Nuclear power plants and childhood leukaemia: lessons from the past and future directions
DOI: https://doi.org/10.4414/smw.2014.13912
Claudia Elisabeth
Kuehni, Ben Daniel
Spycher
Summary
In the 1980s, leukaemia clusters were discovered around nuclear fuel reprocessing plants in Sellafield and Dounreay in the United Kingdom. This raised public concern about the risk of childhood leukaemia near nuclear power plants (NPPs). Since then, the topic has been well-studied, but methodological limitations make results difficult to interpret. Our review aims to: (1.) summarise current evidence on the relationship between NPPs and risk of childhood leukaemia, with a focus on the Swiss CANUPIS (Childhood cancer and nuclear power plants in Switzerland) study; (2.) discuss the limitations of previous research; and (3.) suggest directions for future research.
There are various reasons that previous studies produced inconclusive results. These include: inadequate study designs and limited statistical power due to the low prevalence of exposure (living near a NPP) and outcome (leukaemia); lack of accurate exposure estimates; limited knowledge of the aetiology of childhood leukaemia, particularly of vulnerable time windows and latent periods; use of residential location at time of diagnosis only and lack of data on address histories; and inability to adjust for potential confounders.
We conclude that risk of childhood leukaemia around NPPs should continue to be monitored and that study designs should be improved and standardised. Data should be pooled internationally to increase the statistical power. More research needs to be done on other putative risk factors for childhood cancer such as low-dose ionizing radiation, exposure to certain chemicals and exposure to infections. Studies should be designed to allow examining multiple exposures.
Abbreviations
ALL acute lymphoblastic leukaemia
CANUPIS study childhood cancer and nuclear power plants in Switzerland
CI confidence interval
CL confidence limit
Geocap study childhood leukaemia around French nuclear power plants
IRR incidence rate ratio
KIKK study German study of childhood cancer in the vicinity of nuclear power plants
NPP nuclear power plant
OR odds ratio
SIR standardised incidence ratio
SMR standardised mortality ratio
Introduction
In 1983, public attention was drawn to the possibility of a link between nuclear installations and cancer after the media reported on an excess of leukaemia among young people in Seascale, a coastal village in north-west England situated near the large nuclear fuel reprocessing site Sellafield [1]. Since then, researchers in many countries have investigated the risk of leukaemia and other cancers in children living in the vicinity of nuclear power plants (NPPs) [2–9], with mixed results. Taken as a whole, these studies have found little evidence that living near a NPP increases risk of childhood cancer, but a signal compatible with an increased leukaemia risk was consistently evident for children aged <5 years living <5 km to NPPs across several recent studies [4–9].
It is established that moderate or high doses (above 100mSv) of ionising radiation increase the risk for childhood leukaemia [10]. Recent studies on natural background radiation or radiation from medical examinations support the claim that there is no “safe” threshold for ionising radiation [11, 12]. For people who live in the vicinity of NPPs, average yearly doses attributable to radioactive releases from NPPs are magnitudes lower than those from natural background radiation [1, 13–16]. Many scientists thus believe that when risk of cancer coincides with proximity to NPPs, it can be explained by confounding, i.e. other risk factors for leukaemia which are more common near NPPs.
In this article, we summarise current evidence on the links between residence near NPPs and risk of childhood leukaemia, using as our main example the Swiss CANUPIS (Childhood cancer and nuclear power plants in Switzerland) study. We included studies that we believe are important contributors to the current discussion; this is not a systematic review of the literature. After the summary, we offer our opinions on the limitations of previous research and suggest directions for future studies.
Childhood leukaemia: impact and causes
Leukaemia is the most common childhood cancer, accounting for about 30% of cancer cases among children aged 0–14 years [17, 18]. In Switzerland and other industrialised countries, about five new cases per 100,000 children occur each year [18]. Incidence peaks at the age of 2–3 years with eight to nine cases per 100,000 children annually [19, 20]. Among leukaemia subtypes, acute lymphoblastic leukaemia (ALL) is the most common (80% of all children with leukaemia) [18]. Childhood leukaemias fall into different immunophenotypes and cytogenetic subtypes that vary in prognosis, response to treatments and, possibly, in aetiology [21, 22].
Little is known about the causes of childhood leukaemia. Certain genetic syndromes such as neurofibromatosis and Down’s syndrome increase the risk of leukaemia. Susceptibility to childhood leukaemia is also affected by common genetic variants [23].
Of the potential environmental causes, only ionising radiation in moderate to high doses is a proven risk factor for childhood leukaemia [10, 21, 24]. High-risk groups, including survivors of the atomic bombings in Japan and children receiving radiation therapy, provide sound evidence for this [10]. Recent studies suggest that exposure to low-dose ionising radiation from natural sources (cosmic and terrestrial radiation, indoor radon) or from diagnostic radiology increase the risk of childhood leukaemia in the general population [10–12, 25]. Other possible environmental risk factors include electromagnetic fields (extremely low frequency and radio frequency) [26], pesticides and air pollution (particularly air pollution containing carcinogenic compounds like benzene) [27–29]. However, the results of studies that have investigated these environmental factors are heterogeneous and inconclusive.
When investigating environmental factors, exposure history should be considered from conception to diagnosis, because latency periods of leukaemia are insufficiently known and characteristic chromosomal translocations for leukaemia can occur already in utero [30, 31]. Furthermore, as has been shown for ionising radiation [10], the first years of life may be a critical time window during which children are more susceptible to certain environmental exposures.
Results from some descriptive studies suggest that infections might play a role in the aetiology of childhood cancers, and particularly of ALL [21, 32, 33]. Observations include: a higher incidence of ALL in resource-rich countries where early infections are less common than in resource-poor countries [32]; a characteristic peak in the incidence of ALL at age 2–4 years [20]; reports of local clusters in which the number of diagnosed cases over short periods of time was much greater than the number expected [34]; reports of a general tendency for cases to cluster in space and in space-time [35–37] and of seasonal patterns [38, 39]. As yet, no molecular traces of specific viral agents have been found in biological samples from patients with childhood leukaemia [40, 41].
Available evidence on childhood leukaemia risk and nuclear power plants
Independent investigations commissioned by the British government in the wake of the 1983 discovery confirmed a markedly increased incidence near Sellafield [1] and Dounreay, another nuclear fuel reprocessing site in Scotland [42], but concluded that these facilities released too little radioactivity to explain the observed excess incidence.
Many studies on childhood cancer and nuclear installations followed, in the UK and elsewhere. Most studies, summarised in recent reviews [2, 4, 43, 44], were ecological. Typically, they applied national cancer incidence rates to the population of children that lived in given geographical units (municipalities, communities or wards) and calculated the number of expected cancer cases in each unit. Comparing observed to expected cancer cases returns the standardised incidence ratio (SIR), an estimate of relative risk of children living in an area, compared with the average risk country-wide. About 200 different nuclear facilities, including NPPs, research facilities, nuclear fuel or weapons production sites and reprocessing plants in 10 countries, were investigated in this fashion [2]. Among these, clear excesses were evident around only three sites: the two nuclear reprocessing sites of Sellafield and Dounreay, and around Kruemmel, a power generating plant in Germany [2]. There was no evidence of increased risk at other sites, either singly or in aggregate [2]. A meta-analysis of 136 single sites did report slightly increased risks [43] but its methodology was questionable [45].
Recent studies focused on young children (<5 years at diagnosis) living very close (<5 km) to NPPs [4–8, 46–50]. Elevated leukaemia incidence around NPPs in this group had first been found in a subgroup analysis of an ecological study from Germany [51]. Public concern inspired repeated investigation of this age-group in Germany [5, 48, 52]. The most recent was a case-control study (German KiKK study: study of childhood cancer in the vicinity of nuclear power plants), [5, 48] that used exact distances between home address at diagnosis and the nearest NPP to compare cancer cases with population based controls. Odds ratios (ORs) for leukaemia (OR 2.19, lower one-sided confidence limit [CL] 1.51) and all cancers (OR 1.61, lower CL 1.26) were significantly higher in this age-group [5, 48].
This study refuelled the debate among scientists, politicians and the public. In its wake, the governments of surrounding countries (Switzerland [6], the UK [4], France [7], Finland [50] and Belgium [8]) commissioned studies to investigate leukaemia incidence in under-fives living within 5 km of NPPs. A French case-control study (Geocap: childhood leukaemia around French nuclear power plants) found that children <15 years old, who lived within 5 km of an NPP, had a higher risk for acute leukaemia; for under-fives the increase was not statistically significant (OR 1.6, 95% confidence interval [CI] 0.7–4.1]) [7].
Table 1compares the results of these studies for 0–4 year olds. To make comparison easier, we give results from ecological analyses (SIRs) for all countries. Results were skewed in the same direction for most studies, except in Finland, where no cases were observed within 5 km of a NPP. These studies suggest that leukaemia risk is slightly increased in the 5 km zone, with SIRs ranging from 1.3 to 1.41.
|
Table 1:Leukaemia risk in 0–4 year old children living within 5 km of a nuclear power plant: ecological analyses from recent studies in five countries. |
|
|
Study period
|
Expected cases
|
Observed cases
|
SIR (95% CI)
|
| Germany [5] |
1980–2003 |
24.1 |
34 |
1.41 (0.98–1.97) |
| Finland [50] |
1977–2004 |
NA |
|
NA |
| United Kingdom* [4] |
1969–2004 |
15.4 |
20 |
1.30 (0.79–2.01) |
| Switzerland [6] |
1985–2009 |
7.9 |
11 |
1.40 (0.70–2.50) |
| France [7] |
1990–2007 |
10.2** |
14** |
1.37 (0.75–2.30) |
| CI = confidence interval; NA = not available; SIR = standardised incidence ratio * Distance was calculated from centroid of administrative units (electoral wards in England and Wales, postcode zones in Scotland) instead of from the child’s home. ** Includes only cases of acute leukaemia |
Interpretation of findings
The large majority of previous studies did not confirm that living near a NPP increases the risk of childhood leukaemia. However, we cannot dismiss the possibility that such an association does exist. First, clusters of leukaemia cases have been observed around specific sites and, second, results from studies of multiple sites suggest moderately elevated risks for under-fives who live very close to a NPP.
Different explanations for leukaemia clusters in the vicinity of nuclear facilities have been proposed. The hypothesis that leukaemia clusters are caused by radioactive releases from these facilities is contradicted by studies showing that such releases account for a small fraction of the overall annual radiation dose, which comes mostly from natural sources [2]. The hypothesis that changes in the germ cells of fathers who worked in the facilities caused leukaemia in their children has not been confirmed in independent studies [53]. Clusters might also be explained by the presence of environmental pollutants other than ionising radiation, such as chemicals, but no evidence for this has been found [54].
A possible explanation for leukaemia clusters around nuclear facilities is that rapid population influxes into previously isolated communities (population mixing) may lead to localised epidemics of an as-yet unknown infection that can cause leukaemia [55]. This hypothesis was originally proposed as an explanation for the leukaemia cluster around Sellafield, but might also explain leukaemia clusters in other locations, apart from NPPs [56, 57]. A series of studies have found elevated cancer risks following historic events of intense population mixing, such as the creation of new towns or large construction projects [58]. However, findings from studies using population growth between two census time points or other census-based measures of population mixing show less consistent results [58–61].
Given our current knowledge, we cannot exclude the possibility that living in proximity to NPPs causes a moderate increase in risk of childhood leukaemia. We suggest that these open questions be addressed by more focused research that avoids the limitations of previous studies. The remainder of this article addresses this research and uses the example of the CANUPIS study to show how some challenges might be met.
The Swiss CANUPIS study
CANUPIS is a census-based cohort study that tested the association between residence near one of the five Swiss NPPs and increased risks of leukaemia and other childhood cancers from 1985–2009 (study period) [6]. We used the Swiss Childhood Cancer Registry to identify cancer cases during this period and collected address histories from diagnosis back to birth. For the census years 1990 and 2000, we had access to precise geocoded places of residence for the entire Swiss resident population and could use them to calculate person years at risk for children of different age and sex categories, in concentric zones around the NPPs (<5 km, 5–10 km, 10–15 km, >15 km) (fig. 1). We performed two analyses: a birth cohort analysis that included children born in Switzerland during the study period; and, a resident cohort analysis that included 0–15 year olds who lived in Switzerland for any length of time during the study period. We used addresses at birth and at diagnosis to compare incidence of cancer in the three circles around NPPs with incidence of cancer further away.
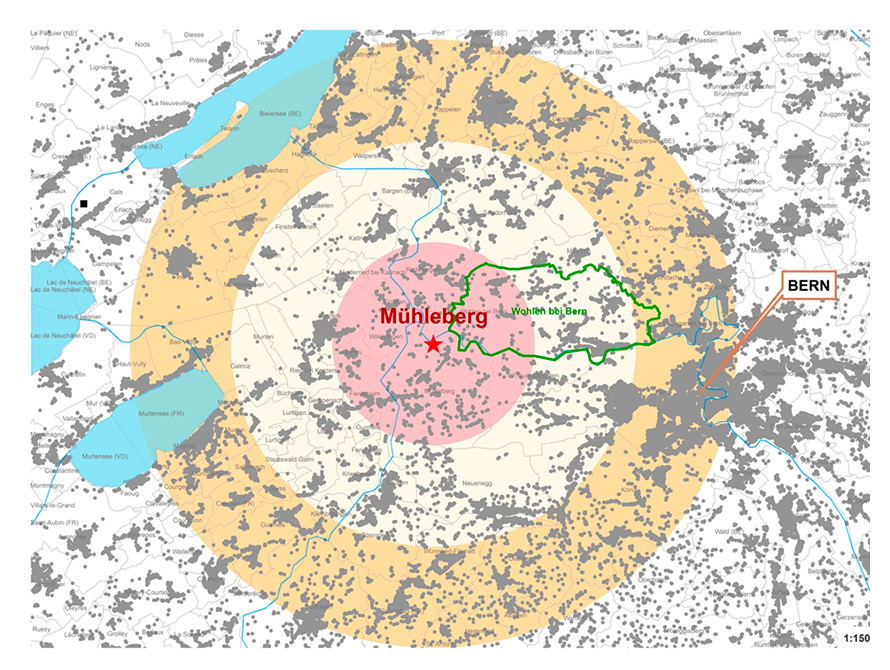
Figure 1
Map showing consecutive 5-km zones (<5 km, 5–10 km, 10–15 km) around the Mühleberg nuclear power plant, residential homes of children from the general population (grey points), community boundaries (grey faint lines, community “Wohlen bei Bern” with green boundaries as an illustrative example).
The birth cohort included 2,925 children with cancer, of whom 953 (32.6%) had leukaemia. Compared with children who were born >15 km away, the incidence rate ratios (IRRs) for leukaemia in children born within 5 km of a NPP were 1.20 (95% CI 0.60–2.41) for 0–4 year olds, and 1.05 95% CI 0.60–1.86) for 0–15 year olds; we found no evidence of a relationship to distance. The resident cohort included 4,090 children with cancer of whom 1,345 (32.9%) had leukaemia. Corresponding IRRs were 1.41 (95% CI 0.78–2.55) for 0-4 year olds, and 1.24 (95% CI 0.80–1.94) for 0–15 year olds. IRRs for all cancers were similar to those for leukaemia. Results remained comparable when we included other nuclear facilities (research and storage sites) and when we adjusted for potential confounders such as socioeconomic status, natural background radiation, electromagnetic fields from radio and television transmitters, distance to major roads, railway tracks, high-voltage power lines and to land-use plots where heavy use of agricultural pesticides was likely.
CANUPIS introduced several improvements over earlier studies. It used nationwide routine datasets with high coverage (census data and national cancer registry) and could thus combine the advantages of ecological studies (large samples, lack of selection bias) with those of case-control studies (exact individual geocoding of residential addresses). The CANUPIS study adjusted for many potential confounding factors, any of which could have affected estimates of the strength of association. The study used address at birth as well as address at diagnosis, which allowed us to account for exposures during prenatal and early postnatal development. Because Switzerland is relatively small, the study had limited statistical power. However, a comparison of total population levels between countries would suggest lower statistical power than the study actually achieved. Statistical power is largely determined by the number of cases observed in the vicinity of NPPs, and Swiss NPPs are situated in densely populated areas.
Challenges in research on nuclear power plants and childhood leukaemia
In table 2, we summarise the main methodological challenges faced by researchers who explore links between nuclear installations and leukaemia risk in children. We describe the main methodological problems and the resulting consequences (columns 1 and 2), and summarise the ways in which previous studies dealt with these issues (column 3).
Rarity of both outcomes and exposures
Childhood cancer is a rare disease and only few children live in the immediate proximity of a NPP. This makes study design challenging. Prospective cohort studies are infeasible because of the exceedingly long wait times required to observe a sufficient number of disease cases among a small group of exposed individuals. Instead, earlier studies relied on routine datasets (census and cancer registry or mortality data) and ecological study designs. In such studies, proximity to NPPs was typically measured from the centroid of the community or county of residence, which did not accurately capture the real distance between the child’s home and the NPP. Large communities might stretch over a wide distance range (illustrated in the example of Wohlen, a Swiss community; see fig. 1). An observed association between leukaemia risk and distance to NPPs at the community level may therefore not correctly reflect such an association at an individual level (ecological fallacy). Furthermore, ecological studies cannot adjust for confounders at an individual level. Some studies could include information on confounders at an aggregate level such as area-based measures of socioeconomic status.
The rarity of outcomes and exposures also greatly affects statistical power. Any given study includes only few exposed cases. Previous studies attempted to increase statistical power by enlarging the areas defined as exposed (e.g. all communities with centroid within 20 km from a NPP), by performing multi-site studies, or by maximising the power of statistical tests [62].
Lack of accurate estimates of exposure to radiation from NPP releases
Complex modelling is required to estimate the effective doses that people receive when radionuclides are released from NPPs (see [15, 63] for an overview). NPPs usually release trace amounts of radionuclides into the environment and their dispersal and deposition depends on factors like wind speed and direction and rainfall. Humans are exposed through various routes that differ in their biological effects, including inhalation (via air transport) or ingestion (via soil deposition and food production), or through external irradiation [63]. Even if doses are modelled for a given location, the cumulative dose children receive will depend on their residence history, possibly from preconception to the time of diagnosis.
In earlier studies, exposure was usually approximated by calculating distance between the centroid of the community of residence and the NPP. More recent studies have used exact distance measurements. Geocodes of residential addresses for the whole population are routinely available only in Nordic countries and Switzerland, but these countries have small populations resulting in limited statistical power [6, 50]. Studies from Germany [5] and France [7], where exact addresses were available from cancer registries for leukaemia cases, but not for the whole population, used case-control designs; they calculated precise distances for cases and selected controls. Geocap used a representative sample of households from a central registry, and the KiKK study obtained controls from registrar’s offices of individual communities. Because willingness to provide addresses was lower in communities near power plants, there is concern that the KiKK study may have been affected by selection bias. However, sensitivity analyses excluding cases and controls from communities that did not fully cooperate in selection of controls still found an increase risk near NPPs, suggesting that results are not explained by selection bias [64, 65].
Few studies have tried to improve measurements of exposure. In an additional analysis, CANUPIS used prevailing wind directions to investigate areas around NPPs in which deposition of radionuclides was more likely, but found no evidence of an association [6]. Geocap defined geographic zones based on distances and based on modelled doses received by residents [66]. There was a statistically significant association between leukaemia in preschool children and distance to NPPs, but this was not evident in the dose-based zoning.
Lack of knowledge on the aetiology of childhood cancer
There is still much uncertainty regarding latency periods or critical time windows of development of leukaemia, environmental risk factors other than ionising radiation, or about whether subtypes of leukaemia differ in their aetiology. To our knowledge only CANUPIS and an earlier ecological study [67] investigated exposure at birth. Other studies focused only on exposure at the time of diagnosis (incidence studies) or death (mortality studies). It is known that certain chromosomal translocations characteristic of leukaemia already occur in utero [31]. Aetiological mechanisms might also differ between subtypes of childhood ALL [21]. Few studies have investigated subtype-specific effects of environmental exposures, which might not show up when subtypes are grouped.
Poor evidence on risk factors for childhood leukaemia also limits our ability to explore relevant confounding factors. In CANUPIS, the availability of precise geocodes allowed us to estimate exposure to a number of potential confounders. None of these adjustments altered the results [6]. However, we ignored potential confounders that were more difficult to assess and could not be estimated based on residential location: individual data on socioeconomic status; health behaviour (smoking, alcohol, exercise, etc.); use of medicines and diagnostic and therapeutic radiation; exposure to household chemicals. The German KiKK study assessed a wide range of potential confounders in a nested questionnaire survey, and found that correcting for these did not markedly change results [48]. However, because response rates to the questionnaire survey varied considerably by distance to NPPs, particularly for controls, no firm conclusions could be drawn regarding possible confounding [48, 68].
|
Table 2:Methodological challenges of studies on childhood cancer and nuclear power plants and proposed solutions. |
|
Problems
|
Consequences
|
Solutions in the past
|
Proposed solutions for the future
|
|
1. Low prevalence of outcomes (childhood cancer)
and exposure (proximity to NPPs) |
Reduced statistical powerProspective cohort studies not feasibleReliance on routine datasets and missing or limited individual level data on exposures and confounders |
Increased size of area defined as exposed (e.g. all communities with centroid ≤20 km from NPP) Multi-site studiesDesigning tests of improved statistical power[62]
Ecological studies using administrative area units (e.g. county, community) and SIR (when incidence data from cancer registries are available) or SMR (using cause specific mortality from mortality registries) |
Standardised methodology and meta-analyses of different studies (using conventional or individual-patient data meta-analyses) Case control studies, preferably register-based (to avoid the problem of selection bias) Census based cohort studies using approximations for person-time at risk (CANUPIS) |
|
2. Difficulty of accurate exposure measurement (This would require correct modelling of transfer pathways of radioactive releases and dosimetric modelling of effective doses to humans [15, 63]) |
Reliance on proxy measures such as distance resulting in misclassification of individual exposure |
Usually distance of centroid of geographic unit or residence (e.g. county community) from NPP |
Use of precise distances between residence and NPPs (German KIKK study and CANUPIS) Alternative functional relationships between distance and cancer risk that better reflect decline of radionuclide concentrations with distance (German KIKK study and CANUPIS) Use of dose-based geographic zoning (French Geocap study) |
|
3. Lack of understanding of aetiology of childhood leukaemiaand childhood cancer in general
|
Uncertainty regarding:Latency and developmental time
windows (pregnancy, early childhood) Potential variability between leukaemia subtypesRelevant confounders
|
Usually, only place of residence at diagnosis was studied No adjustment for confounders, or ecological adjustment for limited sets of confounders |
Exposure during pregnancy and infancy (residence at birth, CANUPIS) Cumulative doses based on complete address historiesAnalysis of leukaemia subtypes
Individual adjustment for confounders that can be estimated using place of residence (CANUPIS) Parallel investigation of “control sites”that may have similar characteristics as NPP sites (e.g. sites where NPPs were planned but not built (CANUPIS) or industrial areas) |
| NPP = nuclear power plant; SIR = standardised incidence ratio; SMR = standardised mortality rate |
Directions for future research on nuclear power plants
The last column in table 2 summarises potential improvements for future studies.
First, researchers should try to harmonise study designs. Data assessed with comparable methods in different countries could then be pooled in an individual patient data meta-analysis. This would improve statistical power and reduce heterogeneity. Meta-analyses might then have enough power to discern between leukaemia subtypes and smaller age groupings, and consider different time-windows of exposure.
At a minimum, we suggest a case control study design that obtains cases from high coverage cancer registries and age and sex matched controls from representative population registers (register-based case controls study). Geocoded addresses of residence of all cases and controls should be available so researchers can examine geographically determined potential confounders or additional risk factors. We also suggest exploring better methods for estimating exposure. Estimations might be improved by straightforward extensions of geographic zoning, such as defining zones of higher exposure according to prevalent wind directions, or spatial dose models like those used in Geocap. Wherever possible, cumulative exposure via residence histories should be obtained, back to conception. Where this cannot be done, maternal address at child’s birth is as a reasonable approximation of residence during pregnancy and the first year of life.
A comparison of risk of leukaemia around NPP sites with “control sites” selected for similarity to NPP sites could correct for unknown risk factors common to NPP sites, but unrelated to releases.
Conclusion
We must continue to monitor childhood cancer incidence in the neighbourhood of NPPs and improve our methodology. Future studies should collect detailed information on multiple exposures and their datasets should be designed to answer more than one research question. Setting an international standard of minimum methodological requirements will facilitate pooled analyses. These measures should allow channelling scarce financial resources into more effective research on the causes of childhood leukaemia.
Research on leukaemia risk near NPPs need not have the highest priority: the argument for harmful effects of NPPs in normal operation has little plausibility. As noted previously, it makes little sense to test a hypothesis if it is a priori considered to be unreasonable [69]. This research will also grow less pressing if countries move away from nuclear energy and toward alternative sources, as is the case in Switzerland. But as long as NPPs remain in use, arguments against a link between radioactive releases during normal operation and childhood cancer must be weighed against the concerns of the population and policy makers. Regardless of whether or not such a link exists, a distrust of nuclear power stemming from the fear of catastrophic events is likely to remain.
We suggest that resources would be best focused on basic research into the aetiology of childhood cancer, including critical developmental processes and the temporal aspects of disease initiation and progression, and on versatile epidemiological studies that can address various hypothesised disease causes in parallel.
Acknowledgement: We thank Kali Tal for her editorial assistance.
References
1 Black D. Investigation of the Possible Increased Incidence of Cancer in West Cumbria; Report of the Independent Advisory Group. London: HMSO; 1984.
2 Laurier D, Jacob S, Bernier MO, Leuraud K, Metz C, Samson E, et al. Epidemiological studies of leukaemia in children and young adults around nuclear facilities: a critical review. Radiat Prot Dosimetry. 2008;132(2):182–90.
3 Laurier D, Grosche B, Hall P. Risk of childhood leukaemia in the vicinity of nuclear installations – findings and recent controversies. Acta Oncol. 2002;41(1):14–24.
4 Committee on Medical Aspects of Radiation in the Environment (COMARE). Fourteenth report. Further consideration of the incidence of childhood leukaemia around nuclear power plants in Great Britain. Chilton: Health Protection Agency; 2011.
5 Kaatsch P, Spix C, Jung I, Blettner M. Childhood leukemia in the vicinity of nuclear power plants in Germany. Dtsch Arztebl Int. 2008;105(42):725–32.
6 Spycher BD, Feller M, Zwahlen M, Roosli M, von der Weid NX, Hengartner H, et al. Childhood cancer and nuclear power plants in Switzerland: a census-based cohort study. Int J Epidemiol. 2011;40(5):1247–60.
7 Sermage-Faure C, Laurier D, Goujon-Bellec S, Chartier M, Guyot-Goubin A, Rudant J, et al. Childhood leukemia around French nuclear power plants – the Geocap study, 2002–2007. Int J Cancer. 2012;131(5):E769–80.
8 Bollaerts K, Fierens S, Simons K, Francart J, Poffijjn A, Sonk M. Monitoring of Possible Health Effects of Living in the Vicinity of Nuclear Sites in Belgium. Brussels: Institut Scientifique de Santé Publique WIV-ISP; 2012.
9 Koerblein A, Fairlie I. French geocap study confirms increased leukemia risks in young children near nuclear power plants. Int J Cancer. 2012;131(12):2970–1.
10 Wakeford R. The risk of childhood leukaemia following exposure to ionising radiation – a review. J Radiol Prot. 2013;33(1):1–25.
11 Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505.
12 Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia. 2013;27(1):3–9.
13 BfS. Epidemiological Study of Childhood Cancer in the Vicinity of Nuclear Power Plants – KiKK Study. Concluding Statement of the Federal Office for Radiation Protection (September 2009). Salzgitter: Federal Office for Radiation Protection; 2009.
14 United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Report 2000, Sources and effects of ionizing radiation, vol. I Sources, Annex B, Exposures from natural radiation sources. United Nations; 2000.
15 United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Report 2000, Sources and effects of ionizing radiation, vol. I Sources, Annex C, Exposures from man-made sources of radiation. United Nations; 2000.
16 Federal Office of Public Health. Umweltradioaktivität und Strahlendosen in der Schweiz. Swiss Federal Office of Public Health, Radiological Protection Division; 2011. German.
17 Stiller CA, Marcos-Gragera R, Ardanaz E, Pannelli F, Almar Marques E, Canada Martinez A, et al. Geographical patterns of childhood cancer incidence in Europe, 1988–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):1952–60.
18 Mitter V, Michel G, Wölfli P, Gianinazzi M, Rüegg C, Sommer G, et al. Swiss Childhood Cancer Registry – Annual Report 2011/12. Bern: Institute of Social and Preventive Medicine (ISPM), University of Bern; 2013.
19 Michel G, von der Weid NX, Zwahlen M, Redmond S, Strippoli MP, Kuehni CE, et al. Incidence of childhood cancer in Switzerland: the Swiss Childhood Cancer Registry. Pediatr Blood Cancer. 2008;50(1):46–51.
20 Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh J-W, Lacour B, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet. 2004;364(9451):2097–105.
21 Wiemels J. Perspectives on the causes of childhood leukemia. Chem Biol Interact. 2012;196(3):59–67.
22 Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–65.
23 Enciso-Mora V, Hosking FJ, Sheridan E, Kinsey SE, Lightfoot T, Roman E, et al. Common genetic variation contributes significantly to the risk of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia. 2012;26(10):2212–5.
24 Portier C. Risk factors for childhood leukaemia. Discussion and summary. Radiat Prot Dosimetry. 2008;132(2):273–4.
25 Little MP, Wakeford R, Kendall GM. Updated estimates of the proportion of childhood leukaemia incidence in Great Britain that may be caused by natural background ionising radiation. J Radiol Prot. 2009;29(4):467–82.
26 Calvente I, Fernandez MF, Villalba J, Olea N, Nunez MI. Exposure to electromagnetic fields (non-ionizing radiation) and its relationship with childhood leukemia: a systematic review. Sci Total Environ. 2010;408(16):3062–9.
27 Pyatt D, Hays S. A review of the potential association between childhood leukemia and benzene. Chem Biol Interact. 2010;184(1-2):151–64.
28 Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Residential exposure to pesticides and childhood leukaemia: a systematic review and meta-analysis. Environ Int. 2011;37(1):280–91.
29 Infante-Rivard C. Chemical risk factors and childhood leukaemia: a review of recent studies. Radiat Prot Dosimetry. 2008;132(2):220–7.
30 Greaves M. Pre-natal origins of childhood leukemia. Rev Clin Exp Hematol. 2003;7(3):233–45.
31 Wiemels J. Chromosomal translocations in childhood leukemia: natural history, mechanisms, and epidemiology. J Natl Cancer Inst Monogr. 2008(39):87–90.
32 McNally RJ, Eden TO. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br J Haematol. 2004;127(3):243–63.
33 Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6(3):193–203.
34 Steinmaus C, Lu M, Todd RL, Smith AH. Probability estimates for the unique childhood leukemia cluster in Fallon, Nevada, and risks near other U.S. Military aviation facilities. Environ Health Perspect. 2004;112(6):766–71.
35 McNally RJ, Alexander FE, Vincent TJ, Murphy MF. Spatial clustering of childhood cancer in Great Britain during the period 1969–1993. Int J Cancer. 2009;124(4):932–6.
36 Schmiedel S, Jacquez GM, Blettner M, Schuz J. Spatial clustering of leukemia and type 1 diabetes in children in Denmark. Cancer Causes Control. 2011;22(6):849–57.
37 Demoury C, Goujon-Bellec S, Guyot-Goubin A, Hemon D, Clavel J. Spatial variations of childhood acute leukaemia in France, 1990–2006: global spatial heterogeneity and cluster detection at “living-zone” level. Eur J Cancer Prev. 2012;21(4):367–74.
38 Basta NO, James PW, Craft AW, McNally RJ. Season of birth and diagnosis for childhood cancer in Northern England, 1968–2005. Paediatr Perinat Epidemiol. 2010;24(3):309–18.
39 Sorensen HT, Pedersen L, Olsen J, Rothman K. Seasonal variation in month of birth and diagnosis of early childhood acute lymphoblastic leukemia. JAMA. 2001;285(2):168–9.
40 Gustafsson B, Honkaniemi E, Goh S, Giraud G, Forestier E, von Dobeln U, et al. KI, WU, and Merkel cell polyomavirus DNA was not detected in guthrie cards of children who later developed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2012;34(5):364–7.
41 O’Connor SM, Boneva RS. Infectious etiologies of childhood leukemia: plausibility and challenges to proof. Environ Health Perspect. 2007;115(1):146–50.
42 Committee on Medical Aspects of Radiation in the Environment (COMARE). Second Report. Investigation of the possible increased incidence of leukaemia in young people near the Dounreay Nuclear Establishment, Caithness, Scotland. London: HMSO; 1988.
43 Baker PJ, Hoel DG. Meta-analysis of standardized incidence and mortality rates of childhood leukaemia in proximity to nuclear facilities. Eur J Cancer Care (Engl). 2007;16(4):355–63.
44 Committee on the Analysis of Cancer Risks in Populations near Nuclear Facilities – Phase 1. Analysis of cancer risks in popoulation near nuclear facilities – Appendix A. Washington, D.C.: Nuclear and Radiation Studies Board, Division of Earth and Life Studies, National Research Council; 2012.
45 Spix C, Blettner M. Re: BAKER P.J. & HOEL D.G. (2007) European Journal of Cancer Care16, 355-363. Meta-analysis of standardized incidence and mortality rates of childhood leukaemia in proximity to nuclear facilities. Eur J Cancer Care (Engl). 2009;18(4):429–30.
46 Laurier D, Hemon D, Clavel J. Childhood leukaemia incidence below the age of 5 years near French nuclear power plants. J Radiol Prot. 2008;28(3):401–3.
47 Bithell JF, Keegan TJ, Kroll ME, Murphy MF, Vincent TJ. Childhood leukaemia near British nuclear installations: methodological issues and recent results. Radiat Prot Dosimetry. 2008;132(2):191–7.
48 Spix C, Schmiedel S, Kaatsch P, Schulze-Rath R, Blettner M. Case-control study on childhood cancer in the vicinity of nuclear power plants in Germany 1980–2003. Eur J Cancer. 2008;44(2):275–84.
49 Bithell JF, Keegan TJ, Kroll ME, Murphy MF, Vincent TJ. Reponse to Letter to the Editor. Radiat Prot Dosimetry. 2010;138(1):87–91.
50 Heinavaara S, Toikkanen S, Pasanen K, Verkasalo PK, Kurttio P, Auvinen A. Cancer incidence in the vicinity of Finnish nuclear power plants: an emphasis on childhood leukemia. Cancer Causes Control. 2010;21(4):587–95.
51 Michaelis J, Keller B, Haaf G, Kaatsch P. Incidence of childhood malignancies in the vicinity of west German nuclear power plants. Cancer Causes Control. 1992;3(3):255–63.
52 Kaatsch P, Kaletsch U, Meinert R, Michaelis J. An extended study on childhood malignancies in the vicinity of German nuclear power plants. Cancer Causes Control. 1998;9(5):529-33.
53 Wakeford R. Childhood leukaemia and radiation exposure of fathers – the end of the road, perhaps? J Radiol Prot. 2003;23(4):359–62.
54 Committee on Medical Aspects of Radiation in the Environment (COMARE). Fourth Report. The incidence of cancer and leukaemia in young people in the vicinity of the Sellafield site, West Cumbria: Further studies and an update of the situation since the publication of the report of the Black Advisory Group in 1984. London: Department of Health; 1996.
55 Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988;2(8624):1323–7.
56 Kinlen L, Doll R. Population mixing and childhood leukaemia: Fallon and other US clusters. Br J Cancer. 2004;91(1):1–3.
57 Francis SS, Selvin S, Yang W, Buffler PA, Wiemels JL. Unusual space-time patterning of the Fallon, Nevada leukemia cluster: Evidence of an infectious etiology. Chem Biol Interact. 2012;196(3):102–9.
58 Kinlen LJ. An examination, with a meta-analysis, of studies of childhood leukaemia in relation to population mixing. Br J Cancer. 2012;107(7):1163–8.
59 Laplanche A, de Vathaire F. Leukaemia mortality in French communes (administrative units) with a large and rapid population increase. Br J Cancer. 1994;69(1):110–3.
60 Koushik A, King WD, McLaughlin JR. An ecologic study of childhood leukemia and population mixing in Ontario, Canada. Cancer Causes Control. 2001;12(6):483–90.
61 Wartenberg D, Schneider D, Brown S. Childhood leukaemia incidence and the population mixing hypothesis in US SEER data. Br J Cancer. 2004;90(9):1771–6.
62 Bithell JF. The choice of test for detecting raised disease risk near a point source. Stat Med. 1995;14(21-22):2309–22.
63 United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Report 2000, Sources and effects of ionizing radiation, vol. I Sources, Annex A, Dose assessment methodologies. United Nations; 2000.
64 Kaatsch P, Spix C, Schmiedel S, Schulze-Rath R, Mergenthaler A, Blettner M. Umweltforschungsplan des Bundesumweltministeriums (UFOPLAN). Reaktorsicherheit und Strahlenschutz. Vorhaben StSch 4334: Epidemiologische Studie zu Kinderkrebs in der Umgebung von Kernkraftwerken (KiKK-Studie). Zusammenfassung/Summary. Bundesamt für Strahlenschutz; 2010. German.
65 Strahlenschutzkommission (SSK). Bewertung der epidemiologischen Studie zu Kinderkrebs in der Umgebung von Kernkraftwerken (KiKK-Studie). Stellungnahme der Strahlenschutzkommission. Berlin: H. Hoffmann GmbH – Fachverlag; 2008..
66 Sermage-Faure C, Demoury C, Rudant J, Goujon-Bellec S, Guyot-Goubin A, Deschamps F, et al. Childhood leukaemia close to high-voltage power lines – the Geocap study, 2002–2007. Br J Cancer. 2013;108(9):1899–906.
67 McLaughlin JR, Clarke EA, Nishri ED, Anderson TW. Childhood leukemia in the vicinity of Canadian nuclear facilities. Cancer Causes Control. 1993;4(1):51–8.
68 Kaatsch P, Spix C, Schulze-Rath R, Schmiedel S, Blettner M. Leukaemia in young children living in the vicinity of German nuclear power plants. Int J Cancer. 2008;122(4):721–6.
69 Wing S, Richardson DB, Hoffmann W. Cancer risks near nuclear facilities: the importance of research design and explicit study hypotheses. Environ Health Perspect. 2011;119(4):417–21.
