Figure 1
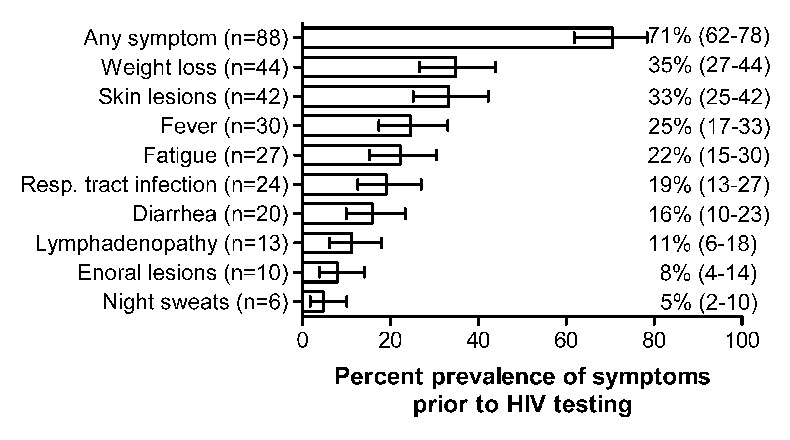
Prevalence of symptoms prior to the HIV test among the 125 late presenters. Error bars represent the 95% exact binomial confidence intervals.
HIV = human immunodeficiency virus
DOI: https://doi.org/10.4414/smw.2014.13961
Presenting late with HIV-1
Untreated infection with the human immunodeficiency virus (HIV) progressively destroys the immune system leading to opportunistic illnesses and death. Since potent combination antiretroviral therapy (cART) has been introduced, morbidity and mortality of HIV-infected people has drastically improved [1–3]. However, a substantial proportion of individuals are not aware of their HIV infection or do not present for care and treatment until the disease is advanced [4]. Late initiation of cART results in less favourable outcomes [5–7] and is associated with increased medical costs [8]. Furthermore, untreated people may contribute to the spread of HIV for many years. In Europe, 33% to 42% and 49% to 54% of individuals were reported not to be diagnosed with HIV until having CD4 cell count values below 200 cells/µl [9–12] and 350 cells/µl [4, 13, 14], respectively, and up to 30% present with an acquired immunodeficiency syndrome (AIDS) defining illness [15]. Unfortunately, different definitions for late presentation complicate direct comparisons of the study findings [16]. A recent initiative resulted in a European consensus definition for late presentation of HIV-infected persons: Individuals presenting for care with a CD4 cell count below 350 cells/µl or with an AIDS-defining illness regardless of CD4 cell count should be classified as late presenters [16]. Late presentation for care comprises two entities which are believed to be quite different from each other in terms of risk factors and interventions: (i.) late HIV testing, which reflects patients who are unaware of their HIV infection, and (ii.) delayed presentation for care, including individuals who are aware of their HIV infection but do not seek care right away.
The aim of this study was to apply this new consensus definition to individuals entering care in a large HIV outpatient clinic and to identify reasons for late HIV testing or presentation for care through a detailed chart review. The structured questionnaire developed during this study could then serve for further prospective studies.
We selected patients from the University Hospital Zurich, the largest centre of the Swiss HIV Cohort Study (SHCS). The SHCS is a nationwide, multicentre cohort with continuous enrollment of HIV-infected individuals and semiannual study visits [17]. The responsible ethics committees of each study centre approved this cohort study and written informed consent was obtained from all individuals included. The retrospective chart review was approved by the Kantonale Ethikkomission Zürich (KEK-ZH-Nr. 2011–0455). For this study we included all individuals who were enrolled in the SHCS from January 2009 to December 2011 at the HIV outpatient clinic of the Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich with available CD4 cell count at enrollment. Persons with CD4 cell counts below 350 cells/µl or with an AIDS-defining illness were considered to be potential late presenters. The other individuals served as a comparison group. During the data collection it became clear that the group of potential late presenters included a considerable number of persons with acute HIV infection whose CD4 cell counts were below 350 cells/µl at the time of enrollment. To ascertain that no individuals with acute HIV infection were classified as late presenter or not late presenter we matched data with the database of the Zurich Primary HIV Infection Study (ZPHI; ClinicalTrials.gov identifier, NCT00537966). This study includes all patients of the HIV outpatient clinic of the Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich with acute or recent HIV-1 infection, defined as (i.) acute retroviral syndrome and negative or indeterminate Westernblot in the presence of a positive p24 antigen and/or detectable plasma HIV-1 ribonucleic acid (RNA), (ii.) documented seroconversion with or without symptoms within 90 days, or (iii.) possible acute seroconversion syndrome (ARS), positive Westernblot and detectable HIV-RNA, and a negative HIV-gp120 avidity, respectively detuned assay. We thus analysed three groups of individuals: patients with an acute or recent HIV infection, chronically infected not late presenters, and late presenters. Demographic characteristics and laboratory results were obtained from the SHCS database.
The development of the questionnaire was partially based on the questionnaire of the HIV Denmark initiative (courtesy of Prof. Jens D. Lundgren, Copenhagen HIV Programme) which has been extended and adapted as a result of a pilot chart review of late presenting patients and feedback from the community. For a report on this project see http://www.hiv-danmark.dk/fileadmin/user_upload/hiv-danmark/pdf/Late_presenters-FINAL.pdf. As the questionnaire also had to be suitable for further studies with prospective patient interviews, we included items which were unlikely to be adequately documented in the clinical records, such as symptoms and structural factors at the time of the first HIV test, fear and emotional response to HIV infection, stigma and individual risk appraisal. Information about the following items was included for further analysis: (i.) setting in which the first positive HIV test was performed, (ii.) reasons for the performance of the test, (iii.) HIV-related symptoms before first HIV test, (iv.) number of physicians seen because of the symptoms before first positive HIV test, and (v.) duration between first positive test and entering into medical care.
Diagnostic procedures for newly diagnosed HIV-1 infections include a genotypic resistance test to detect potentially transmitted drug resistance mutations, which have to be considered when choosing the initial antiretroviral treatment. As recently described by researchers from the SHCS (www.shcs.ch) and the Zurich Primary HIV Infection Study (ZPHI; ClinicalTrials.gov identifier, NCT00537966), the results from these resistance tests can also be used to estimate the duration of the HIV infection by determining the fraction of ambiguous nucleotides [18]. In bulk sequencing, ambiguous nucleotide calls arise when different viral strains do not permit a precise determination of the nucleotide at a specific position. The fraction of ambiguous nucleotides thus reflects the polymorphism of the HIV population within a patient and is an indirect measure of the age of infection. To evaluate the suitability of ambiguity scores for distinguishing the three patient groups, we applied this method for all individuals who had results from enrollment resistance tests available from the SHCS resistance database.
We used EpiData V3.1 (http://www.epidata.dk, The EpiData Association, Odense Denmark) for data collection and Stata/SE 13.1 (StataCorp, College Station, Texas, USA) for analyses. Comparisons across groups for categorical variables were done with Chi-square tests, or Fisher's exact test if an expected cell count was less than five. Continuous variables were analysed with Wilcoxon rank-sum tests (two groups) or Kruskal-Wallis tests (three groups). Factors associated with late presentation compared with the combined groups of not late presentation and acute HIV infection were further analysed with univariable and multivariable logistic regression. Results of the logistic regression were reported as odds ratios (ORs) with 95% confidence intervals (CIs). We considered sex, transmission categories (men who have sex with men [MSM] and heterosexual intercourse), living in a stable partnership, ethnicity, age (<30, 30–39, 40–49, 50+ years) and educational level in the models. Transmission categories of injection drug use (IDU) and of other risks were excluded from the logistic regression because of small numbers. We allowed for interactions between sex, transmission category and type of partnership by creating a combined variable with six levels: (i.) MSM without stable partner, (ii.) male heterosexual without stable partner, (iii.) female heterosexual without stable partner, (iv.) MSM with stable partner, (v.) male heterosexual with stable partner, and (vi.) female heterosexual with stable partner. Patients were defined as living in stable partnerships if they answered affirmatively on the question of whether they had had a stable partnership for the preceding 6 months in an interviewer-administered questionnaire at a regular SHCS follow-up visit. A p-value <0.05 was considered statistically significant.
| Table 1:Characteristics of the 281 individuals enrolled at the Zurich centre of the Swiss HIV Cohort Study between 01 January 2009 and 07 December 2011 and included in this analysis. Late presenters (n = 125) and people with acute HIV infection (n = 21) had CD4 cell counts below 350 cells/µ or AIDS at enrollment. The remaining 135 persons represent the comparison group. | ||||||
| Not late | Late | Acute HIV infection | Total | p-value1 | p-value2 | |
| Total individuals included | 135 (48) | 125 (45) | 21 (7) | 281 (100) | – | – |
| Sex: Male Female | 112 (83) 23 (17) | 97 (78) 28 (22) | 21 (100) 0 (0) | 230 (82) 51 (18) | 0.027 | 0.348 |
| Median age at HIV diagnosis (IQR) [years] | 34 (29–42) | 38 (31–46) | 34 (30–38) | 35 (30–44) | 0.010 | 0.004 |
| Risk group: Heterosexual Male Female IDU Male Female MSM Other | 30 (22) 9 (7) 21 (15) 4 (3) 3 (2) 1 (1) 98 (73) 3 (2) | 49 (39) 25 (20) 24 (19) 4 (3) 2 (1) 2 (1) 67 (54) 5 (4) | 4 (19) 4 (19) 0 (0) 0 (0) 0 (0) 0 (0) 17 (81) 0 (0) | 83 (29) 38 (13) 45 (16) 8 (3) 5 (2) 3 (1) 182 (65) 8 (3) | 0.0103 | 0.0073 |
| Ethnicity: Caucasian African Hispanic Asian | 109 (81) 9 (7) 10 (7) 7 (5) | 90 (72) 15 (12) 6 (5) 14 (11) | 14 (67) 4 (19) 3 (14) 0 (0) | 213 (76) 28 (10) 19 (7) 21 (7) | 0.052 | 0.102 |
| Education: School not completed Mandatory school Apprenticeship Higher professional education University degree | 4 (3) 14 (10) 68 (51) 18 (13) 31 (23) | 10 (8) 19 (15) 47 (37) 27 (22) 22 (18) | 2 (9) 1 (5) 9 (43) 1 (5) 8 (38) | 16 (6) 34 (12) 124 (44) 46 (16) 61 (22) | 0.032 | 0.039 |
| Stable partner: Yes No | 84 (62) 51 (38) | 74 (59) 51 (41) | 13 (62) 8 (38) | 171 (61) 110 (39) | 0.912 | 0.703 |
| First CD4 cell count: Median (IQR) [cells/µl] <100 100–199 200–349 350–499 500+ Missing | 471 (376–580) 0 (0) 0 (0) 0 (0) 55 (40) 59 (44) 214 (16) | 169 (55–261) 43 (34) 36 (29) 46 (37) 0 (0) 0 (0) 0 (0) | 246 (193–278) 0 (0) 6 (29) 15 (71) 0 (0) 0 (0) 0 (0) | 300 (152–447) 43 (15) 42 (15) 61 (22) 55 (20) 59 (21) 21 (7) | <0.001 | <0.001 |
| AIDS-defining illness at HIV diagnosis | 0 (0) | 35 (28) | 1 (5) | 36 (11) | <0.001 | <0.001 |
| First HIV RNA value5 Median (IQR) [log10 copies/ml] | 4.5 (4.0–5.0) | 5.0 (4.5–5.4) | 5.7 (5.2–6.6) | 4.8 (4.3–5.3) | <0.001 | <0.001 |
| Known source of infection6 | 93 (70) | 73 (60) | 18 (86) | 184 (67) | 0.035 | 0.114 |
| AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus; lDU = injection drug use; IQR = interquartile range; MSM = men who have sex with men; RNA = ribonucleic acid Numbers in parentheses are percentages unless otherwise stated. 1 p-values for differences across all three groups from Fisher's exact tests for categorical variables and Kruskal-Wallis tests for continuous variables. 2 p-values for differences between late and non-late presenters from Fisher's exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. 3 This p-value represents the comparison across all six groups. 4 CD4 values at HIV diagnosis not available for 21 patients in care elsewhere prior to enrollment. 5 HIV-1 RNA values from individuals not on antiretroviral therapy. 6 Based on 276 individuals with available information. | ||||||
From 01 January 2009 to 07 December 2011, 318 patients were enrolled in the Zurich centre of the SHCS and had a CD4 cell count available. The chart review revealed that 37 individuals did not qualify as late presenters, mostly because referral letters showed that they had been in care for a longer period elsewhere prior to enrollment in the SHCS. These people were excluded from the analysis since we had no information about their initial CD4 cell counts. Of the remaining 281 patients, 146 (52%) had a CD4 cell count below 350 cells/µl and/or an AIDS-defining disease at registration and were potential late presenters. However 21 (14%) of these potential late presenters were found to have a low CD4 cell count during the acute phase of HIV infection. We therefore analysed three groups consisting of 125/281 (45%) persons identified as true late presenters, 135/281 (48%) as not late presenters and 21/281 (7%) as acutely infected individuals.

Figure 1
Prevalence of symptoms prior to the HIV test among the 125 late presenters. Error bars represent the 95% exact binomial confidence intervals.
HIV = human immunodeficiency virus
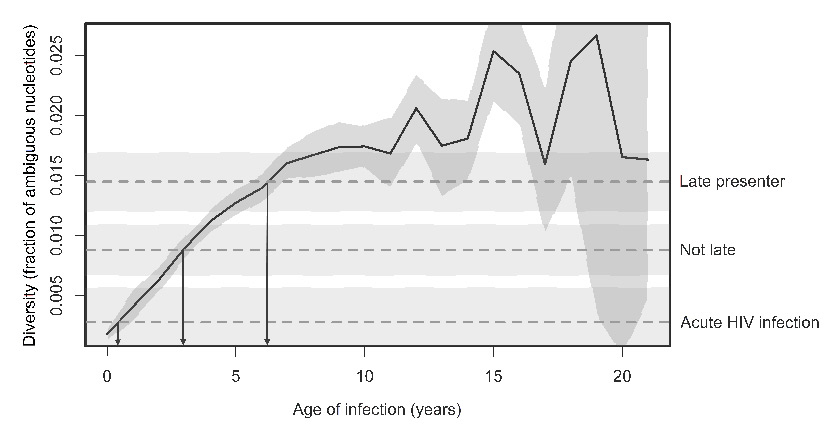
Figure 2
Relationship between the year of infection and the fraction of ambiguous nucleotides. The solid black line represents the mean fraction as a function of the age of infection, where data points have been binned according to the age of infection in years (n = 3,307 patients). (Figure adapted from [18] Roger D. Kouyos et al.: Ambiguous Nucleotide Calls From Population-based Sequencing of HIV-1 are a Marker for Viral Diversity and the Age of Infection, Clinical Infectious Diseases, 2011, 52(4):532–539, by permission of Oxford University Press.) The dashed horizontal lines depict the mean fraction from the three groups of our study and the shaded area represents the 95% confidence intervals. The three arrows from the intersections of the curve with the horizontal lines point to estimated durations of HIV infection of approximately 6, 3, and <1 years for the three groups.
HIV = human immunodeficiency virus
The characteristics of these 281 individuals are summarised in table 1. Patients were primarily male (82%) and Caucasian (76%). Late presentation was more frequent among females than males (55% vs 42%, respectively), in heterosexually infected individuals (59%) compared with other risk groups (38%), and in the African and Asian populations (54% and 67%, respectively) compared with Caucasians (42%), but less common in Hispanics (32%). The frequency of late presentation was low among individuals with a university degree or a completed apprenticeship (36% and 38%, respectively) and high among individuals who didn't finish school or had only completed mandatory school (62% and 56%, respectively). However patients with higher professional education showed quite a high percentage of late presentation (59%).
The 21 individuals with acute HIV infection selected in this study were all male and predominantly MSM (81%). Noteably, 38% had a university degree compared with only 18% among the late presenters. CD4 cell counts of persons with acute HIV infection were substantially below 350 cells/µl, but significantly higher than late presenters (246 [interquartile range {IQR} 193–278] vs 169 [IQR 55–261] cells/µl). Of the latter group, 43 and 36 individuals had CD4 cell counts below 100 and from 100 to 199 cells/µl, respectively, resulting in a total of 79/125 (63%) late presenters with fewer than 200 cells/µl.
The chart review of the 125 late presenters revealed that 75 (60%) had their first positive HIV test performed in a private practice. Six patients (4.8%) had their first test at an anonymous testing venue and three women (2.4%) were diagnosed during pregnancy screening. A total of 26 (21%) of the positive tests were performed in a hospital setting and two (1.6%) were incidental findings in the context of a blood donation. Only very few patients (6.4%) had their first positive test performed outside of Switzerland. In 60% of individuals the test was performed following a physician's suggestion, in 7.2% each because of a specific risk situation and characteristic symptoms and in 6.4% because the partner had been tested HIV positive.
Potentially HIV-related symptoms prior to the test were present in 88 (70%) patients. The prevalence of the most frequent symptoms is shown in figure 1. A total of 79 patients (63%) consulted a physician because of these symptoms. Before an HIV test was performed, a median of two different physicians (IQR 1–3) were seen because of the symptoms. However, once HIV was diagnosed, the majority (88%) of individuals entered medical care within 1 month, and only 4.8% waited for 1 year or more.
Data about the fraction of ambiguous nucleotides was available in 71 (53%) of the control group, in 65 (52%) of true late presenters and 15 (71%) of patients with acute HIV infection. Ambiguity scores were highest in late presenters (0.014, 95% CI 0.012–0.17)), followed by not late presenters (0.007, 95% CI 0.005–0.010) and lowest for persons with acute HIV infection (0.003, 95% CI 0.000–0.006). All pairwise comparisons gave a p <0.01 (Wilcoxon rank-sum tests). As shown by the arrows in figure 2, these scores correspond to estimated durations of HIV infection of approximately 6, 3, and <1 years for the three groups.
We analysed risk factors for late presentation in 265 individuals infected via homo- or heterosexual contacts, using the combined groups of not late presenters and acutely HIV-infected persons as comparator. Results from univariable and multivariable logistic regression analyses are shown in table 2. Compared with younger individuals (age <30 years), persons above 50 years of age had 3.16–fold increased odds (95% CI 1.23‒8.17, p = 0.017), and Asian people, as compared with Caucasians, had 3.5–fold increased odds of presenting late (95% CI 1.21–10.14, p = 0.021). Compared with MSM without stable partnership, MSM in a stable partnership were significantly less likely to present late (OR 0.50, 95% CI 0.27–0.95, p = 0.034), whereas heterosexual men in a stable partnership had 2.72–fold increased odds to present late (95% CI 1.004–7.37, p = 0.049).
| Table 2: Logistic regression analyses of cofactors associated with late presentation (n = 116) compared with the combined groups of not late presentation and acute HIV infection (n = 149). Persons infected via injection drug use (n = 8) and other routes (n = 8) were omitted. | ||
| Univariable logistic regression | Multivariable logistic regression | |
| Factor | OR (95% CI) p-value | OR (95% CI) p-value |
| Interaction1 of sex, transmission category and partnership: Without stable partner MSM Male heterosexual Female heterosexual With stable partner MSM Male heterosexual Female heterosexual Ethnicity: Caucasian Afroamerican Hispanoamerican Asian Age group (years): <30 30–39 40–49 50+ Education: Apprenticeship School not completed Mandatory school Higher professional education University degree | 1.00 (reference) 1.25 (0.34–4.65) 0.739 1.25 (0.37–4.21) 0.719 0.55 (0.30–1.02) 0.057 3.13 (1.23–7.92) 0.016 1.5 (0.67–3.38) 0.329 1.00 (reference) 1.64 (0.72–3.74) 0.235 0.54 (0.19–1.58) 0.262 2.82 (1.09–7.29) 0.032 1.00 (reference) 0.97 (0.51–1.83) 0.914 1.46 (0.73–2.92) 0.282 3.05 (1.26–7.4) 0.013 1.00 (reference) 2.62 (0.87–7.85) 0.086 2.47 (1.08–5.66) 0.032 2.29 (1.13–4.64) 0.021 1.04 (0.54–1.98) 0.912 | 1.00 (reference) 0.98 (0.25–3.95) 0.984 0.90 (0.23–3.53) 0.883 0.50 (0.27–0.95) 0.034 2.72 (1.004–7.37) 0.049 1.05 (0.40–2.74) 0.923 1.00 (reference) 1.33 (0.49–3.57) 0.576 0.72 (0.24–2.21) 0.566 3.5 (1.21–10.14) 0.021 1.00 (reference) 0.94 (0.47–1.86) 0.853 1.49 (0.71–3.12) 0.295 3.16 (1.23–8.17) 0.017 Not included2 |
| CI = confidence interval; HIV = human immunodeficiency virus; MSM = men who have sex with men; OR = odds ratio 1 To allow for interactions between sex, transmission category and type of partnership we created a combined variable with six levels: (i.) MSM without stable partner (reference), (ii.) male heterosexual without stable partner, (iii.) female heterosexual without stable partner, (iv.) MSM with stable partner, (v.) male heterosexual with stable partner, (vi.) female heterosexual with stable partner. 2 Education was not included in the multivariable analysis because of collinearity with transmission category and sex: The education level of MSM was on average one category higher. | ||
A total of 45% of the 281 newly enrolled HIV-infected individuals in our clinic between 01 January 2009 and 07 December 2011 were late presenters according to the European consensus definition, with a CD4 cell count threshold of below 350 cells/µl or an AIDS-defining illness [16]. This is considerably less than in the reports from Germany, England and New Zealand with 49.5%, 49% and 50%, respectively [13, 14, 19]. COHERE, a large European collaborative study involving over 80,000 patients from 35 countries recently even found a prevalence of 52% late presenters in 2010/2011 [4]. Without a careful chart review, we would have included 21 persons who presented with acute HIV infection, resulting in 146 of 281 persons (52%). Although the consensus definition advises repeating CD4 cell count determinations to exclude transient lymphopenia, such confirmation is not mandatory and may explain some of the variations between reports with data sources from different settings.
With routinely performed genotypic resistance tests we demonstrated that the fraction of ambiguous nucleotides [18] successfully discriminated the three groups in our study and that it is a valid tool for further epidemiological analyses.
The analysis of risk factors for late presentation revealed that the HIV transmission categories, sex, and partnership status, are not independent. We created a combined variable of the above characteristics, which allowed us to dissect differential effects. For example, MSM in a stable partnership are much less likely to present late than heterosexual men in a stable partnership. This finding is in concordance with a French study where, among non-migrants, longstanding heterosexual couples had the highest risk for late presentation [10]. Similarly, the association of late presentation with older age has been described in several other studies [10, 12, 13, 20, 21]. Furthermore, a higher risk for late presentation in immigrants and ethnic groups other than Caucasians has been shown previously [9, 11, 12, 20]. Whereas Africans and Hispanics showed no difference in late presentation compared to Caucasians in our study, Asians were at significantly higher risk for late presentation.
The chart review revealed that the majority of late presenting patients had symptoms and were diagnosed in private practices. Only 7.2% of individuals specifically asked for an HIV test because of the symptoms and a further 7.2% because they were aware of a risk situation. The fact that in our study almost 90% entered medical care within 1 month after diagnosis shows that access to healthcare is excellent in Switzerland. A study on delayed entry into HIV medical care between 2000 and 2004 in the United States found that 28% of newly diagnosed persons had a delay of more than 3 months [22]. However, we also found that the HIV risk perception of late presenters was suboptimal, as more than half of them were tested after a physician's suggestion and fewer than 10% because of a perceived risk situation. Thus, late presentation in Switzerland seems to be driven by late HIV testing as opposed to delayed presentation for care after a positive HIV test.
The small number of people infected via injection drug use in our study of newly enrolled patients since 2009 documents the success of the harm reduction programmes such as needle exchange and opiate substitution programmes since 1985 in Switzerland. The numbers of injection drug users as well as people infected via other transmission routes were too small to be included in the analysis of risk factors.
The major limitation of the chart review was the large variation in the level of detail in the medical histories. Anamnestic information was often unstructured and did not cover information about knowledge, individual risk appraisal, fear, the role of stigma and other emotional reactions to a potential HIV infection. We therefore recently started a larger multicentre study in which late presenters and not late presenters are interviewed prospectively regarding the circumstances of their HIV infection and diagnosis. A further potential limitation is the fact that not all HIV-infected individuals agreed to participate in the SHCS. However, a recent survey showed that the SHCS includes 84% of all HIV-infected persons treated at the study centres [23].
Our findings suggest that delayed entry into medical care after an HIV diagnosis is rare. The frequency of late testing, however, could be improved by promoting awareness among older individuals and especially heterosexual men in stable partnerships. Such strategies might complement the current testing policies aimed at populations and persons at risk.
The members of the Swiss HIV Cohort Study are:
Aubert V, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Gorgievski M, Günthard H (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos R, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, Rauch A (Chairman of the Scientific Board), Regenass S, Rickenbach M (Head of Data Centre), Rudin C (Chairman of the Mother & Child Substudy), Schöni-Affolter F, Schmid P, Schultze D, Schüpbach J, Speck R, Staehelin C, Tarr P, Telenti A, Trkola A, Vernazza P, Weber R, Yerly S.
1 Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ. 1997;315(7117):1194–9. PubMed PMID: 9393221; PubMed Central PMCID: PMCPMC2127760.
2 Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. PubMed PMID: 9516219.
3 Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–9. PubMed PMID: 12853195.
4 Mocroft A, Lundgren JD, Sabin ML, Monforte AdA, Brockmeyer N, Casabona J, et al. Risk Factors and Outcomes for Late Presentation for HIV-Positive Persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013;10(9):e1001510. doi: 10.1371/journal.pmed.1001510.
5 Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–8. doi: 10.1097/QAD.0b013e3282f7cb76. PubMed PMID: 18427202; PubMed Central PMCID: PMCPMC3618460.
6 Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8(2):88–97. PubMed PMID: 16848276.
7 Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1–infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140–6736(09)60612–7. PubMed PMID: 19361855; PubMed Central PMCID: PMCPMC2670965.
8 Krentz HB, Gill MJ. The Direct Medical Costs of Late Presentation (<350/mm) of HIV Infection over a 15–Year Period. AIDS Res Treat. 2012;2012:757135. doi: 10.1155/2012/757135. PubMed PMID: 21904673; PubMed Central PMCID: PMCPMC3166713.
9 Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis and consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000–2004). AIDS. 2006;20(18):2371–9. doi: 10.1097/QAD.0b013e32801138f7. PubMed PMID: 17117024.
10 Delpierre C, Dray-Spira R, Cuzin L, Marchou B, Massip P, Lang T, et al. Correlates of late HIV diagnosis: implications for testing policy. Int J STD AIDS. 2007;18(5):312–7. doi: 10.1258/095646207780749709. PubMed PMID: 17524190; PubMed Central PMCID: PMCPMC2486458.
11 Borghi V, Girardi E, Bellelli S, Angeletti C, Mussini C, Porter K, et al. Late presenters in an HIV surveillance system in Italy during the period 1992–2006. J Acquir Immune Defic Syndr. 2008;49(3):282–6. doi: 10.1097/QAI.0b013e318186eabc. PubMed PMID: 18845959.
12 Wolbers M, Bucher HC, Furrer H, Rickenbach M, Cavassini M, Weber R, et al. Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med. 2008;9(6):397–405. doi: 10.1111/j.1468–1293.2008.00566.x. PubMed PMID: 18410354.
13 Iwuji CC, Churchill D, Gilleece Y, Weiss HA, Fisher M. Older HIV-infected individuals present late and have a higher mortality: Brighton, UK cohort study. BMC Public Health. 2013;13:397. doi: 10.1186/1471–2458–13–397. PubMed PMID: 23622568; PubMed Central PMCID: PMCPMC3651303.
14 Zoufaly A, an der Heiden M, Marcus U, Hoffmann C, Stellbrink H, Voss L, et al. Late presentation for HIV diagnosis and care in Germany. HIV Med. 2012;13(3):172–81. doi: 10.1111/j.1468–1293.2011.00958.x. PubMed PMID: 22093171.
15 Baratin D, Marceillac E, Trepo C, Cotte L, Peyramond D, Chidiac C, et al. Characteristics of patients diagnosed with AIDS shortly after first detection of HIV antibodies in Lyon University hospitals from 1985 to 2001. HIV Med. 2004;5(4):273–7. doi: 10.1111/j.1468–1293.2004.00220.x. PubMed PMID: 15236616.
16 Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61–4. doi: 10.1111/j.1468–1293.2010.00857.x. PubMed PMID: 20561080.
17 Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Günthard HF, Telenti A, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39(5):1179–89. doi: 10.1093/ije/dyp321. PubMed PMID: 19948780.
18 Kouyos RD, von Wyl V, Yerly S, Böni J, Rieder P, Joos B, et al. Ambiguous nucleotide calls from population-based sequencing of HIV-1 are a marker for viral diversity and the age of infection. Clin Infect Dis. 2011;52(4):532–9. doi: 10.1093/cid/ciq164. PubMed PMID: 21220770; PubMed Central PMCID: PMCPMC3060900.
19 Dickson N, McAllister S, Sharples K, Paul C. Late presentation of HIV infection among adults in New Zealand: 2005–2010. HIV Med. 2012;13(3):182–9. doi: 10.1111/j.1468–1293.2011.00959.x. PubMed PMID: 22093231.
20 Lemoh C, Guy R, Yohannes K, Lewis J, Street A, Biggs B, et al. Delayed diagnosis of HIV infection in Victoria 1994 to 2006. Sex Health. 2009;6(2):117–22. doi: 10.1071/SH08028. PubMed PMID: 19457290.
21 Mojumdar K, Vajpayee M, Chauhan NK, Mendiratta S. Late presenters to HIV care and treatment, identification of associated risk factors in HIV-1 infected Indian population. BMC Public Health. 2010;10:416. doi: 10.1186/1471–2458–10–416. PubMed PMID: 20626905; PubMed Central PMCID: PMCPMC2912818.
22 Reed JB, Hanson D, McNaghten AD, Bertolli J, Teshale E, Gardner L, et al. HIV testing factors associated with delayed entry into HIV medical care among HIV-infected persons from eighteen states, United States, 2000–2004. AIDS Patient Care STDS. 2009;23(9):765–73. doi: 10.1089/apc.2008.0213. PubMed PMID: 19694550.
23 Thierfelder C, Weber R, Elzi L, Furrer H, Cavassini M, Calmy A, et al. Participation, characteristics and retention rates of HIV-positive immigrants in the Swiss HIV Cohort Study. HIV Med. 2012;13(2):118–26. doi: 10.1111/j.1468–1293.2011.00949.x. PubMed PMID: 22107170.
Funding / potential competing interests: Part of the collection of patient data and resistance test results for this work has been performed within the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant # 134277).