More than skin-deep: the many dimensions of the psoriatic disease
DOI: https://doi.org/10.4414/smw.2014.13968
Wolf-Henning
Boehncke, Sandra
Boehncke
Summary
Psoriasis is among the most common skin diseases, exhibiting a wide spectrum of clinical manifestations. Joint involvement in the form of psoriatic arthritis is readily recognised, but both frequency and severity of this manifestation have long been underestimated. More recently, additional important diseases have been found to be associated with psoriasis, including the metabolic syndrome (or components thereof), cardiovascular diseases, lymphoma, and anxiety/depression. In the past, psoriasis treatment aimed at suppressing acute rashes. Current concepts regard psoriasis as a chronic systemic inflammatory condition and cardiovascular risk factor. In the light of this concept, long-term disease control through systemic maintenance therapy is increasingly recommended by experts. This approach became feasible with the approval of numerous biologics for the treatment of psoriasis. But to really address all medical needs of psoriasis patients, a truly interdisciplinary, comprehensive management approach is needed. Several national societies have already published algorithms to ensure that this need will be implemented in the daily practice of dermatologists and nondermatologists alike.
The many dimensions of psoriasis as a skin disease
Skin diseases are special in as much as it is next to impossible to hide them. Visible disfiguration often triggers reactions ranging from irritation to disgust among others, explaining to some extend the readily measurable psychological burden of disease: Psoriasis ranks second only to depression when the impact of major diseases on quality of life is quantified [1]. Importantly, the physical burden of disease is also at least comparable to other major diseases such as cancer or arthritis [1]. This result is not surprising considering symptoms such as itch, pain or bleeding, all of which are regularly reported by patients [2]. With a prevalence believed to be in the order of 2% in Europe and North America, psoriasis is a common disease [3], showing a linear increase of prevalence over time, with a prevalence at the age of 18 already in the order of 1% [4]. A manifestation during childhood or adolescence is thus not unusual.
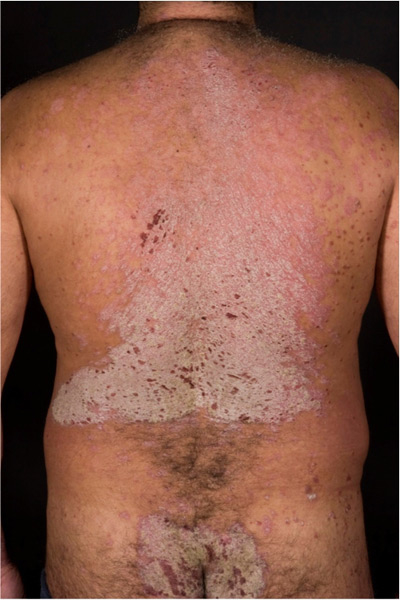
Figure 1
Chronic plaque-type psoriasis. This classical clinical manifestation of psoriasis is characterised by well demarkated, red, infiltrated plaques, covered with a coarse silvery scaling. Here, the lumbar region as a predilection site is affected.
The classical clinical manifestations of psoriasis are well demarked: red, infiltrated plaques, covered with a coarse silvery scaling (fig. 1). Predilection sites include elbows and knees, scalp, and periumbilical and lumbar regions, but psoriasis can affect any anatomical site, even at the same time, thus resulting in the clinical picture of erythroderma. In this case, nail changes might point towards the correct diagnosis, as psoriasis patients often exhibit pitting or yellow-brownish patches underneath the nail plate, depending on whether the psoriatic plaques are located in the nail matrix or the nail bed, respectively. A manifestation sparing predilection sites, but affecting intertriginous areas such as axillae or groins, is called inverse psoriasis. Diagnosis may sometimes be difficult, as scales are usually absent. Guttate psoriasis is an acute exanthematic form, starting with disseminated red keratotic papules, which eventually grow to small plaques. In addition, there are numerous types of psoriasis that do not exhibit plaques, but rather pustules, with acute generalised pustular psoriasis considered a medical emergency needing immediate therapy.
In the past, the treatment decision was primarily based on the extent of the disease. Patients with “mild” psoriasis would preferentially be treated with topical drugs, while those with “moderate-to-severe” psoriasis would be considered candidates for photo- or systemic therapies. The cut-off point was regarded to be in the order of 10% of the body surface being affected by the disease. As all therapeutic options made it difficult to suggest long-term maintenance therapy, be it for reasons of practicability (topical and phototherapies) or safety (phototherapies, acitretin, ciclosporin A, methotrexate), the treatment goal was to rapidly control acute rashes (table 1) [5]. Over the last decade, numerous biologics have been developed and approved for the treatment of psoriasis. Currently, three biologics inhibiting the function of tumour necrosis factor-alpha are approved for this indication, and a fourth approved drug blocks interleukins (ILs) 12 and 23; it is thought specifically that inhibition of IL-23 interferes with the development of T helper-17 (Th17) lymphocytes, currently regarded as particularly important effector cells in psoriatic inflammation. Their efficacy during induction therapy seems to be superior to that of conventional systemic drugs, and lack of cumulative toxicity and drug-drug interactions makes biologics valuable for maintenance therapy. This approach is feasible, given their favourable safety profile, with a slight increase in opportunistic infections (table 2) [5].
This much-improved therapeutic arsenal allowed the therapeutic approach towards psoriasis to be revisited. First, experts suggested redefining disease severity, as it is clear that the burden of disease depends not only on the absolute extent of skin lesions, but on other aspects as well, such as localisation of lesions [6]. Consequently, many dermatologists no longer regard psoriasis to be “moderate-to-severe” only in patients with extensive skin disease in terms of affected body surface, but also whenever psoriasis has a significant impact on a patient’s quality of life. One such constellation is significant nail involvement, which – based on criteria such as percentage of affected body surface area – would previously not have been considered an indication for a treatment using biologics. Taking into account the patient’s substantially reduced quality of life gives allows the physician also to consider systemic therapies including biologics, for which there is good evidence for satisfactory efficacy. Second, long-term control of cutaneous signs and symptoms through maintenance therapy is increasingly being propagated in the light of convincing safety data, again specifically of the biologics. Indeed, questionnaire-based studies over the last 10 years showed that patients were not at all satisfied with the former approach that aimed at controlling rashes [2]. Third, with ever more potent biologics being developed for this indication, the concept of complete (or almost complete) clearance of skin symptoms becomes feasible.
|
Table 1: Summary of currently available nonbiological antipsoriatic therapies (modified from reference [5]). |
|
Drug
|
Efficacy*
|
Safety/tolerability during induction therapy#
|
Safety/tolerability during maintenance therapy#
|
Limitations for maintenance therapy / important end-organ toxicities
|
| Indicated for mild psoriasis |
| Glucocorticosteroids |
++++ |
+++ |
+ |
Skin atrophy
Time consuming |
| Vitamin D derivatives |
+++ |
+++ |
+++ |
Time consuming |
| Calcineurin inhibitors |
++ |
++ |
Not indicated |
Time consuming
Off label |
| Indicated for moderate-to-severe psoriasis |
| UVB phototherapy |
+++ |
+++ |
Not recommended |
Time consuming
Carcinogenic? |
| PUVA photochemotherapy |
++++ |
+ |
Not recommended |
Time consuming
Carcinogenic |
| Acitretin |
+ |
+ |
+ |
Dyslipidaemia
Teratogenicity |
| Ciclosporin A |
++ |
+ |
+ |
Nephrotoxicity
Hypertension |
| Methotrexate |
++ |
+ |
++ |
Hepatotoxicity
Contraindicated during pregnancy |
| PUVA = psoralen plus ultraviolet A; UVB = ultraviolet B
* Semiquantitative grading of efficacy:
Topical therapies: “+” 15% PASI75, “++” 30%, “+++” 45% PASI75
Systemic therapies: “+” 30% PASI75, “++” 50% PASI75, “+++” 70% PASI 75, “++++” 90% PASI75
(PASI = psoriasis area severity index)
# Semiquantitative grading of safety/tolerability: global assessment based on consensus of the authors of the German S3 psoriasis guidelines [5] |
|
Table 2: Summary of currently available biological antipsoriatic therapies (modified from reference [5]). |
|
Drug
|
Type of molecule and target structure
|
Efficacy*
|
Safety during induction therapy#
|
Safety during maintenance therapy#
|
Comments
|
| Adalimumab |
Fully human anti-TNF-α monoclonal antibody |
+++ |
++ |
++ |
Current market leader |
| Etanercept |
Fusion molecule |
+ |
++ |
++ |
Sole biologic approved to date for treating children/adolescents |
| Infliximab |
Chimeric anti-TNF-α monoclonal antibody |
++++ |
+ |
++ |
Recommended for treating generalised pustular psoriasis (off-label) |
| Ustekinumab |
Fully human anti-p40 (common subunit of Il-12 and Il-23) monoclonal antibody |
+++ |
++ |
++ |
Represents a different mode of action; so-far no tuberculosis under ustekinumab |
| IL = interleukin; TNF = tumour necrosis factor
* Semiquantitative grading of efficacy: “+” 30% PASI75, “++” 50% PASI75, “+++” 70% PASI 75, “++++” 90% PASI75 (PASI = psoriasis area severity index)
# Semiquantitative grading of safety/tolerability: global assessment based on consensus of the authors of the German S3 psoriasis guidelines [5] |
The many dimensions of psoriasis as a systemic inflammatory condition
As a group, psoriasis patients exhibit numerous other important diseases more often than expected on the basis of the prevalence of the respective diseases (table 3) [7, 8]. These include psoriatic arthritis, Crohn’s disease, malignancies, depression, nonalcoholic fatty liver disease, the metabolic syndrome (or components of it), and cardiovascular disorders [9, 10]. These so-called comorbidities contribute substantially to morbidity and mortality among psoriasis patients. As comorbidities demand treatment, the number of drugs taken by psoriasis patients is significantly higher when compared with controls [11]. The list of comedications taken by psoriasis patients often comprises antidepressants, highlighting the so-far somewhat neglected importance of depression as a comorbidity [12]. Moreover, and like other chronic inflammatory diseases, the patients’ life expectancy is substantially reduced, with cardiovascular diseases contributing most [13].
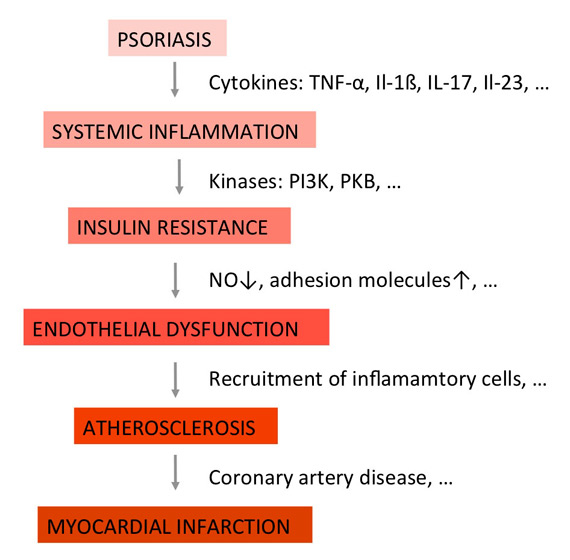
Figure 2
The concept of the “psoriatic march” (adapted from reference [25]). Psoriasis is considered a state of chronic systemic inflammation. Proinflammatory cytokines typically associated with psoriasis induce insulin resistance in endothelial cells, characterized by a reduced release of vasodilating factors such as NO. The resulting state of endothelial dysfunction comprises additional features such as expression of adhesion molecules, and provides the basis for the formation of atherosclerotic plaques.
IL = interleukin; NO = nitric oxide; PI3K = phosphoinositide 3-kinase; PKB = protein kinase B; TNF = tumour necrosis factor
The association of psoriasis with at least some of these comorbidities might be explained in part on the grounds of shared genetics. Around 40 genes have been found to be associated with psoriasis [14]. Although the function of numerous of these genes is so far unknown, many of them point towards a central role of both the adaptive and the innate immune systems [15, 16]. The importance of T-cells in general and Th17 lymphocytes in particular is underlined by variants in the genes encoding the IL-23 receptor and in the untranslated region of IL-12B as indicators of psoriasis risk [17, 18]. Numerous of these psoriasis susceptibility genes, including IL23R, are associated with psoriasis and psoriatic arthritis [19, 20]. The gene CDKAL1 is found in association with psoriasis and type 2 diabetes mellitus as well as Crohn’s disease [21].
The association of some of these comorbidities with psoriasis might thus be due in part to common genetics, although this is still under debate [22]. With regard to malignancies, namely lymphoma and skin cancer, it is unclear whether they are related to psoriasis itself, or its treatment [23]. Finally, the increased cardiovascular mortality has long been attributed to cumulating traditional cardiovascular risk factors among psoriasis patients [24]. However, this hypothesis cannot explain why only patients with severe, but not mild, psoriasis exhibit this increased cardiovascular risk [25]. Moreover, in a case-control study, coronary artery calcification – as an indicator for coronary artery disease – has been shown to be significantly more frequent and more pronounced among psoriasis patients compared with controls [26].
To explain the increased cardiovascular mortality of patients with severe psoriasis, the concept of the so-called “psoriatic march” has been proposed (fig. 2) [27]: According to this concept, psoriasis is a state of systemic inflammation, as numerous biomarkers for inflammation can not only readily be detected in the patients’ blood, but also correlate well with disease activity. Systemic inflammation in turn induces insulin resistance, that is, reduced signalling of the insulin receptor upon binding of its ligand. As the insulin receptor is expressed on metabolic and nonmetabolic cells alike, it has effects beyond controlling blood glucose. At the level of endothelial cells, it enhances blood flow and vasodilatation through changes nitric oxide (NO). Insulin resistance, induced by proinflammatory cytokines, therefore results in reduced release of vasodilating factors such as NO. The resulting vascular stiffness is known as endothelial dysfunction. It comprises additional features, such as expression of adhesion molecules, and provides the basis for the formation of atherosclerotic plaques. Depending on their localisation, resulting diseases include myocardial infarction and stroke, both of them known to be associated with psoriasis. Numerous groups have independently published evidence in favour of this hypothesis [28]. Importantly, it has been shown that the clinical signs and symptoms of psoriasis can effectively be treated using the insulin-sensitising drug glucagon-like peptide 1 (GLP1), thus pointing towards insulin resistance as a central phenomenon in inflammation [29, 30]. In line with these observations, Bürger et al. demonstrated that insulin resistance directly contributes to the epidermal phenotype – hyperproliferation and altered differentiation of keratinocytes – seen in psoriasis, thus further underlining the role of factors involved in insulin receptor signalling as potential targets for innovative antipsoriatic therapies [31, 32]. However, the question regarding the “effect size” of psoriatic inflammation versus inflammation from other sources such as obesity has yet to be established. The concept of the “psoriatic march” should, therefore, at present be considered a hypothesis that hopefully stimulates further research to test whether influencing insulin resistance might represent a sufficiently powerful therapeutic strategy to yield clinically meaningful effects.
|
Table 3: Important comorbidities and screening recommendations. |
|
Comorbidity
|
Odds ratio [6, 7]
|
Suggested screening [31–34]#
|
| Part of the so-called metabolic syndrome |
| Diabetes mellitus |
2.0 |
Fasting blood glucose |
| Arterial hypertension |
1.7 |
Two consecutive blood pressure measurements |
| Obesity |
1.7 |
Body mass index
Waist circumference |
| Dyslipidaemia |
1.8 |
Fasting blood lipids |
| Other |
| Cardiovascular disease |
1.8* |
Screening for the components of the metabolic syndrome |
| Liver disease |
1.4 |
Transaminases†
|
| Psoriatic arthritis |
Around 25% of psoriasis patients [35, 36] |
Screening questionnaire (e.g. ToPAS, PASE, PEST)
Ask/look for tender/swollen joints
Ask for inflammatory back pain‡
|
| PASE = psoriatic arthritis screening and evaluation; PEST = psoriasis epidemiology screening tool; ToPAS = Toronto psoriatic arthritis screening
* Coronary artery disease.
# Recommendations for dermatologists (recommendations in the respective guidelines may vary).
† Nonalcoholic fatty liver disease (NAFLD) cannot be ruled out on the basis of laboratory tests alone.
‡ Typically at night, eases with physical activity. |
From treatment to management: towards a comprehensive approach to control the psoriatic disease
In the age of biological therapies, complete or almost complete clearance of cutaneous signs and symptoms of psoriasis is about to become a feasible goal. Moreover, long-term maintenance therapy is readily being accepted as an approach to guaranteeing a low burden of disease over long periods of time for patients with psoriasis. However, to fully address the medical needs of psoriasis patients, their comorbidities need to be considered at the same time. This requires routine screening at least for the most important comorbidities of psoriasis patients. Once diagnosed, these comorbidities need to be addressed by a well-structured cooperation between the respective experts (table 3) [33–36]. The early detection of psoriatic arthritis is of particular practical relevance. As most patients develop psoriasis (of the skin) many years before their first joint symptoms [37, 38], dermatologists are in the ideal situation to serve as sentinels in this regard. Given the limited clinical experience of most dermatologists, questionnaires have been developed to help dermatologists to screen for psoriatic arthritis [39].
As stated above, cardiovascular comorbidities contribute most to the patients’ increased mortality [13]. Whether or not certain therapeutic approaches might provide the additional benefit of reducing a patient’s cardiovascular risk, is, therefore, a question of considerable interest. Such an effect might be expected from long-term systemic anti-inflammatory treatment, considering that atherosclerosis is driven by systemic inflammation [27, 40, 41]. To date, evidence supporting this hypothesis comes from retrospective analyses and some small prospective studies on biomarkers for cardiovascular risk [42–45]. However, conclusive data on hard clinical endpoints, from registries, or trials are not yet available, meaning that a protective effect of systemic anti-inflammatory therapies on cardiovascular disease has yet to be proven.
In summary, there is increasing evidence that psoriasis goes beyond the skin and should be regarded a chronic systemic inflammatory condition, perhaps better called the “psoriatic disease”. The latter comprises at least skin and joints, and there might even be a direct pathogenetic link via insulin resistance with cardiovascular diseases. Irrespective of the current state of the scientific discussion around pathogenetic concepts, a truly interdisciplinary, comprehensive management approach is needed to address the medical needs of patients suffering from the psoriatic disease.
References
1 Rapp SR, Feldman SR, Exum ML, Fleischer jr. AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7.
2 Dubertret L, Mrowietz U, Ranki A, van de Kerkhof PC, Chimenti S, Lotti T, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient memerbship survey. Br J Dermatol. 2006;155:729–36. 2008.
3 Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–85.
4 Augustin M, Glaeske G, Radtke MA, Schaefer I, Radtke M. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–6.
5 Nast A, Boehncke WH, Mrowietz U, Ockenfels HM, Philipp S, Reich K, et al. S3–guidelines on the treatment of psoriasis vulgaris. Update. J Dtsch Dermatol Ges. 2012;Suppl. 2:S1–95.
6 Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, Nast A, et al. Definiiton of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10.
7 Augustin M, Reich K, Glaeske G, Schäfer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venerol. 2010;90:147–51.
8 Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–9.
9 Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–6.
10 Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71.
11 Gerdes S, Zahl VA, Knopf H, Weichenthal M, Mrowietz U. Comedication related to comorbidities: a study in 1203 hospitalized patients with severe psoriasis. Br J Dermatol. 2008;159:1116–23.
12 Dowlatshahi EA, Wakkee M, Herings RM, Hollestein LM, Nijsten T. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venerol. 2013;93:544–50.
13 Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study. In the U.K. Br J Dermatol. 2010;163:586–92.
14 Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–26.
15 Capon F, Burden AD, Trembath RC, Barker JN. Psoriasis and other complex trait dermatoses: from loci to functional pathways. J Invest Dermatol. 2012;132:915–22.
16 Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nature Genet. 2012;44:1341–8.
17 Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–90.
18 Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–6.
19 Rahman P, Imman RD, Maksymovwych WP, Reeve JP, Peddle L, Gladman DD. Association of interleukin 23 receptor variants with psoriatic arthritis. J Rheumatol. 2009;36:137–40.
20 Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013;72(Suppl. 2): ii104–10.
21 Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes und Crohn’s Disease. J Med Genet. 2008;45:114–6.
22 Gupta Y, Möller S, Zillikens D, Boehncke WH, Ibrahim SM, Ludwig RJ. Genetic control of psoriasis is relatively distinct from that of metabolic syndrome and coronary artery disease. Exp Dermatol. 2013;22:552–3.
23 Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126:2194–201.
24 Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68–73.
25 Malbris L, Akre O, Granath F, Yin L, Lindelöf B, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19:225–30.
26 Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271–6.
27 Boehncke WH, Boehncke S, Schön MP. Managing comorbid disease in patients with psoriasis. Br Med J. 2010;340:b5666.
28 Boehncke WH, Boehncke S, Tobin AM, Kirby B. The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–7.
29 Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–54.
30 Ahern T, Tobin AM, Corrigan M, Hogan A, Sweeney C, Kirby B, O’Shea D. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2012 (e-pub ahead of print)
31 Buerger C, Richter B, Woth K, Salgo R, Malisiewicz B, Diehl S, et al. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. J Invest Dermatol. 2012;132:2206–14.
32 Buerger C, Malisiewicz B, Eiser A, Hardt K, Boehncke WH. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br J Dermatol. 2013;169:156–9.
33 Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58:1031–42.
34 Boehncke WH, Boehncke S. Cardiovascular mortality in psoriasis and psoriatic arthritis: epidemiology, pathomechanisms, therapeutic implications, and perspectives. Curr Rheumatol Rep. 2012;14:343–8
35 Daudén E, Castañeda S, Suárez C, García-Campayo J, Blasco AJ, Aguilar MD, et al. Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis. J Eur Acad Dermatol Venerol. 2013;27:1387–404.
36 Wohlrab J, Fiedler G, Gerdes S, Nast A, Philipp S, Radtke MA, et al. Recommendations for detection of individual risk for comorbidities in patients with psoriasis. Arch Dermatol Res. 2013;305:91–8.
37 Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA): an analysis of 220 patients. Q J Med 1987;62:127–41.
38 Veale DJ, Fitzgerald O. Psoriatic arthritis: pathogenesis and epidemiology. Clin Exp Rheumatol. 2002;20:S27–33.
39 Dominguez P, Gladman DD, Helliwell P, Mease PJ, Husni ME, Qureshi AA. Development of screening tools to identify psoriatic arthritis. Curr Rheum Rep. 2010;12:295–9.
40 Hansson GK. Inflamamtion, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
41 Kim J Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904.
42 Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011;25:1187–93.
43 Boehncke S, Fichtlscherer S, Salgo R, Garbaraviciene J, Beschmann H, Diehl S, et al. Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch Dermatol Res. 2011;303:381–8.
44 Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197–204.
45 Armstrong AW, Brezinski EA, Follansbee MR, Armstrong EJ. Effects of Biologic Agents and Other Disease-Modifying Antirheumatic Drugs on Cardiovascular Outcomes in Psoriasis and Psoriatic Arthritis: A Systematic Review. Curr Pharm Des. 2013; e-pub ahead of print.

