed Central PMCID: 1782924. Epub 2003/04/02.
23 Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175(9):5827–38. PubMed PMID: 16237075. Epub 2005/10/21.
24 Toporovski R, Morrow MP, Weiner DB. Interferons as potential adjuvants in prophylactic vaccines. Expert Opin Biol Ther. 2010;10(10):1489–500. PubMed PMID: 20836750. Epub 2010/09/15.
25 Halloran PF, Urmson J, Van der Meide PH, Autenried P. Regulation of MHC expression in vivo. II. IFN-alpha/beta inducers and recombinant IFN-alpha modulate MHC antigen expression in mouse tissues. J Immunol. 1989;142(12):4241–7. PubMed PMID: 2498428. Epub 1989/06/15.
26 Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nature Reviews Immunology. 2012:doi:10.1038/nri3152.
27 Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20(2):97–113. PubMed PMID: 19268625.
28 Clegg CH, Roque R, Van Hoeven N, Perrone L, Baldwin SL, Rininger JA, et al. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17585–90. PubMed PMID: 23045649. Pubmed Central PMCID: 3491477.
29 Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25(19):3871–8. PubMed PMID: 17337102.
30 Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39(3–4):147–64. PubMed PMID: 12200047.
31 Mari N, Hercor M, Denanglaire S, Leo O, Andris F. The capacity of Th2 lymphocytes to deliver B-cell help requires expression of the transcription factor STAT3. Eur J Immunol. 2013. PubMed PMID: 23504518. Epub 2013/03/19.
32 Kamburova EG, Koenen HJ, Boon L, Hilbrands LB, Joosten I. In vitro effects of rituximab on the proliferation, activation and differentiation of human B cells. Am J Transplant. 2012;12(2):341–50. PubMed PMID: 22070501. Epub 2011/11/11.
33 Moschovakis GL, Bubke A, Dittrich-Breiholz O, Braun A, Prinz I, Kremmer E, et al. Deficient CCR7 signaling promotes TH2 polarization and B-cell activation in vivo. Eur J Immunol. 2012;42(1):48–57. PubMed PMID: 21969271. Epub 2011/10/05.
34 Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006 Sep;6(9):644–58. PubMed PMID: 16932750. Epub 2006/08/26.
35 Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat Rev Immunol. 2005;5(2):125–35. PubMed PMID: 15688040. Epub 2005/02/03.
36 Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–65. PubMed PMID: 17525754. Epub 2007/05/26.
37 McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012;12(1):24–34. PubMed PMID: 22158414. Epub 2011/12/14.
38 King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9(11):757–66. PubMed PMID: 19855402. Epub 2009/10/27.
39 Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012 Aug 2;120(5):985–93. PubMed PMID: 22692510. Pubmed Central PMCID: 3412336. Epub 2012/06/14.
40 Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol. 2011;186(11):6173–81. PubMed PMID: 21531891. Pubmed Central PMCID: 3170914.
41 Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2(7):465–75. PubMed PMID: 12094221. Epub 2002/07/03.
42 Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–33. PubMed PMID: 19541535. Epub 2009/06/23.
43 Rappuoli R, Dormitzer PR. Influenza: options to improve pandemic preparation. Science. 2012 Jun 22;336(6088):1531–3. PubMed PMID: 22723412. Epub 2012/06/23.
44 Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11(12):865–72. PubMed PMID: 22051890. Epub 2011/11/05.
45 Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–29. PubMed PMID: 19768417. Epub 2009/09/22.
46 Liu WM, van der Zeijst BA, Boog CJ, Soethout EC. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7 Suppl:94–8. PubMed PMID: 21301210. Epub 2011/02/09.
47 Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206(8):1250–9. PubMed PMID: 22904335. Epub 2012/08/21.
48 Nicoll A, Sprenger M. Low effectiveness undermines promotion of seasonal influenza vaccine. Lancet Infect Dis. 2013;13(1):7–9. PubMed PMID: 23257219. Epub 2012/12/22.
49 Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. PubMed PMID: 16213065. Epub 2005/10/11.
50 Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165–74. PubMed PMID: 16198765. Epub 2005/10/04.
51 Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–81. PubMed PMID: 17914038. Epub 2007/10/05.
52 Baluch A, Humar A, Eurich D, Egli A, Liacini A, Hoschler K, et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13(4):1026–33. PubMed PMID: 23406320. Epub 2013/02/15.
53 Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–9. PubMed PMID: 19822627. Epub 2009/10/14.
54 Parish CR, Liew FY. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972;135(2):298–311. PubMed PMID: 5060292. Pubmed Central PMCID: 2180527. Epub 1972/02/01.
55 Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286(5447):2159–62. PubMed PMID: 10591648. Epub 1999/12/11.
56 Serre K, Mohr E, Benezech C, Bird R, Khan M, Caamano JH, et al. Selective effects of NF-kappaB1 deficiency in CD4(+) T cells on Th2 and TFh induction by alum-precipitated protein vaccines. Eur J Immunol. 2011;41(6):1573–82. PubMed PMID: 21469117. Epub 2011/04/07.
57 Harris N, Gause WC. To B or not to B: B cells and the Th2–type immune response to helminths. Trends Immunol. 2011;32(2):80–8. PubMed PMID: 21159556. Pubmed Central PMCID: 3076625. Epub 2010/12/17.
58 Nauta JJ, Beyer WE, Osterhaus AD. On the relationship between mean antibody level, seroprotection and clinical protection from influenza. Biologicals. 2009;37(4):216–21. PubMed PMID: 19268607. Epub 2009/03/10.
59 Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. PubMed PMID: 20210985. Pubmed Central PMCID: 2851702. Epub 2010/03/10.
60 Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6(1):e16333. PubMed PMID: 21298114. Pubmed Central PMCID: 3027669. Epub 2011/02/08.
61 Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, et al. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm Res. 2009;26(6):1477–85. PubMed PMID: 19255727. Epub 2009/03/04.
62 Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes. XVII–XXIV. J Pathol Bacteriol. 1926;29:31–40.
63 Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29(5):807–18. PubMed PMID: 18993083. Pubmed Central PMCID: 2642965. Epub 2008/11/11.
64 Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–82. PubMed PMID: 18362170. Pubmed Central PMCID: 2292225. Epub 2008/03/26.
65 Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–6. PubMed PMID: 18496530. Epub 2008/05/23.
66 Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178(8):5271–6. PubMed PMID: 17404311. Epub 2007/04/04.
67 Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. PubMed PMID: 18566365. Pubmed Central PMCID: 2587213. Epub 2008/06/21.
68 Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61(1):143–51. PubMed PMID: 3876178. Pubmed Central PMCID: 1577243. Epub 1985/07/01.
69 Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69(2):1151–9. PubMed PMID: 11160013. Pubmed Central PMCID: 97997. Epub 2001/02/13.
70 Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25(23):4575–85. PubMed PMID: 17485153. Epub 2007/05/09.
71 Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2(10):947–50. PubMed PMID: 11547333. Epub 2001/09/08.
72 Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362(6417):245–8. PubMed PMID: 8384701. Epub 1993/03/18.
73 Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4–deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26(9):2062–6. PubMed PMID: 8814247. Epub 1996/09/01.
74 Manzoli L, De Vito C, Salanti G, D'Addario M, Villari P, Ioannidis JP. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One. 2011;6(9):e24384. PubMed PMID: 21915319. Pubmed Central PMCID: 3167852. Epub 2011/09/15.
75 Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–96. PubMed PMID: 20948547. Epub 2010/10/16.
76 Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186(1):18–27. PubMed PMID: 9637761. Epub 1998/06/25.
77 Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011;108(27):11169–74. PubMed PMID: 21690334. Pubmed Central PMCID: 3131326. Epub 2011/06/22.
78 He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–45. PubMed PMID: 20676093. Pubmed Central PMCID: 3047500. Epub 2010/08/03.
79 Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–96. PubMed PMID: 7551221. Epub 1995/01/01.
80 Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, et al. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol. 2001;31(10):2910–8. PubMed PMID: 11592066. Epub 2001/10/10.
81 Hui G, Hashimoto C. The requirement of CD80, CD86, and ICAM-1 on the ability of adjuvant formulations to potentiate antibody responses to a Plasmodium falciparum blood-stage vaccine. Vaccine. 2007;25(51):8549–56. PubMed PMID: 18006124. Pubmed Central PMCID: 2211737. Epub 2007/11/17.
82 O'Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 – an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–8. PubMed PMID: 22682289. Epub 2012/06/12.
83 Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105(30):10501–6. PubMed PMID: 18650390. Pubmed Central PMCID: 2483233. Epub 2008/07/25.
84 Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29(13):2461–73. PubMed PMID: 21256188. Epub 2011/01/25.
85 Dupuis M, McDonald DM, Ott G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine. 1999;18(5–6):434–9. PubMed PMID: 10519932. Epub 1999/10/16.
86 Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R. MF59–adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18–60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine. 2007;25(20):3955–61. PubMed PMID: 17383057. Epub 2007/03/27.
87 Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59–adjuvanted vaccine. Vaccine. 2001;19(17–19):2673–80. PubMed PMID: 11257408. Epub 2001/03/21.
88 Gasparini R, Pozzi T, Montomoli E, Fragapane E, Senatore F, Minutello M, et al. Increased immunogenicity of the MF59–adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol. 2001;17(2):135–40. PubMed PMID: 11599686. Epub 2001/10/16.
89 Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003;21(11–12):1268–74. PubMed PMID: 12559808. Epub 2003/02/01.
90 Banzhoff A, Nacci P, Podda A. A new MF59–adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003;49(3):177–84. PubMed PMID: 12679609. Epub 2003/04/08.
91 Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O'Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009;28(7):563–71. PubMed PMID: 19561422. Epub 2009/06/30.
92 Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3(85):85ra48. PubMed PMID: 21632986. Pubmed Central PMCID: 3501657. Epub 2011/06/03.
93 Beran J, Abdel-Messih IA, Raupachova J, Hobzova L, Fragapane E. A phase III, randomized, open-label study to assess the tolerability and immunogenicity of an H5N1 influenza vaccine administered to healthy adults with a 1-, 2-, 3-, or 6-week interval between first and second doses. Clin Ther. 2010;32(13):2186–97. PubMed PMID: 21316535. Epub 2011/02/15.
94 Vesikari T, Karvonen A, Tilman S, Borkowski A, Montomoli E, Banzhoff A, et al. Immunogenicity and safety of MF59–adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics. 2010;126(4):e762–70. PubMed PMID: 20819892. Epub 2010/09/08.
95 Stephenson I, Gust I, Kieny MP, Pervikov Y. Development and evaluation of influenza pandemic vaccines. Lancet Infect Dis. 2006;6(2):71–2. PubMed PMID: 16439326. Epub 2006/01/28.
96 Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357(9272):1937–43. PubMed PMID: 11425416. Epub 2001/06/27.
97 Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, et al. An MF59–adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine. 2006;24(16):3063–5. PubMed PMID: 16464520. Epub 2006/02/09.
98 Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis. 2005;191(8):1210–5. PubMed PMID: 15776364. Epub 2005/03/19.
99 Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, et al. Safety and cross-reactive immunogenicity of candidate AS03–adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201(11):1644–53. PubMed PMID: 20423222. Epub 2010/04/29.
100 Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28(25):4123–9. PubMed PMID: 20433807. Epub 2010/05/04.
101 Arguedas A, Soley C, Abdelnour A, Sales V, Lindert K, Della Cioppa G, et al. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin. 2011;7(1):58–66. PubMed PMID: 21285531. Epub 2011/02/03.
102 Elkayam O, Amir S, Mendelson E, Schwaber M, Grotto I, Wollman J, et al. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res (Hoboken). 2011;63(7):1062–7. PubMed PMID: 21425247. Epub 2011/03/23.
103 Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59–adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–35. PubMed PMID: 19745215. Epub 2009/09/12.
104 Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–6. PubMed PMID: 20466528. Epub 2010/05/15.
105 Martin JT. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals. 1997;25(2):209–13. PubMed PMID: 9236054. Epub 1997/06/01.
106 Iob A, Brianti G, Zamparo E, Gallo T. Evidence of increased clinical protection of an MF59–adjuvant influenza vaccine compared to a non-adjuvant vaccine among elderly residents of long-term care facilities in Italy. Epidemiol Infect. 2005;133(4):687–93. PubMed PMID: 16050515. Pubmed Central PMCID: 2870297. Epub 2005/07/30.
107 Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, Yang PC, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27(52):7428–35. PubMed PMID: 19683087. Epub 2009/08/18.
108 Leroux-Roels G. Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther. 2009;9(8):1057–71. PubMed PMID: 19555313. Epub 2009/06/27.
109 Schwarz TF, Horacek T, Knuf M, Damman HG, Roman F, Drame M, et al. Single dose vaccination with AS03–adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27(45):6284–90. PubMed PMID: 19856521. Epub 2009/10/27.
110 Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. Bmj. 2010;340:c2649. PubMed PMID: 20508026. Pubmed Central PMCID: 2877808. Epub 2010/05/29.
111 Manuel O, Pascual M, Hoschler K, Giulieri S, Alves D, Ellefsen K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03–adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52(2):248–56. PubMed PMID: 21288852. Epub 2011/02/04.
112 Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, et al. Immunogenicity profile of a 3.75–mug hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis. 2011;203(8):1054–62. PubMed PMID: 21450995. Pubmed Central PMCID: 3068020. Epub 2011/04/01.
113 Carmona A, Omenaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, et al. Immunogenicity and safety of AS03–adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 2010;28(36):5837–44. PubMed PMID: 20600478. Epub 2010/07/06.
114 Garcia-Sicilia J, Gillard P, Carmona A, Tejedor JC, Aristegui J, Merino JM, et al. Immunogenicity and safety of AS03–adjuvanted H1N1 pandemic vaccines in children and adolescents. Vaccine. 2011;29(26):4353–61. PubMed PMID: 21504774. Epub 2011/04/21.
115 Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, Crowcroft N, et al. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. Bmj. 2011;342:c7297. PubMed PMID: 21292718. Pubmed Central PMCID: 3033439. Epub 2011/02/05.
116 Saenz R, Souza Cda S, Huang CT, Larsson M, Esener S, Messmer D. HMGB1–derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine. 2010;28(47):7556–62. PubMed PMID: 20800114. Pubmed Central PMCID: 2963688. Epub 2010/08/31.
117 Clawson C, Huang CT, Futalan D, Seible DM, Saenz R, Larsson M, et al. Delivery of a peptide via poly(D,L-lactic-co-glycolic) acid nanoparticles enhances its dendritic cell-stimulatory capacity. Nanomedicine. 2010;6(5):651–61. PubMed PMID: 20348031. Pubmed Central PMCID: 2947606. Epub 2010/03/30.
118 Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–8. PubMed PMID: 12908518. Epub 2003/08/12.
119 Enoksson M, Ejendal KF, McAlpine S, Nilsson G, Lunderius-Andersson C. Human cord blood-derived mast cells are activated by the Nod1 agonist M-TriDAP to release pro-inflammatory cytokines and chemokines. J Innate Immun. 2011;3(2):142–9. PubMed PMID: 21099203. Epub 2010/11/26.
120 Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–32. PubMed PMID: 17569868. Epub 2007/06/16.
121 Raman VS, Bhatia A, Picone A, Whittle J, Bailor HR, O'Donnell J, et al. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol. 2010;185(3):1701–10. PubMed PMID: 20601594. Pubmed Central PMCID: 3109724. Epub 2010/07/06.
122 Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, et al. Toll-like receptor 6–independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35(1):282–9. PubMed PMID: 15580661. Epub 2004/12/08.
123 Yan W, Chen WC, Liu Z, Huang L. Bryostatin-I: a dendritic cell stimulator for chemokines induction and a promising adjuvant for a peptide based cancer vaccine. Cytokine. 2010;52(3):238–44. PubMed PMID: 20869878. Epub 2010/09/28.
124 Sasaki S, Fukushima J, Hamajima K, Ishii N, Tsuji T, Xin KQ, et al. Adjuvant effect of Ubenimex on a DNA vaccine for HIV-1. Clin Exp Immunol. 1998;111(1):30–5. PubMed PMID: 9472658. Pubmed Central PMCID: 1904860. Epub 1998/02/24.
125 Rey-Ladino J, Ross AG, Cripps AW, McManus DP, Quinn R. Natural products and the search for novel vaccine adjuvants. Vaccine. 2011;29(38):6464–71. PubMed PMID: 21787827. Epub 2011/07/27.
126 Flower DR. Systematic identification of small molecule adjuvants. Expert Opin Drug Discov. 2012;7(9):807–17. PubMed PMID: 22724523. Epub 2012/06/26.
127 Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114(1):135–41. PubMed PMID: 10620129. Epub 2000/01/05.
128 Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6(5):835–47. PubMed PMID: 17931162. Epub 2007/10/13.
129 Ma F, Zhang J, Zhang C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol. 2010;7(5):381–8. PubMed PMID: 20543857. Epub 2010/06/15.
130 Klinman D, Shirota H, Tross D, Sato T, Klaschik S. Synthetic oligonucleotides as modulators of inflammation. J Leukoc Biol. 2008;84(4):958–64. PubMed PMID: 18430787. Pubmed Central PMCID: 2538593. Epub 2008/04/24.
131 Luganini A, Caposio P, Landolfo S, Gribaudo G. Phosphorothioate-modified oligodeoxynucleotides inhibit human cytomegalovirus replication by blocking virus entry. Antimicrob Agents Chemother. 2008;52(3):1111–20. PubMed PMID: 18180342. Pubmed Central PMCID: 2258505. Epub 2008/01/09.
132 Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167(9):4878–86. PubMed PMID: 11673492. Epub 2001/10/24.
133 Stacey KJ, Blackwell JM. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 1999;67(8):3719–26. PubMed PMID: 10417129. Pubmed Central PMCID: 96645. Epub 1999/07/23.
134 Cui Z, Qiu F. Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model. Cancer Immunol Immunother. 2006;55(10):1267–79. PubMed PMID: 16362407. Epub 2005/12/20.
135 Buschow SI, Lasonder E, van Deutekom HW, Oud MM, Beltrame L, Huynen MA, et al. Dominant processes during human dendritic cell maturation revealed by integration of proteome and transcriptome at the pathway level. J Proteome Res. 2010;9(4):1727–37. PubMed PMID: 20131907. Epub 2010/02/06.
136 Poland GA, Ovsyannikova IG, Kennedy RB, Haralambieva IH, Jacobson RM. Vaccinomics and a new paradigm for the development of preventive vaccines against viral infections. OMICS. 2011;15(9):625–36. PubMed PMID: 21732819. Pubmed Central PMCID: 3166201. Epub 2011/07/08.
137 Haralambieva IH, Oberg AL, Dhiman N, Ovsyannikova IG, Kennedy RB, Grill DE, et al. High-dimensional gene expression profiling studies in high and low responders to primary smallpox vaccination. J Infect Dis. 2012;206(10):1512–20. PubMed PMID: 22949304. Pubmed Central PMCID: 3475634. Epub 2012/09/06.
138 Haralambieva IH, Ovsyannikova IG, Pankratz VS, Kennedy RB, Jacobson RM, Poland GA. The genetic basis for interindividual immune response variation to measles vaccine: new understanding and new vaccine approaches. Expert Rev Vaccines. 2013;12(1):57–70. PubMed PMID: 23256739. Pubmed Central PMCID: 3570049. Epub 2012/12/22.
139 Germain RN, Meier-Schellersheim M, Nita-Lazar A, Fraser ID. Systems biology in immunology: a computational modeling perspective. Annu Rev Immunol. 2011;29:527–85. PubMed PMID: 21219182. Pubmed Central PMCID: 3164774. Epub 2011/01/12.
140 Umlauf BJ, Ovsyannikova IG, Haralambieva IH, Kennedy RB, Vierkant RA, Pankratz VS, et al. Correlations between vaccinia-specific immune responses within a cohort of armed forces members. Viral Immunol. 2011;24(5):415–20. PubMed PMID: 21958369. Pubmed Central PMCID: 3236101. Epub 2011/10/01.
141 Kennedy RB, Ovsyannikova IG, Pankratz VS, Haralambieva IH, Vierkant RA, Jacobson RM, et al. Genome-wide genetic associations with IFNgamma response to smallpox vaccine. Hum Genet. 2012;131(9):1433–51. PubMed PMID: 22661280. Epub 2012/06/05.
142 Ovsyannikova IG, Kennedy RB, O'Byrne M, Jacobson RM, Pankratz VS, Poland GA. Genome-wide association study of antibody response to smallpox vaccine. Vaccine. 2012;30(28):4182–9. PubMed PMID: 22542470. Pubmed Central PMCID: 3367131. Epub 2012/05/01.
143 Davis NA, Crowe JE, Jr., Pajewski NM, McKinney BA. Surfing a genetic association interaction network to identify modulators of antibody response to smallpox vaccine. Genes Immun. 2010;11(8):630–6. PubMed PMID: 20613780. Pubmed Central PMCID: 3001955. Epub 2010/07/09.
144 Haralambieva IH, Oberg AL, Ovsyannikova IG, Kennedy RB, Grill DE, Middha S, et al. Genome-wide characterization of transcriptional patterns in high and low antibody responders to rubella vaccination. PLoS One. 2013;8(5):e62149. PubMed PMID: 23658707. Pubmed Central PMCID: 3641062. Epub 2013/05/10.
145 Fellay J, Frahm N, Shianna KV, Cirulli ET, Casimiro DR, Robertson MN, et al. Host genetic determinants of T cell responses to the MRKAd5 HIV-1 gag/pol/nef vaccine in the step trial. J Infect Dis. 2011;203(6):773–9. PubMed PMID: 21278214. Pubmed Central PMCID: 3071133. Epub 2011/02/01.
146 Png E, Thalamuthu A, Ong RT, Snippe H, Boland GJ, Seielstad M. A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum Mol Genet. 2011;20(19):3893–8. PubMed PMID: 21764829. Epub 2011/07/19.
147 Miller EK, Dumitrescu L, Cupp C, Dorris S, Taylor S, Sparks R, et al. Atopy history and the genomics of wheezing after influenza vaccination in children 6–59 months of age. Vaccine. 2011;29(18):3431–7. PubMed PMID: 21396408. Pubmed Central PMCID: 3334304. Epub 2011/03/15.
148 Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. PubMed PMID: 19029902. Epub 2008/11/26.
149 Henn AD, Wu S, Qiu X, Ruda M, Stover M, Yang H, et al. High-resolution temporal response patterns to influenza vaccine reveal a distinct human plasma cell gene signature. Sci Rep. 2013;3:2327. PubMed PMID: 23900141.
150 Bekeredjian-Ding I, Jego G. Toll-like receptors-sentries in the B-cell response. Immunology. 2009;128(3):311–23. PubMed PMID: 20067531. Pubmed Central PMCID: 2770679. Epub 2010/01/14.
151 Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536. PubMed PMID: 22470453. Pubmed Central PMCID: 3314666. Epub 2012/04/04.
152 Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. Bmj. 2013;346:f794. PubMed PMID: 23444425. Epub 2013/02/28.
153 Hattotuwagama CK, Davies MN, Flower DR. Receptor-ligand binding sites and virtual screening. Curr Med Chem. 2006;13(11):1283–304. PubMed PMID: 16712470. Epub 2006/05/23.
154 McInnes C. Virtual screening strategies in drug discovery. Curr Opin Chem Biol. 2007;11(5):494–502. PubMed PMID: 17936059. Epub 2007/10/16.
155 Ripphausen P, Nisius B, Peltason L, Bajorath J. Quo vadis, virtual screening? A comprehensive survey of prospective applications. J Med Chem. 2010;53(24):8461–7. PubMed PMID: 20929257. Epub 2010/10/12.
156 Bajorath J. Computational studies, virtual screening, and theoretical molecular models. J Med Chem. 2010;53(1):1–2. PubMed PMID: 20000613. Epub 2009/12/17.
157 Charoenvit Y, Goel N, Whelan M, Rosenthal KS, Zimmerman DH. CEL-1000–-a peptide with adjuvant activity for Th1 immune responses. Vaccine. 2004;22(19):2368–73. PubMed PMID: 15193396. Epub 2004/06/15.
158 Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998 Feb;338(7):436–45. PubMed PMID: 9459648. Epub 1998/02/12.
159 Rees S, Morrow D, Kenakin T. GPCR drug discovery through the exploitation of allosteric drug binding sites. Receptors Channels. 2002;8(5–6):261–8. PubMed PMID: 12690954. Epub 2003/04/15.
160 Gether U, Asmar F, Meinild AK, Rasmussen SG. Structural basis for activation of G-protein-coupled receptors. Pharmacol Toxicol. 2002;91(6):304–12. PubMed PMID: 12688373. Epub 2003/04/12.
161 Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7. PubMed PMID: 8629022. Epub 1996/05/10.
162 Ehrlich HJ, Muller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med. 2008;358(24):2573–84. PubMed PMID: 18550874. Epub 2008/06/14.
163 Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–64. PubMed PMID: 16714186. Epub 2006/05/23.
164 Leroux-Roels I, Van der Wielen M, Kafeja F, Vandermeulen C, Lazarus R, Snape MD, et al. Humoral and cellular immune responses to split-virion H5N1 influenza vaccine in young and elderly adults. Vaccine. 2009;27(49):6918–25. PubMed PMID: 19761837. Epub 2009/09/19.
165 Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, Chen WH, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine. 2009;27(37):5091–5. PubMed PMID: 19577636. Pubmed Central PMCID: 2730950. Epub 2009/07/07.
166 Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis. 2008;198(9):1309–16. PubMed PMID: 18808338. Pubmed Central PMCID: 3044935. Epub 2008/09/24.
167 Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106(19):7962–7. PubMed PMID: 19416838. Pubmed Central PMCID: 2674105. Epub 2009/05/07.
168 Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, et al. Safety and immunogenicity of nonadjuvanted and MF59–adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43(9):1135–42. PubMed PMID: 17029131. Epub 2006/10/10.
169 Vesikari T, Groth N, Karvonen A, Borkowski A, Pellegrini M. MF59–adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine. 2009;27(45):6291–5. PubMed PMID: 19840662. Epub 2009/10/21.
170 Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1–seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15(2):253–9. PubMed PMID: 18003811. Pubmed Central PMCID: 2238067. Epub 2007/11/16.
171 Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365(15):1406–16. PubMed PMID: 21995388. Epub 2011/10/15.
172 De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, et al. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine. 2012;30(30):4490–8. PubMed PMID: 22561143. Epub 2012/05/09.
173 Moser C, Amacker M, Zurbriggen R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev Vaccines. 2011;10(4):437–46. PubMed PMID: 21506642. Epub 2011/04/22.
174 Ambrosch F, Wiedermann G, Jonas S, Althaus B, Finkel B, Gluck R, et al. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine. 1997;15(11):1209–13. PubMed PMID: 9286045. Epub 1997/08/01.
175 Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12–15 year old girls. PLoS One. 2013;8(5):e61825. PubMed PMID: 23650505. Pubmed Central PMCID: 3641072. Epub 2013/05/08.
176 Hoebe CJ, Vermeiren AP, Dukers-Muijrers NH. Revaccination with Fendrix(R) or HBVaxPro(R) results in better response rates than does revaccination with three doses of Engerix-B(R) in previous non-responders. Vaccine. 2012;30(48):6734–7. PubMed PMID: 22981848. Epub 2012/09/18.
177 Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04–adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4–year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. PubMed PMID: 22075171. Epub 2011/11/15.
178 Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04–adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4–year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):100–10. PubMed PMID: 22075170. Epub 2011/11/15.
179 Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3(2):e1665. PubMed PMID: 18301743. Pubmed Central PMCID: 2253495. Epub 2008/02/28.
180 Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Drame M, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28(3):849–57. PubMed PMID: 19835828. Epub 2009/10/20.
181 Powell TJ, Tang J, Derome ME, Mitchell RA, Jacobs A, Deng Y, et al. Plasmodium falciparum synthetic LbL microparticle vaccine elicits protective neutralizing antibody and parasite-specific cellular immune responses. Vaccine. 2013;31(15):1898–904. PubMed PMID: 23481177. Pubmed Central PMCID: 3611586. Epub 2013/03/14.
182 McNally B, Willette M, Ye F, Partida-Sanchez S, Flano E. Intranasal administration of dsRNA analog poly(I:C) induces interferon-alpha receptor-dependent accumulation of antigen experienced T cells in the airways. PLoS One. 2012;7(12):e51351. PubMed PMID: 23236482. Pubmed Central PMCID: 3517467. Epub 2012/12/14.
183 Ichinohe T, Ainai A, Tashiro M, Sata T, Hasegawa H. PolyI:polyC12U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine. 2009;27(45):6276–9. PubMed PMID: 19840660. Epub 2009/10/21.
184 Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, et al. Recombinant Rabies Viruses Expressing GM-CSF or Flagellin Are Effective Vaccines for Both Intramuscular and Oral Immunizations. PLoS One. 2013;8(5):e63384. PubMed PMID: 23700422. Pubmed Central PMCID: 3658976. Epub 2013/05/24.
185 Liu G, Song L, Reiserova L, Trivedi U, Li H, Liu X, et al. Flagellin-HA vaccines protect ferrets and mice against H5N1 highly pathogenic avian influenza virus (HPAIV) infections. Vaccine. 2012;30(48):6833–8. PubMed PMID: 23000130. Epub 2012/09/25.
186 Taylor DN, Treanor JJ, Sheldon EA, Johnson C, Umlauf S, Song L, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012;30(39):5761–9. PubMed PMID: 22796139. Epub 2012/07/17.
187 Burns RP, Jr., Ferbel B, Tomai M, Miller R, Gaspari AA. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. Clin Immunol. 2000;94(1):13–23. PubMed PMID: 10607486. Epub 1999/12/23.
188 Shukla NM, Mutz CA, Malladi SS, Warshakoon HJ, Balakrishna R, David SA. Toll-like receptor (TLR)-7 and -8 modulatory activities of dimeric imidazoquinolines. J Med Chem. 2012;55(3):1106–16. PubMed PMID: 22239408. Pubmed Central PMCID: 3276691. Epub 2012/01/14.
189 Rhee JW, Kim D, Park BK, Kwon S, Cho S, Lee I, et al. Immunization with a hemagglutinin-derived synthetic peptide formulated with a CpG-DNA-liposome complex induced protection against lethal influenza virus infection in mice. PLoS One. 2012;7(11):e48750. PubMed PMID: 23144954. Pubmed Central PMCID: 3492448. Epub 2012/11/13.
190 Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. PubMed PMID: 21506647. Pubmed Central PMCID: 3108434. Epub 2011/04/22.
191 Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. PubMed PMID: 20042088. Pubmed Central PMCID: 2806383. Epub 2010/01/01.
192 Garcon N, Heppner DG, Cohen J. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines. 2003;2(2):231–8. PubMed PMID: 12899574. Epub 2003/08/06.
193 Montana M, Verhaeghe P, Ducros C, Terme T, Vanelle P, Rathelot P. Safety review: squalene and thimerosal in vaccines. Therapie. 2010;65(6):533–41. PubMed PMID: 21176760. Epub 2010/12/24.
194 Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19(1):2–11. PubMed PMID: 19515003. Epub 2009/06/12.
195 Lingnau K, Riedl K, von Gabain A. IC31 and IC30, novel types of vaccine adjuvant based on peptide delivery systems. Expert Rev Vaccines. 2007;6(5):741–6. PubMed PMID: 17931154. Epub 2007/10/13.
196 McCluskie MJ, Weeratna RD, Evans DM, Makinen S, Drane D, Davis HL. CpG ODN and ISCOMATRIX adjuvant: a synergistic adjuvant combination inducing strong T-Cell IFN-gamma responses. Biomed Res Int. 2013;2013:636847. PubMed PMID: 23586050. Pubmed Central PMCID: 3618927. Epub 2013/04/16.
197 Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol. 2012;61(Pt 7):935–43. PubMed PMID: 22442293. Epub 2012/03/24.
198 Vujanic A, Snibson KJ, Wee JL, Edwards SJ, Pearse MJ, Scheerlinck JP, et al. Long-term antibody and immune memory response induced by pulmonary delivery of the influenza Iscomatrix vaccine. Clin Vaccine Immunol. 2012;19(1):79–83. PubMed PMID: 22072721. Pubmed Central PMCID: 3255945. Epub 2011/11/11.
199 Kulkarni PS, Manjunath K, Agarkhedkar S. Safety and immunogenicity of an adjuvanted whole virion, inactivated A (H1N1) 2009 influenza vaccine in young and elderly adults, and children. Vaccine. 2012;31(1):20–2. PubMed PMID: 23131207. Epub 2012/11/08.
200 Lin JT, Li CG, Wang X, Su N, Liu Y, Qiu YZ, et al. Antibody persistence after 2–dose priming and booster response to a third dose of an inactivated, adjuvanted, whole-virion H5N1 vaccine. J Infect Dis. 2009;199(2):184–7. PubMed PMID: 19067606. Epub 2008/12/11.
201 Nolan TM, Richmond PC, Skeljo MV, Pearce G, Hartel G, Formica NT, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26(33):4160–7. PubMed PMID: 18599164. Epub 2008/07/05.
202 Nicholson KG, Thompson CI, Klap JM, Wood JM, Batham S, Newman RW, et al. Safety and immunogenicity of whole-virus, alum-adjuvanted whole-virus, virosomal, and whole-virus intradermal influenza A/H9N2 vaccine formulations. Vaccine. 2009;28(1):171–8. PubMed PMID: 19799843. Epub 2009/10/06.
203 Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361(25):2414–23. PubMed PMID: 19846844. Epub 2009/10/23.
204 Nakayama T, Kumagai T, Ishii KJ, Ihara T. Alum-adjuvanted H5N1 whole virion inactivated vaccine (WIV) induced IgG1 and IgG4 antibody responses in young children. Vaccine. 2012;30(52):7662–6. PubMed PMID: 23102974. Epub 2012/10/30.
205 Ikeno D, Kimachi K, Kino Y, Harada S, Yoshida K, Tochihara S, et al. Immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1, NIBRG-14) vaccine administered by intramuscular or subcutaneous injection. Microbiol Immunol. 2010;54(2):81–8. PubMed PMID: 20377741. Epub 2010/04/10.
Funding / potential competing interests: D.K. has received research support from Hoffmann-LaRoche. A.E. research was supported by Nachwuchsförderung Universität Basel (2013). D.M.S. research was supported by Canadian Institutes of Health Research (CIHR) and Alberta Innovates Health Solutions (AIHS) postdoctoral fellowships. A.L. is supported by the Banting Postdoctoral Fellowship Program, administered by the Government of Canada and by AIHS postdoctoral fellowship. M.H. is supported by the Canada Excellence Research Chair (CERC) in Virology award. D.L.J.T. is supported by a CIHR grant.