Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target?
DOI: https://doi.org/10.4414/smw.2014.13957
Philipp
Schütz, Martina
Bally, Zeno
Stanga, Ulrich
Keller
Summary
Loss of appetite and ensuing weight loss is a key feature of severe illnesses. Protein-energy malnutrition (PEM) contributes significantly to the adverse outcome of these conditions. Pharmacological interventions to target appetite stimulation have little efficacy but considerable side effects. Therefore nutritional therapy appears to be the logical step to combat inadequate nutrition. However, clinical trial data demonstrating benefits are sparse and there is no current established standard algorithm for use of nutritional support in malnourished, acutely ill medical inpatients. Recent high-quality evidence from critical care demonstrating harmful effects when parenteral nutritional support is used indiscriminately has led to speculation that loss of appetite in the acute phase of illness is indeed an adaptive, protective response that improves cell recycling (autophagy) and detoxification. Outside critical care, there is an important gap in high quality clinical trial data shedding further light on these important issues. The selection, timing, and doses of nutrition should be evaluated as carefully as with any other therapeutic intervention, with the aim of maximising efficacy and minimising adverse effects and costs. In light of the current controversy, a reappraisal of how nutritional support should be used in acutely ill medical inpatients outside critical care is urgently required. The aim of this review is to discuss current pathophysiological concepts of PEM and to review the current evidence for the efficacy of nutritional support regarding patient outcomes when used in an acutely ill medical patient population outside critical care.
An area of current uncertainty
Introduction
Although nutritional support using either oral nutritional supplements (ONS) or enteral feeding is one of the most common interventions in medicine, there is no current standard algorithm for the use in unselected, polymorbid, acutely ill medical inpatients at risk of protein-energy malnutrition (PEM). In the light of recent high-quality evidence from critical care demonstrating contrasting results, either late beneficial effects [1], lack of benefit [2] or harmful effects [3, 4] when clinical nutrition is used indiscriminately and/or too aggressively, a reappraisal of how nutritional therapy should be used in medical inpatients is now required.

Figure 1
Association of PEM measured with the NRS 2002 and 30 days mortality in a 6-month observational cohort study performed in the Medical University Clinic at the Kantonsspital Aarau, Switzerland [7]. In red, 30-day mortality is shown. In black, the number of patients in different NRS risk classes are displayed.
NRS = nutritional risk screening; PEM = protein-energy malnutrition
Several considerations support the current approach of systematically screening inpatients for PEM risk and of starting nutritional therapy in at-risk patients. Epidemiological studies from various countries and healthcare settings have shown strong associations between PEM and patient outcomes. For example, an observational cohort study of nutrition practices in 167 intensive care units (ICUs) across 37 countries including 2,772 mechanically ventilated patients found that an increase of 1,000 calories per day was associated with a significant reduction in mortality (odds ratio for 60-day mortality 0.76; 95% confidence interval 0.61–0.95, p = 0.014) [5]. The authors found also similar results for protein intake, with significant associations with adverse patient outcomes. Interestingly, the effect of nutritional therapy on patient outcomes differed according to body mass index (BMI): effects were largest in patients with BMI <25 or >35 kg/m2 with only little evidence of a treatment effect in patients in the midrange (BMI 25–35). Similar results have also been reported for noncritical-care settings with significant associations of PEM (defined by the nutritional risk score [NRS]) with higher risk for complications, mortality and longer hospital length-of-stay [6]. An observational study from the Medical University Clinic of the Kantonsspital Aarau, Switzerland, found increased risk for PEM in about 30% of patients and a stepwise increase in all-cause 30-day mortality (fig. 1) [7]. In addition, several observational studies found that there was a vicious cycle, with PEM resulting in increased risk for infection that in turn reduced food intake and worsened PEM (reviewed in [8]). Multinational studies found strong associations of decreased food intake and increased all-cause mortality in hospitalised patients [9]. It has been demonstrated that inadequate dietary intake leads to dysfunction of the immune system and to mucosal damage in the gut, with risk for invasion of pathogens (translocation). In acutely ill patients nutritional status may be further aggravated by diarrhoea, vomiting, malabsorption, loss of appetite, diversion of nutrients for the immune response and urinary nitrogen loss, all of which increase nutrient losses and further damage the body`s defence mechanisms [8]. In addition, PEM is associated with adverse metabolic consequences, such as catabolism and muscle wasting (reviewed in [10]; fig. 2).
Despite these associations, causal inferences remain largely unproven and whether provision of nutritional therapy in the acute phase of illness has the potential to reverse these adverse effects associated with PEM in the noncritical-care inpatient setting remains unclear.
The aim of this review article is to discuss current pathophysiological concepts of the effects of PEM and nutritional therapy using ONS or tube feeding on patient outcomes when used in an acutely ill medical inpatient population outside critical care.
Nutritional risk screening
Nutrition screening aims at identifying patients with nutritional deficits who benefit from further detailed nutritional status assessment and nutritional therapy interventions [11]. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines state that the purpose of nutrition screening is to predict the probability of a better or worse outcome due to nutrition factors and whether nutritional treatment is likely to influence this. Optimally, patients admitted to acute-care hospitals should be screened for risk of PEM within 24 hours. There are several screening tools validated for this purpose in the acute-care setting [12]. Many institutions trigger automatic nutritional support when certain screening criteria are met for in-depth assessment of patients.
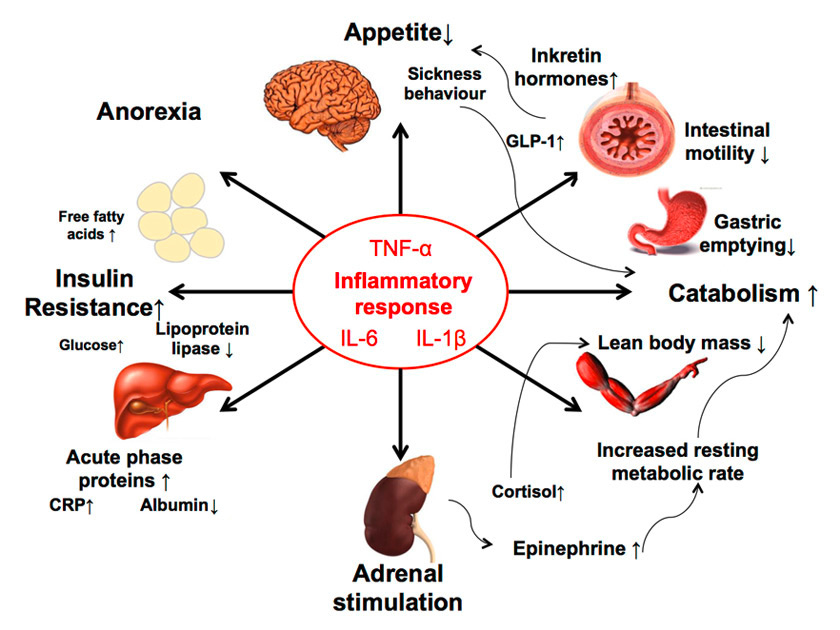
Figure 2
The complex interaction of acute illness and cachexia is mediated by various mechanisms (adapted from [10]).
CRP = C-reactive protein; GLP-1 = glucagon-like peptide-1; IL = interleukin; TNF-α = tumour necrosis factor-α
Various nutrition screening tools are used in hospitals, but many of them have not been well validated for the acute-care setting. Thus, it is unclear if they appropriately identify patients who need further nutrition assessment and potentially nutritional therapy. The most widely used tools are the Nutritional Risk Screening (NRS) 2002, the Malnutrition Universal Screening Tool (MUST), the subjective global assessment of nutritional status (SGA) and the Mini Nutritional Assessment® (MNA) (reviewed in [12]). These screening instruments differ in the variables included and patient populations to be targeted, and either assess patients for the presence of malnutrition (SGA, MNA) or for being at risk for malnutrition (MUST, NRS). Among them, the NRS 2002 is recommended by ESPEN as the preferred screening tool for hospitalised patients. The NRS 2002 was developed by Kondrup and colleagues based on a retrospective analysis of controlled trials [13]. Their premise was that the indications for nutritional support should depend on two factors: (i.) the severity of impaired nutritional status and (ii.) the increase in nutrition requirements resulting from disease (stress metabolism). For this reason, the NRS 2002 includes both a measure of current potential undernutrition and a measure of disease severity. In addition, older age is considered a risk factor, with an additional point added for age ≥70 years. A score of ≥3 is the generally accepted cut-off, which indicates the need to start nutritional therapy.
The NRS 2002 tool performed well in a validation study including 128 controlled nutrition support trials and was capable in identifying patients who would or would not benefit from nutritional intervention [13]. In fact, the likelihood ratio for a positive effect at cut-offs of 3.0 and 4.0 points were 1.7 and 5.0. As an important limitation to the medical inpatient setting, most of the included trials were surgical trials and none of them included the typical acutely ill medical inpatient population outside the ICU. In addition, a subsequent prospective, controlled trial with 212 hospitalized patients did not show significant effects of nutritional therapy in regard to mortality, hospital length-of-stay or quality of life [14]. Thus, there is remaining uncertainty about the potential of the NRS 2002 to identify patients who will benefit from nutritional therapy in the medical inpatient setting.
Pathophysiology of PEM due to acute and chronic illness
Acute and chronic illness is associated with loss of appetite and poor nutritional intake, frequently associated with a proinflammatory state, and loss of lean and adipose tissue. Cachexia is the result of various metabolic pathways and mechanisms [10, 15]. Weight loss associated with acute and chronic illness may result directly from caloric deprivation due to loss of appetite, as well as from dehydration and sarcopenia. Also, hormonal imbalance with an increase in glucocorticoid hormones and a decrease in testosterone and other sexual steroids may further enhance catabolism and aggravate PEM (fig. 2). Nonetheless, although numerous acute medical diseases are associated with cachexia, the underlying pathophysiological mechanisms remain ill defined.
Recent investigations point to the central role of cytokines in the pathogenesis of cachexia. This relationship between acute disease and cachexia may well be bidirectional, with illness affecting nutritional status, and dietary factors influencing the inflammatory response and the course of illness. Cytokines are released in the body as part of the systemic inflammatory response associated with acute illnesses and have been implicated in the aetiology of anorexia, weight loss, cognitive dysfunction, anaemia, and frailty mediated by different mechanisms. Cytokines, such as interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), influence brain circuitries that control food intake, delayed gastric emptying and skeletal muscle catabolism [16, 17]. Cytokines not only activate nuclear transcription factor κB (NF-κB) resulting in decreased muscle protein synthesis, but also reduce MyoD protein, a transcription factor that modulates signalling pathways involved in muscle development (fig. 2) [18, 19]. TNF-α and interferon-γ act synergistically to inhibit the activation of messenger ribonucleic acid (RNA) for myosin heavy chain synthesis and thereby stimulate the proteolysis of myosin heavy chains [19]. Cytokines also activate the ubiquitin-mediated proteolytic system, which plays a key role in disease-related hypercatabolism [20]. Ubiquinated proteins and subsequent muscle proteolysis provide amino acids that are consumed in hepatic synthesis of acute-phase proteins such as C-reactive protein. Additionally, cytokines are able to modulate the response of the hypothalamic-pituitary-adrenal axis at each level and stimulate the release of various stress hormones including cortisol and catecholamines, which in turn lead to an increase in resting metabolic rate [21, 22]. Other hormones that may be important for the development of cachexia are incretin hormones such as glucagon-like peptide-1 (GLP-1) released directly from gut tissues. Recent evidence found a cross-talk of inflammatory cytokines (mainly IL-6 and IL-1β) and GLP-1 and its analogues that resulted in reduced food intake and thus eventually weight loss [23]. Although this effect is beneficial in obese diabetic patients, it may negatively affect malnourished acutely ill medical patients. Synergistically, these various activated pathways during acute illness result in negative energy balance and weight loss – ultimately resulting in cachexia and PEM.
Loss of appetite may develop during hospital stays either as a consequence of an underlying medical condition (e.g., pneumonia) or medical treatments (e.g., pain medication, chemotherapy), or may pre-exist as a primary condition owing to depression, social isolation and advanced age. This distinction maybe important as it impacts the effects of nutritional interventions. As loss of appetite secondary to acute disease may be seen as an evolutionary process, there is an ongoing debate about possible biological explanations why the human body adapts in such a manner during acute disease. Interestingly, there are some preclinical and clinical studies suggesting that starvation may indeed improve cell recycling by induction of autophagy, a survival mechanism serving to recycle intracellular nutrients such as toxic protein aggregates and damaged organelles [24]. In a recent animal study, early parenteral nutrition – particularly proteins and lipids – suppressed the ubiquitin-proteasome pathway [25]. This contributes to the preservation of muscle mass, but also leads to autophagy deficiency in liver and skeletal muscle. Thus the maintenance of muscle mass might come at the price of accumulation of toxic protein aggregates that ultimately compromises cell function. A similar observation was also made in critically ill patients [26] and was recently shown to contribute to mitochondrial dysfunction, organ failure and adverse outcome in a rabbit model of critical illness [27]. Based on these observations, it is tempting to speculate that starvation during the early phase of acute illness may indeed have some beneficial effects and improves the cell recycling system. At which time point these beneficial effects may become harmful as a result of progressive catabolism and PEM remains unclear today.
|
Table 1:Overview of most recent randomised controlled trials evaluating nutritional therapy in medical inpatients. Trials using parenteral nutrition are excluded. |
|
Author, year
|
Population
and No.
|
Treatment of the nutritional intervention group
|
Treatment of the control group
|
Endpoints
|
Limitations
|
Main results
|
| Rüfenacht U, 2010 [29] |
Adult patients admitted to a general medical ward, NRS ≥3
n = 53 |
ONS 400 ml daily, 600 kcal, 24 g protein and individual nutritional counselling |
Only ONS 400 ml daily, 600 kcal, 24 g protein |
Energy and protein intake, LOS, QoL (functional assessment anorexia cancer therapy [FAACT] and VAS) |
QoL difference only at one time point, confounding by intensive nutritional care possible |
Significant increase in energy and protein-intake as well as QoL |
| Starke J, 2011 [51] |
Adult patients admitted to a general medical ward, NRS ≥3
n = 132 |
Individual nutritional care, including detailed nutritional assessment, individual food supply, fortification of meals, in-between snacks and ONS |
Standard nutritional care |
Daily energy and protein intake, weight, complications, antibiotic therapy of infectious complications, LOS, QoL, hospital readmission, mortality, compliance with supplement consumption, Levels of 25-OH-vitamin D3, ascorbic acid and glutathione |
No functional outcome assessed |
Intervention beneficial for nutritional status Qol, fewer complications and rehospitalisations |
| Potter JM, 2001
[49] |
Patients admitted to a “general medical ward for the elderly” (>60 y) any kind of nutritional status
n = 381 |
Protein energy sip feed supplement containing 1.5 kcal/ml energy intended to provide 22.5 g protein and 540 kcal/d. Three times daily 120 ml |
Normal ward diet and snacks, dietetic intervention available to all patients in the study |
Weight, arm muscle circumference, triceps skinfold thickness, BMI, 20-point Barthel ADL, survival, discharge destination, LOS, energy intake |
|
Lower mortality, better functional outcome in severely malnourished patients after intervention |
| Gariballa S, 2006 [52] |
Patients over age 65 y, medical or surgical (not GI surgery) diagnosis, any kind of nutritional status
n = 445 |
400 ml ONS in addition to standard hospital diet |
Placebo with minimal calorie content (60 kcal) |
Barthel score, nonelective readmissions, LOS, discharge destination, morbidity and mortality |
Nonsignificant results in primary endpoints |
No significant effect of intervention |
| Hickson M, 2004 [53] |
Patients ≥65 years of age, admitted to a “medicine for the elderly ward”, any kind of nutritional status
n = 592 |
Encouragement of patients, offering of snacks and drinks |
Standard hospital diet alone |
LOS, Barthel, weight, MAC, TSF, albumin, abbreviated mental test, intake, infection rate measured by antibiotic use, fluid use, in hospital mortality. |
Intervention conducted by nondieticians (healthcare assistants) |
Employing extra healthcare assistants in preventing weight loss is not effective |
| Johansen N, 2004 (12) |
Adult patients admitted for medical disease or surgical diagnosis or interventions, nutritionally at risk
n = 212 |
Nutritional team for motivation, estimation of requirements, advice and assurance of adequate nutrition |
Usual care |
LOS-NDI (nutritional discharge index) SF-36 QoL, intake of energy and protein, use of antibiotics |
Inclusion of surgical and medical patients |
No differences in rate of complications and LOS |
| ADL = activities of daily living; BMI = body mass index; d = day(s); GI = gastrointestinal; h = hours(s); LOS = length of stay; m = month(s); NRS = nutritional risk screening; ONS = oral nutritional supplement; QoL = quality of life; VAS = visual analogue scale |
Clinical trials
Different randomised controlled trials (RCTs) have investigated the effects of nutritional support using ONS and/or enteral feeding on patient outcomes in the medical inpatient setting. These trials have looked at different types of outcomes including clinical outcome (physician focus), quality of life and recovery duration (patient focus), and costs (healthcare system focus). Table 1 shows a summary of the most recent RCTs focusing on different patient populations. The two trials conducted in Switzerland [28, 29] included an individual nutritional therapy intervention aiming to improve energy and protein intake. According to individual patient's preferences and needs, hospital standard food combined with dietician counselling and interventions: fortification measures and beverages, snacks, protein powder, maltodextrin and/or ONS. The control group in both trials received either energy-dense ONS without dietician counselling [29] or standard nutritional care including ONS or nutritional therapy on request if considered necessary by the independent treating physician [28]. As a result, protein intake [28] or energy and protein intake [29] was increased in the intervention group compared with the control group during study period. This provides strong evidence that individualised nutritional counselling and support improves energy intake in malnourished medical inpatients. Owing to the small sample size, it remains unclear from these trials whether or not an increase in energy and protein intake results in improved patient outcome. Importantly, quality of life measured with either the Function Assessment Anorexia-Cancer Therapy (FAACT) tool and visual analogue scale (VAS) [29] or the SF36 questionnaire [28] was improved in the intervention group in both trials. As a limitation, improvement in quality of life may also be attributable to the intervention per se, i.e., the higher attention provided to patients during the study period.
The RCT conducted by Starke et al. [28] with a sample size of 134 patients (67 intervention group, 67 control group) found a lower rate of in-hospital complications, use of antibiotics and readmissions associated with the intervention. However, the trial failed to demonstrate a benefit in regard to mortality or hospital length-of-stay (LOS). Again, the sample size was small and the trial thus underpowered to find such effects.
Other trials used more standardised nutritional protocols instead of individualised nutritional treatment strategies. In 2006, Gariballa et al. [30] published the results from a large, randomised, double-blind, placebo-controlled trial comparing daily energy- and protein-rich ONS with a placebo drink containing a minimal amount of calories and without any protein in a geriatric acutely ill medical patient population. Overall, adherence to the intervention (ONS as well as placebo) was low, with only around 50% of the study population consuming more than half of the amount provided to them. The authors found significant changes in red-cell folate and vitamin B12 after 6 weeks, as well as lower readmission rates after 6 months, in the intervention group, whereas all other patient-relevant outcomes including LOS, disability measured with the Barthel Index, mortality and infections remained similar in both groups.
Outside the acute care medical inpatient setting, where ONS treatment resulted in only subtle improvements in patient outcomes, ONS was associated with important improvements in long-term institutionalised malnourished geriatric patients and in perioperative patients in some trials [31, 32]. But also in this setting, not all trials found such effects and the benefit of ONS remains somewhat unclear [33]. A major shortcoming in the above mentioned trials was low protocol adherence and thus nutritional targets were not met. Whether this was the main reason for the lack of significant results remains debated. Outside critical care, trials using enteral and/or parenteral nutrition to reach nutritional targets and that could shed a light on this important issue are lacking.
Current evidence from RCT data is inconsistent regarding the effectiveness of different nutritional strategies in the acutely ill inpatient setting. However, all the above-mentioned trials were relatively small, highly heterogeneous in design, patient populations and type of intervention, and lacked statistical power to demonstrate safety and, altogether, produced inconclusive results. Blinding of therapy was not performed since this is not feasible when nutritional interventions are used. This could also have resulted in an investigator bias. Unsurprisingly, two previous aggregate data meta-analyses confirm the important lack of high-quality evidence to endorse or refute nutritional support [33, 34]. Both meta-analyses, however, were based on aggregate data only and did not specifically examine the effect of early nutritional therapy in medical inpatients. Rather, one meta-analysis focused on such therapy in critical-care/perioperative patients [34], and the other only considered general protein and energy supplementation in the elderly [33]. Moreover, having been published in 2007 and 2009, respectively, neither meta-analysis captures the most recent work.
Guideline recommendations of European and American professional societies
Considerable efforts have been made to standardise nutritional treatment on an inpatient and outpatient basis. National and international consensus committees developed and published guidelines focusing each on distinct medical conditions and different indications [35–46]. The most important guidelines are from the American Society for Parenteral and Enteral Nutrition (ASPEN) and ESPEN. Most of the recommendations, however, are based on small trials in selected patient populations, and recommendations for general medical inpatients do not exist currently. Due to the lack of high quality clinical data, most guidelines give only weak recommendations and no unequivocal instructions on patient selection, the time-points when to start and stop, route, amount and duration of nutritional support. As a result and despite widespread acceptance, implementation of nutritional guidelines is still insufficient [47]. Also, the substantial differences between national and international guidelines may further slow down the widespread implementation of guidelines. In the case of renal failure, for instance, the ASPEN guidelines [37] recommend adapting energy and protein targets based on the requirements measured by indirect calorimetry and nitrogen balance. In contrast, ESPEN guidelines [38] specify general targets for micro- and macronutrients for each stage of acute and chronic renal failure. However, neither guideline gives specific recommendations about the time-point, route of delivery and amount of nutritional therapy (energy) in this situation. Importantly, malnutrition may exists before hospital admission, and develop and/or aggravate during the hospital stay, which should also influence the medical strategy for treatment of the patients in question [48, 49]. In addition, to our knowledge few published data included analysis of the barriers to the practical implementation of nutritional guidelines [50]. However, it can be assumed these would include unawareness and insufficient knowledge, ethical considerations and patient resistance among others.
Conclusions and outlook
Although the use of nutritional therapy involving dietary counselling, ONS or enteral nutrition is one of the most common interventions in medical inpatients, there is no current scientific clear evidence of its efficacy, and standard algorithms for its use in acutely ill medical inpatients at risk of PEM are generally lacking. In light of recent high-quality evidence from parenteral nutrition in critical care, a reappraisal of how nutritional support should be implemented in acutely ill medical patients is now required. The selection, timing, dose and feasibility of nutritional treatment should be evaluated as carefully as with any other therapeutic interventions, with the aim of maximising efficacy and minimising side effects and costs.
References
1 Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–93.
2 Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2013;309(20):2130–8.
3 Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17.
4 Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–97.
5 Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive care medicine. 2009;35(10):1728–37.
6 Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krahenbuhl L, Meier R, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clinical nutrition. 2008;27(3):340–9.
7 Schuetz P, Hausfater P, Amin D, Haubitz S, Fassler L, Grolimund E, et al. Optimizing triage and hospitalization in adult general medical emergency patients: the triage project. BMC Emerg Med. 2013;13(1):12.
8 Katona P, Katona-Apte J. The interaction between nutrition and infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46(10):1582–8.
9 Hiesmayr M, Schindler K, Pernicka E, Schuh C, Schoeniger-Hekele A, Bauer P, et al. Decreased food intake is a risk factor for mortality in hospitalised patients: the NutritionDay survey 2006. Clinical nutrition. 2009;28(5):484–91.
10 Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83(4):735–43.
11 Kirkland LL, Kashiwagi DT, Brantley S, Scheurer D, Varkey P. Nutrition in the hospitalized patient. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2013;8(1):52–8.
12 Anthony PS. Nutrition screening tools for hospitalized patients. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2008;23(4):373–82.
13 Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clinical nutrition. 2003;22(3):321–36.
14 Johansen N, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clinical nutrition. 2004;23(4):539–50.
15 Kubrak C, Jensen L. Malnutrition in acute care patients: a narrative review. Int J Nurs Stud. 2007;44(6):1036–54.
16 Kuhlmann MK, Levin NW. Potential interplay between nutrition and inflammation in dialysis patients. Contrib Nephrol. 2008;161:76–82.
17 Oner-Iyidogan Y, Gurdol F, Kocak H, Oner P, Cetinalp-Demircan P, Caliskan Y, et al. Appetite-regulating hormones in chronic kidney disease patients. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2011;21(4):316–21.
18 Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS, Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363–6.
19 Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(3):370–8.
20 Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–905.
21 Pende A, Musso NR, Vergassola C, Puppo F, Ioverno A, Criscuolo D, et al. Neuroendocrine effects of interferon alpha 2–a in healthy human subjects. Journal of biological regulators and homeostatic agents. 1990;4(2):67–72.
22 Schuetz P, Muller B. The hypothalamic-pituitary-adrenal axis in critical illness. Endocrinol Metab Clin North Am. 2006;35(4):823–38, x.
23 Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–9.
24 Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Critical care. 2013;17(1):302.
25 Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, et al. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology. 2012;153(5):2267–76.
26 Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Guiza F, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96(4):E633–45.
27 Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van den Berghe G, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41(1):182–94.
28 Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin Nutr. 2011;30(2):194–201.
29 Rufenacht U, Ruhlin M, Wegmann M, Imoberdorf R, Ballmer PE. Nutritional counseling improves quality of life and nutrient intake in hospitalized undernourished patients. Nutrition. 2010;26(1):53–60.
30 Gariballa S, Forster S, Walters S, Powers H. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am J Med. 2006;119(8):693–9.
31 Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet. 1990;335(8696):1013–6.
32 Larsson J, Unosson M, Ek AC, Nilsson L, Thorslund S, Bjurulf P. Effect of dietary supplement on nutritional status and clinical outcome in 501 geriatric patients – a randomised study. Clin Nutr. 1990;9(4):179–84.
33 Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane database of systematic reviews. 2009; (2): CD003288.
34 Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007;102(2):412–29; quiz 68.
35 Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr. 2006;25(2):245–59.
36 August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33(5):472–500.
37 Brown RO, Compher C. A.S.P.E.N. clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr. 2010;34(4):366–77.
38 Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, et al. ESPEN Guidelines on Parenteral Nutrition: adult renal failure. Clin Nutr. 2009;28(4):401–14.
39 Choban P, Dickerson R, Malone A, Worthington P, Compher C. A.S.P.E.N. Clinical Guidelines: Nutrition Support of Hospitalized Adult Patients With Obesity. JPEN J Parenter Enteral Nutr. 2013.
40 McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr. 2013;37(1):23–36.
41 Mueller C, Compher C, Ellen DM. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16–24.
42 Plauth M, Cabre E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25(2):285–94.
43 Sobotka L, Schneider SM, Berner YN, Cederholm T, Krznaric Z, Shenkin A, et al. ESPEN Guidelines on Parenteral Nutrition: geriatrics. Clin Nutr. 2009;28(4):461–6.
44 Vanek VW, Borum P, Buchman A, Fessler TA, Howard L, Jeejeebhoy K, et al. A.S.P.E.N. position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2012;27(4):440–91.
45 Vanek VW, Matarese LE, Robinson M, Sacks GS, Young LS, Kochevar M. A.S.P.E.N. position paper: parenteral nutrition glutamine supplementation. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2011;26(4):479–94.
46 Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, Milne A, et al. ESPEN Guidelines on Enteral Nutrition: Geriatrics. Clin Nutr. 2006;25(2):330–60.
47 Tangvik RJ, Guttormsen AB, Tell GS, Ranhoff AH. Implementation of nutritional guidelines in a university hospital monitored by repeated point prevalence surveys. Eur J Clin Nutr. 2012;66(3):388–93.
48 Guest JF, Panca M, Baeyens JP, de Man F, Ljungqvist O, Pichard C, et al. Health economic impact of managing patients following a community-based diagnosis of malnutrition in the UK. Clin Nutr. 2011;30(4):422–9.
49 Schneider SM, Veyres P, Pivot X, Soummer AM, Jambou P, Filippi J, et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr. 2004;92(1):105–11.
50 Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Lindorff K, Jorgensen LM, et al. A method for implementation of nutritional therapy in hospitals. Clin Nutr. 2006;25(3):515–23.
51 Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin Nutr. 2011;30(2):194–201.
52 Gariballa S, Forster S, Walters S, Powers H. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am J Med. 2006;119(8):693–9.
53 Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, et al. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in-patients? – a randomised control trial. Clin Nutr. 2004;23(1):69–77.

