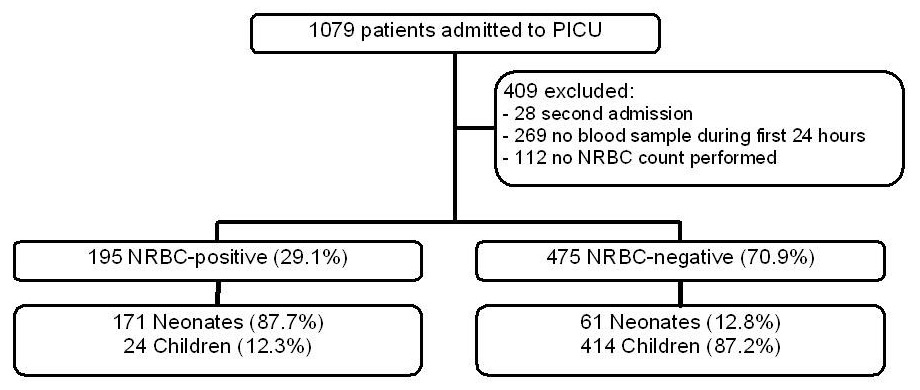
Figure 1
Inclusion and exclusion criteria.
NRBC = nucleated red blood cell; PICU = paediatric intensive care unit
DOI: https://doi.org/10.4414/smw.2014.13944
Nucleated red blood cells (NRBCs) are a normal finding in the peripheral blood of the foetus and neonate. The more premature an infant, the higher the NRBC count at birth [1, 2]. The normal NRBC count in the term infant is around 0.1–0.2x109/l (0.1–0.2 G/l). Beyond the neonatal period, NRBCs are no longer detectable in the peripheral blood [1, 2]. In neonates, the NRBC count correlates with the severity of chronic prepartum and acute intrapartum states of impaired oxygen transport [1–5]. In healthy infants, children and adults NRBCs are not present in the peripheral blood [6]. In critically ill adult patients, the presence of NRBCs is a strong predictor of mortality [6, 7]. In these patients, NRBCs may be considered as a parameter that sums hypoxic and inflammatory injuries [6]. It has been shown in children and in adults that there is an association between NRBCs and length of cardiopulmonary bypass [8, 9], and NRBCs have been found shortly after cardiopulmonary arrest in children [10]. There is no study on the prognostic value of NRBC in the paediatric population beyond the neonatal period.
We undertook this study to investigate if there is an association between the appearance of NRBCs in the peripheral blood smear of critically ill paediatric patients and bad outcomes.
This prospective observational study was performed in the multidisciplinary 18 bed tertiary neonatal-paediatric intensive care unit (PICU) at the University Children’s Hospital in Zurich, from January to December 2008. There are about 1,000 admissions a year. In the neonatal age group the unit admits mainly term, outborn infants with cardiac or surgical problems.
Ethical approval to conduct this study was granted by the Ethical Committee of Canton Zurich (reference number: StV-01/08).
We included all patients admitted to PICU and excluded patients who were admitted for the second time. In the neonatal age group, 24% of infants were premature (<36 6/7 weeks of gestation) at PICU admission.
Blood samples for measurement of absolute counts of NRBCs, leucocytes and platelets, as well as haemoglobin concentration, were taken during the first 24 hours of PICU admission. We took the first results performed (not the worst or a second one). Blood counts were measured using a Sysmex XE-2100 blood analyser (Sysmex, Horgen, Switzerland). NRBCs were expressed as an absolute number per litre and not per 100 white blood cells because of the risk of confounding effects related to the leucocyte count. Processes that increase the leucocyte count will result in a misleadingly low value of NRBCs when reported relative to the white blood cell count, and processes that decrease leucocyte count will produce misleadingly high NRBC counts [11]. NRBC positivity was defined as an absolute count >0. There was a special arrangement to measure the absolute count of NRBCs during the period of data collection, because NRBC counts had not been routinely performed before this study.
Demographic, diagnostic, severity of illness and outcome parameters referred to the entire stay in PICU.
Demographic parameters: age and sex.
Diagnostic variables: diagnosis was coded according to the ANZPIC registry [12]. We referred to the main diagnosis; each patient had only one diagnosis. Some diagnoses were represented by a very low number of patients so we collapsed the diagnoses to three groups as follows: postprocedural, cardiac surgery open and cardiovascular; gastrointestinal, renal and respiratory; neurological, injury and miscellaneous. We analysed the diagnostic subgroup “post open cardiac surgery” separately and therefore excluded it from the “postprocedural” diagnostic group.
Severity of illness variable: expected PICU mortality (PIM2: Paediatric Index of Mortality [13]). Length of stay (LOS) in PICU was regarded as a separate patient characteristic.
Outcome parameters: observed PICU mortality, mechanical ventilation (invasive as well as noninvasive ventilation / continuous positive airway pressure [CPAP]), renal replacement therapy and use of inotropic support (none, one, or multiple drugs).
All these parameters were drawn from the electronic dataset of the PICU.
The aim of this study was to investigate whether there is an association between the presence and/or the number of NRBCs in the peripheral blood and outcomes in critically ill children. In addition to NRBCs, other possible explanatory variables for outcomes were analysed, such as demographic, diagnostic, illness severity and other haematological parameters.
We compared the NRBC-positive with the NRBC-negative patient group regarding the above mentioned haematological, demographic, diagnostic, illness severity and outcome parameters. Because NRBCs are a normal finding in neonates, we formed two age subgroups: neonates (≤28 days of age) and children (>28 days of age). We were also interested in the specific NRBC count of patients who underwent open cardiac surgery and therefore analysed this subgroup separately.
To associate a nominal variable to another nominal variable, we provided a frequency table as well as a p-value of either a chi-square or Fisher's exact test, whichever was appropriate. Since most of the continuous variables we were looking at were not normally distributed, we used the Wilcoxon test to compare a continuous variable between two groups.
Composite endpoint: given the rarity of each of the outcome events (death in PICU, ventilation, renal replacement therapy and inotropic support), a composite event was created to capture “bad outcomes” in the PICU, and was used to overcome concerns of sparsity in the multivariate modelling. The composite endpoint took the value yes if at least one of the four outcome variables was assigned with a yes.
We compared haematological, demographic and severity of illness parameters between groups built by mortality yes/no, ventilation yes/no, renal replacement therapy yes/no and inotropic support yes/no. Because age is an important stratification [2, 11], we analysed the data separately for neonates and children.
Multiple logistic regression for the composite endpoint was performed to find independently associated risk factors for bad outcome. There were 425 patients with a “yes” and 245 patients with a “no” for the composite endpoint. Independent variables were NRBC positivity, age (newborn), diagnosis (gastrointestinal/renal/respiratory and neurological/injury/miscellaneous, respectively), sex (male), the haematological values (haemoglobin, platelets and leucocyte count), PIM2 and LOS.
All p-values ≤0.05 were considered statistically significant.
Computations were done with R (R Development Core Team, 2010) [14].
| Table 1: Demographic, diagnostic, severity of illness and outcome data (outcome data referring to the entire stay at PICU) and haematological parameters (on the first day of admission) in NRBC-positive and NRBC-negative patients. For nominal variables, we provide absolute (n) and relative (%) frequency. For continuous variables, we provide median and interquartile range. | |||||
| NRBC positive (n = 195) | NRBC negative (n = 475) | p-value | |||
| Nominal variables | n | % | n | % | |
| Age group | 171 neonates 24 children1 | 87.7 12.3 | 61 neonates 414 children2 | 12.8 87.2 | <0.001 |
| PICU mortality | 13 patients | 6.7 | 6 patients | 1.3 | <0.001 |
| Ventilation (invasive and noninvasive) | 136 | 69.7 | 247 | 52.0 | <0.001 |
| Renal replacement therapy | 10 | 5.1 | 17 | 3.6 | 0.39 |
| Inotropic support – one drug – multiple drugs | 104 49 55 | 53.3 25.1 28.2 | 143 62 81 | 30.1 13.1 17.0 | <0.001 <0.001 0.002 |
| ANZPIC diagnosis | 72 respiratory 38 cardiovascular 22 miscellaneous 17 postprocedural 16 neurological 10 gastrointestinal 9 open card. s. 8 renal 3 injury | 36.9 19.5 11.3 8.7 8.2 5.1 4.6 4.1 1.5 | 53 respiratory 19 cardiovascular 42 miscellaneous 150 postprocedural 40 neurological 9 gastrointestinal 104 open card. s. 8 renal 50 injury | 11.2 4.0 8.8 31.6 8.4 1.9 21.9 1.7 10.5 | <0.001 |
| Sex | 80 female 115 male | 41.1 58.9 | 183 female 292 male | 38.5 61.5 | 0.60 |
| Continuous variables | Median | IQR | Median | IQR | |
| NRBC (G/l) | 0.28 | 0.62 | <0.001 | ||
| Haemoglobin (g/l) | 159.5 | 55.25 | 114.0 | 33.0 | <0.001 |
| Platelets (G/l) | 211 | 112 | 221 | 176 | 0.14 |
| Leucocytes (G/l) | 14.9 | 9.75 | 10.1 | 6.1 | <0.001 |
| PIM2 (%) | 5.49 | 8.9 | 1.51 | 1.64 | <0.001 |
| Length of stay (days) | 3.5 | 5.13 | 1.69 | 2.25 | <0.001 |
| Mean | (95% CI) | Mean | (95% CI) | ||
| Standardised mortality ratio | 0.7 | (0.41‒1.21) | 0.42 | (0.19‒0.94) | |
| ANZPIC = Australian and New Zealand Paediatric Intensive Care Society; CI = confidence interval; IQR = interquartile range; NRBC = nucleated red blood cell; Open card. s. = open cardiac surgery; PICU = paediatric intensive care unit; PIM = paediatric index of mortality 1 median age: 0.81 years (range 13.2); 2 median age: 3.57 years (range 24.8) Neonates: ≤28 days of age; children: >28 days of age. | |||||
1,079 patients were admitted to our PICU during the study period. Measurement of blood parameters for 7 days after PICU admission was foreseen. Median length of stay in PICU was 3.5 days for the NRBC-positive and 1.7 days for the NRBC-negative patient group. This means that many patients had only a short PICU stay. In order to include as many patients as possible, we thus limited the analysis of blood parameters to the first 24 hours of PICU admission. Unfortunately, NRBCs were not always evaluated, especially during the first months of the study and in the end we had to exclude 409 patients (missing blood counts and multiple admissions). After excluding these patients, we could perform the study with 670 patients (fig. 1).

Figure 1
Inclusion and exclusion criteria.
NRBC = nucleated red blood cell; PICU = paediatric intensive care unit
We found 195 (29.1%) NRBC-positive and 475 (70.9%) NRBC-negative patients with 171 NRBC-positive patients (73.7%) in the neonatal age group (n = 232) and 24 NRBC-positive patients (5.5%) in the child age group (n = 438). Standardised mortality ratio for all patients was 0.58 (SMR = observed mortality divided by expected mortality; observed mortality: 19/670 = 2.8%, expected mortality (mean PIM2 score of all patients): 4.9%).
Demographic, outcome and haematological parameters for the NRBC-positive and NRBC-negative patients are shown in table 1. PICU mortality was significantly higher in NRBC positive patients (p <0.001). These patients were younger (p <0.001), more often ventilated (p <0.001) and needed more inotropic support with one or multiple drugs (p <0.001 and p = 0.002, respectively). In addition, they suffered mostly from respiratory problems (p <0.001), had a significantly higher PIM2 score (p <0.001) and a longer length of stay in the PICU (p <0.001).
Comparison between outcome variables and haematological, demographic, severity of illness parameters and LOS stratified by age subgroup can be found in table 2. In the neonatal age group, patients who died, were ventilated or received inotropic support had significantly more NRBCs in their blood than patients without these conditions, and there was a significant association between NRBC positivity, inotropic support and the composite endpoint. In children >1 month of age there were scarcely any NRBC-positive patients. The only significant association between NRBCs and outcome was found for renal replacement therapy. There was no significant association between outcome events and the other haematological parameters in neonates, whereas some of the (bad) outcome events correlate significantly with lower platelet and lower leucocyte counts in the child age group (table 2b). In most cases, severity of illness parameter (PIM2) and LOS correlated significantly with outcome parameters in both age groups.
Results of multiple logistic regression are displayed in table 3. The odds for a NRBC-positive patient to have a yes for the composite endpoint was increased by a factor of 1.4 (95% confidence interval 0.62‒3.41) compared with a NRBC-negative patient adjusted for all the other variables in the model. However, this association was not significant (p = 0.40). The variables that independently explain the composite endpoint are diagnosis, expected mortality (PIM2) and LOS.
In the subgroup of post open cardiac surgery, there were 9 NRBC-positive patients and 104 NRBC-negative patients. Seven of the nine NRBC-positive patients were in the neonatal age group.
There were 381 patients excluded because of missing NRBC determination in the first 24 hours. This group was composed as follows: 18.6% neonates (<1 month of age). The three most frequent diagnoses were “respiratory” (n = 67, 17.6%), “injury” (n = 54, 14.2%) and “miscellaneous” (n = 34, 8.9%), respectively. Mortality was 1.6% (6 patients died), 103 patients (27%) were ventilated. LOS was 0.86 days (median) and PIM2 score was 2.83% (mean). In the study sample (n = 670), the respective percentages were: neonates 34.6%, “respiratory” 18.6%, “injury” 7.9%, “miscellaneous” 9.6%, mortality 2.8%, ventilation 57.2%. Median LOS was 1.92 days and mean PIM2 was 4.9%.
| Table 2:Univariate analysis based upon clinically important outcomes compared with demographic and haematological variables as well as severity of illness parameters. For nominal variables, relative frequencies are shown; for continuous variables, median is displayed. Composite endpoint: death and/or ventilation and/or renal replacement therapy and/or inotropic support. All p-values <0.05 are in bold type. | ||||||||||
| Table 2a: Neonates (≤28 days of age; n = 232). | ||||||||||
| Outcome parameters | NRBC (G/l) | NRBC positive (%) | Hb (g/l) | Platelets (G/l) | Leucocytes (G/l) | Sex male (%) | PIM2 (%) | LOS (days) | ||
| Mortality D (n = 15) A (n = 217) p-value | 0.50 0.18 0.032 | 86.7 72.8 0.36 | 159.0 159.0 0.92 | 179.0 225.0 0.066 | 19.6 13.4 0.38 | 33.3 63.1 0.04 | 22.26 2.96 <0.001 | 6.05 3.36 0.11 | ||
| Ventilation Y (n = 161) N (n = 71) p-value | 0.21 0.15 0.038 | 77.6 64.8 0.06 | 157.0 166.0 0.19 | 223.0 239.0 0.16 | 14.2 12.7 0.43 | 59.6 64.8 0.55 | 7.56 1.62 <0.001 | 4.76 1.29 <0.001 | ||
| Renal replacement therapy Y (n = 7) N (n = 225) p-value | 0.17 0.19 0.57 | 71.4 73.8 1.00 | 161.0 158.5 0.48 | 176.5 224.0 0.54 | 8.7 13.5 0.17 | 57.1 61.3 1.00 | 5.60 3.77 0.43 | 6.72 3.36 0.14 | ||
| Inotropic support Y (n = 117) N (n = 115) p-value | 0.21 0.16 0.029 | 83.8 63.5 <0.001 | 157.0 161.0 0.54 | 215.0 239.0 0.078 | 13.7 13.4 0.39 | 59.0 63.5 0.57 | 7.96 2.25 <0.001 | 5.60 2.07 <0.001 | ||
| Composite endpoint Y (n = 175) N (n = 57) p-value | 0.21 0.12 0.056 | 77.7 61.4 0.02 | 157.0 166.0 0.49 | 222.5 246.0 0.15 | 14.2 12.2 0.25 | 60.0 63.2 0.85 | 6.84 1.72 <0.001 | 4.66 1.19 <0.001 | ||
| A = alive; D = dead; Hb = haemoglobin; LOS = length of stay; N = no; NRBC = nucleated red blood cells; PIM = paediatric index of mortality; Y = yes | ||||||||||
| Table 2b: Children (>28 days of age; n = 438). | ||||||||||
| Outcome parameters | NRBC (G/l) | NRBC positive (%) | Hb (g/l) | Platelets (G/l) | Leucocytes (G/l) | Sex male (%) | PIM2 (%) | LOS (days) | ||
| Mortality D (n = 4) A (n = 434) p-value | 0 0 0.63 | 0 5.5 1.0 | 103.0 111.0 0.66 | 197.0 215.0 0.74 | 10.9 10.1 0.55 | 75.0 60.4 1.00 | 31.62 1.44 0.002 | 15.27 1.59 0.93 | ||
| Ventilation Y (n = 222) N (n = 216) p-value | 0 0 0.63 | 5.0 6.0 0.78 | 111.0 110.5 0.11 | 181.0 248.0 <0.001 | 9.3 10.95 0.004 | 61.3 59.7 0.82 | 1.94 1.02 <0.001 | 2.10 0.84 <0.001 | ||
| Renal replacement therapy Y (n = 20) N (n = 418) p-value | 0 0 < 0.001 | 25.0 4.5 0.003 | 116.0 111.0 0.94 | 137.0 218.0 0.012 | 10.65 10.10 0.79 | 45.0 61.2 0.22 | 2.82 1.44 0.038 | 6.96 1.56 <0.001 | ||
| Inotropic support Y (n = 130) N (n = 308) p-value | 0 0 0.58 | 4.6 5.8 0.77 | 117.5 109.0 <0.001 | 142.5 223.0 <0.001 | 8.90 10.50 0.007 | 60.0 60.7 0.97 | 2.03 1.22 <0.001 | 2.88 0.94 <0.001 | ||
| Composite endpoint Y (n = 250) N (n = 188) p-value | 0 0 0.34 | 6.4 4.3 0.44 | 110.0 111.0 0.71 | 182.0 249.0 <0.001 | 9.40 11.05 <0.001 | 60.0 61.2 0.88 | 1.88 0.98 <0.001 | 2.01 0.80 <0.001 | ||
| A = alive; D = dead; Hb = haemoglobin; LOS = length of stay; N = no; NRBC = nucleated red blood cells; PIM = paediatric index of mortality; Y = yes | ||||||||||
| Table 3:Multiple logistic regression for the composite endpoint (dependent variable). The composite endpoint (death and/or ventilation and/or renal replacement therapy and/or inotropic support) had a value yes if at least one of the four outcome variables mortality, ventilation, renal replacement therapy or inotropic support was assigned with a yes. Interpretation for “newborn yes” (binary variable): the odds for a “yes" for the composite endpoint are decreased by a factor of exp(–0.47) = 0.627 for patients with a newborn status of “yes" compared to those with a “no" for newborn. Interpretation for “haemoglobin (g/l)” (continuous variable): the odds for having a “yes" for the composite endpoint are decreased by a factor of exp(–0.002) = 0.998 for an increase of haemoglobin by one unit. This effect is multiplicative, i.e., for a value of haemoglobin that is by a factor of 2 higher, the odds for a “yes" are decreased by a factor of exp(2*-0.002) = 0.997. Interpretation for “log(PIM2)”: an increase of log(PIM2) by 1% increases the odds for having a “yes" for the composite endpoint by a factor of exp(1.333) = 3.794. | ||||
| Estimate | Odds ratio | p-value | 95% confidence interval for odds ratio | |
| NRBC positive | 0.37 | 1.44 | 0.40 | (0.62‒3.41) |
| Newborn yes | –0.47 | 0.63 | 0.33 | (0.24‒1.59) |
| Diagnosis gastrointestinal/renal/respiratory | –2.51 | 0.08 | <0.001 | (0.04‒0.16) |
| Diagnosis neurological/injury/miscellaneous | –3.03 | 0.05 | <0.001 | (0.02‒0.09) |
| Sex male | –0.13 | 0.88 | 0.61 | (0.54‒1.44) |
| Haemoglobin (g/l), per one unit increase | –0.002 | 1.00 | 0.74 | (0.99‒1.01) |
| Platelets (G/l), per one unit increase | –0.002 | 1.00 | 0.42 | (1.00‒1.00) |
| log (Leucocytes [G/l]), per one unit increase | –0.23 | 0.79 | 0.31 | (0.50‒1.23) |
| log (PIM2 [%]), per one unit increase | 1.33 | 3.79 | <0.001 | (2.87‒5.12) |
| log (LOS [days]), per one unit increase | 1.37 | 3.94 | <0.001 | (2.90‒5.47) |
| LOS = length of stay; NRBC = nucleated red blood cell; PIM = paediatric index of mortality | ||||
NRBCs may have a prognostic significance in the first month of life. In neonates, most of the bad outcome parameters correlated with the absolute number of NRBCs and/or NRBC positivity (table 2a). In older children, we found a correlation only for renal replacement therapy but the small percentage of NRBC-positive patients in the child age group (5.5%) precludes reliable statistical results for children older than 28 days. Critically ill neonates may be more prone to NRBC production/release than critically ill children. A possible explanation might be a difference in interleukin-6, erythropoietin (EPO) or catecholamine production between these two age groups. Additionally, younger children have a lower threshold for NRBC release into the circulation than older children or adults [8]. NRBCs may be considered as a parameter that sums hypoxic and inflammatory injuries [6]. Interleukin-6 is a mediator of the EPO upregulation in children with systemic inflammatory response syndrome triggered by septic shock [15]. A former study showed that there is a significant and positive correlation between EPO levels and NRBC counts [16]. Suppression of EPO by red blood cell transfusion has been shown to decrease NRBC counts significantly [17]. Catecholamines may also play a role in increasing NRBC count. In animal experiments, catecholamines have been shown to increase EPO production [18, 19]. Therefore, the significant association between inotropic support and NRBC in neonates may be due to critical illness (needing inotropes) or may be a direct consequence of the drug.
A possible explanation for the only significant correlation of NRBCs with renal replacement therapy in the older age group (>28 days of age) might be the very critical state of these patients. In our unit, renal replacement therapy is usually done in patients with a history of several days of critical illness, evolving towards multiple organ failure. This may be a trigger strong enough for NRBC release with a trigger threshold probably higher than in the neonatal period. Interestingly, bad outcome parameters were associated with low platelet counts and low leucocyte counts in the child age group. In our study, these haematological parameters were more sensitive markers of bad outcome than NRBCs.
In both age groups, there was a good association between the outcome events we chose for this study and the severity of illness parameter (PIM2) as well as LOS (table 2). This is at least an indication that the outcome parameters may in fact describe bad outcomes.
Parameters which independently explained bad outcomes in multiple logistic regression were diagnosis, severity of illness and LOS. There was no significant independent prognostic role of NRBC positivity (odds ratio 1.44, 95% confidence interval 0.62–3.41). However, NRBC positivity might show a trend as an independent predictor of bad outcomes.
A recently published study in adults admitted to a surgical intensive care unit [20] found that any positive NRBC value was associated with a poor outcome and that increasing NRBC values were associated with an increasing mortality. Additionally, there were trends in NRBC values and a return to zero was protective in comparison with values that never returned to baseline. Our study was not designed to detect any trend of NRBC values, but results in this field are very interesting and further investigation in the paediatric population is needed.
We could not reproduce the finding that patients after open cardiac surgery with cardiopulmonary bypass have more NRBCs in their peripheral blood smear [8]. This may be explained by the duration of the observation period; Frey et al. [8] took the NRBC count for the first to the tenth postoperative day, showing higher peak levels in the later postoperative course; we analysed only the first 24 hours after PICU admission.
We had to exclude 381 patients because of missing NRBC determination in the first 24 hours of PICU admission and are well aware of a possible bias. The excluded patients had lower illness severity than the analysed patients (lower PIM2 score, lower LOS and lower proportion of ventilated patients). The relatively good condition of these patients may explain why no blood film was taken at admission. Secondly, for statistical reasons, diagnostic groups had to be arbitrarily collapsed for multiple logistic regression calculations. Therefore, we are not able to say which specific diagnoses are independently associated with NRBCs. Thirdly, mortality is typically low in paediatric intensive care, which makes it more difficult to find an association between NRBC and bad outcomes, compared with adult intensive care. The composite endpoint in our study was dominated by the more frequent outcomes “ventilation” and “inotropic support”.
The association of NRBCs in the first 24 hours after admission and outcomes of critically ill children is age dependent. In the first month of life of mainly term infants, increased numbers of NRBCs had a prognostic value in regard to mortality, ventilation and inotropic support. In children >1 month of age, the association between NRBCs and outcome is much less pronounced. However, PIM2 score, LOS and diagnosis independently predicted bad outcome (death and/or ventilation and/or renal replacement therapy and/or inotropic support). Further investigations are needed to assess trends in NRBC values for critically ill children.
Acknowledgement:The authors are grateful to Dr. K. Rufibach, Division of Biostatistics, Institute for Social and Preventive Medicine, University of Zurich, Switzerland, for the statistical contributions to this study.
1 Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE. Nathan and Oski’s Hematology of infancy and childhood. 7th ed. Philadelphia: 2009; p. 1838 and 1840.
2 Christensen RD, Henry E, Andres RL, Bennett ST. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology. 2011;99:289–94.
3 Green DW, Mimouni F. Nucleated erythrocytes in healthy infants and in infants of diabetic mothers. J Pediatr. 1990;116:129–31.
4 Phelan JP, Ahn MO, Korst LM, Marti GI. Nucleated red blood cells: a marker of fetal asphyxia? Am J Obstet Gynecol. 1995;173:1380–4.
5 Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia and erythroblastosis in growth retarded fetuses. BMJ. 1987;294:1051–3.
6 Stachon A, Segbers E, Holland-Letz T, Kempf R, Hering S, Krieg M. NRBC in the blood of medical intensive care patients indicate increased risk: a prospective cohort study. Critical Care. 2007;11:R62.
7 Stachon A, Sondermann N, Imohl M, Krieg M. Nucleated red blood cells indicate high risk of in-hospital mortality. J Lab Clin Med. 2002;140:407–12.
8 Frey B, Duke T, Horton SB. Nucleated red blood cells after cardiopulmonary bypass in infants and children: is there a relationship to the systemic inflammatory response syndrome? Perfusion. 1999;14:173–80.
9 Stachon A, Böning A, Krismann M, Weisser H, Lacskovics A, Skipka G, Krieg M. Prognostic significance of the presence of erythroblasts in blood after cardiothoracic surgery. Clin Chem Lab Med. 2001;39:239–43.
10 Frey B, Horisberger T. Release of nucleated red blood cells early after cardiorespiratory arrest. J Pediatr Hematol Oncol. 2003;25:180–1.
11 Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child. Feta. Neonata. Ed. 2001;84:F211–15.
12 Slater A, Shann F, McEniery J. The ANZPIC registry diagnostic codes: a system for coding reasons for admitting children to intensive care. Intensive Care Med. 2003;29:271–7.
13 Slater A, Shann F, Pearson G. PIM2: a revised version of the paediatric index of mortality. Intensive Care Med. 2003;29:278–85.
14 Leisch F. Sweave: dynamic generation of statistical reports using literate data analysis. Compstat – Proceedings in Computational Statistics. Heidelberg: 2002;p:575–80.
15 Krafte-Jacobs B, Bock GH. Circulating erythropoietin and interleukin-6 concentrations increase in critically ill children with sepsis and septic shock. Crit Care Med. 1996;24:1455–9.
16 Ferber A, Fridel Z, Weissmann-Brenner A, Minior VK, Divon MY. Are elevated fetal nucleated red blood cell counts an indirect reflection of enhanced erythropoietin activity? Am J Obstet Gynecol. 2004;190:1473–5.
17 Frey B. Transfusion in premature infants impairs production and/or release of red blood cells, white blood cells and platelets. J Paediatr Child Health. 2002;38:265–7.
18 Fisher JW. Erythropoietin: pharmacology, biogenesis and control of production. Pharmacol Rev. 1972;24:459–508.
19 Gross DM, Fisher JW. Effects of terbutaline, a synthetic beta adrenoceptor agonist, on in vivo erythropoietin production. Arch Int Pharmacodyn Ther. 1978;236:192–201.
20 Shah R, Reddy S, Horst M, Stassinopoulos J, Jordan J, Rubinfeld I. Getting back to zero with nucleated red blood cells: following trends is not necessarily a bad thing. The American Journal of Surgery. 2012;203:343–46.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.