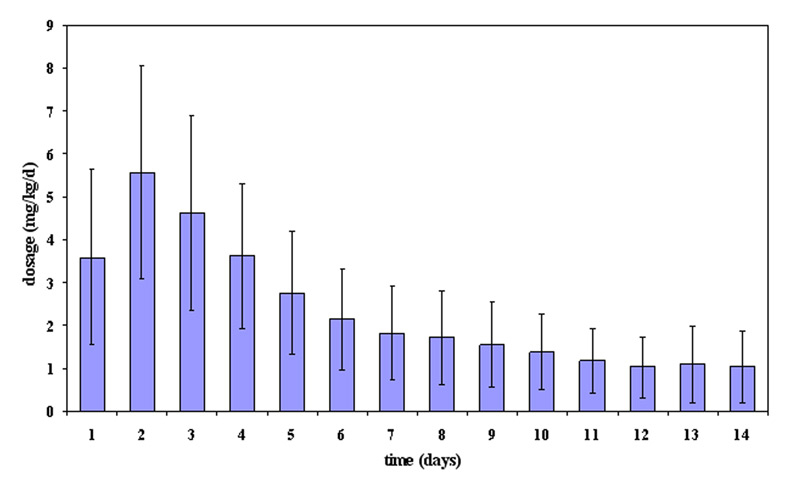
Figure 1
Mean daily hydrocortisone dosage (mg/kg/day) ± standard deviation during the first 14 days of treatment.
DOI: https://doi.org/10.4414/smw.2014.13954
Abbreviations
BPD bronchopulmonary dysplasia
ELBW extremely low birth weight
FIP focal intestinal perforation
HCM hypertrophic cardiomyopathy
IUGR intrauterine growth restriction
NDI neurodevelopmental impairment
NICU neonatal intensive care unit
PDA persistent ductus arteriosus
PNS postnatal steroids
RDS respiratory distress syndrome
ROP retinopathy of prematurity
VLBW very low birth weight
The use of systemic postnatal steroids (PNS) in preterm neonates has been discouraged because of a number of severe adverse events, most importantly poor neurological outcome including cerebral palsy [1–3]. In 2002, the American Academy of Pediatrics and the Canadian Paediatric Society published recommendations against routine use of PNS to prevent bronchopulmonary dysplasia (BPD) [4]. These academic societies also recommended limiting use of PNS to exceptional clinical circumstances and in carefully designed randomised controlled trials, and obtaining informed consent from parents prior to administration of PNS, and suggested performing further clinical trials investigating the use of alternative anti-inflammatory corticosteroids [4]. Moreover, it is of utmost importance to apply expert clinical judgement when attempting to balance the potential adverse effects of glucocorticoid treatment with those of BPD [5]. Conversely, some data recently published indicate that low-dose hydrocortisone therapy may be beneficial with regard to neurodevelopmental outcome in extremely low birth weight (ELBW) infants [6].
Following these guidelines, a substantive reduction in the use of PNS administration was noted [7]. In 2004, the Canadian Neonatal Network (CNN) reported a decrease in PNS utilisation to 2.6% of infants <33 weeks at birth [3]. However, and of importance, this trend has been reversed lately with an increase in PNS administration (8.7% of preterm infants in the CNN annual report <33 weeks gestation in 2010). In parallel, a decrease in dexamethasone use between a 1997–1999 cohort and a 2004–2006 cohort was seen by the Pediatrix network in the USA, whereas hydrocortisone use increased [8]. This trend may be caused by a number of factors: First, steroids increase blood pressure, leading to their use in hypotensive conditions and shock [9, 10]. Second, given the association of BPD and neurodevelopmental impairment (NDI), the use of PNS may be beneficial in neonatal cohorts with a very high risk of developing BPD, thus improving survival without BPD and without NDI [11, 12]. Third, PNS are often given for weaning from the respirator [13] or during episodes of respiratory instability.
In addition to long-term sequelae associated with steroid use, other relevant short-term adverse events may be seen, including arterial hypertension, hyperglycaemia, hypernatraemia, hypokalaemia, transient hypertrophic cardiomyopathy (HCM), and a procoagulant state with an increased risk of thrombus formation. HCM has been described in preterm infants after the use of steroids such as dexamethasone and methylprednisolone in the neonatal period and betamethasone in the prenatal period [14, 15]. However, there are only a few case reports/series of HCM occurring after treatment with hydrocortisone [16].
Despite resurgence in PNS use, only a limited number of reports are available in published form that have critically assessed local practice [13], and have specifically examined the incidence of other, non-neurological complications. Moreover, it must be noted that there has been a switch to using hydrocortisone instead of dexamethasone in recent years [8]. Whereas most reports published in the current literature have focused on NDI associated with the use of systemic steroids, the aim of this prospective audit in our neonatal intensive care unit (NICU) was to assess short-term complications, most importantly the incidence of HCM, thrombus formation, blood glucose and electrolyte disturbances, and infectious complications. Of note, in our NICU there has been a history of using steroids in relatively high frequency and dose, and it was our intention to scrutinise this practice. This practice in our unit was, in part, based on promising results from recent clinical studies demonstrating beneficial effects of hydrocortisone therapy on neurodevelopmental outcome while improving respiratory function [6]. Other relevant parameters that were assessed included: risk/predictive factors for steroid use, type of steroid (hydrocortisone, dexamethasone), dosage, indication for use, time interval and time point when given. Furthermore, we assessed important standard variables including retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), necrotising enterocolitis (NEC) / focal intestinal perforation (FIP), BPD, and basic growth parameters/anthropometric data (body weight, length, head circumference).
This prospective cohort analysis was performed at the University Children’s Hospital of Saarland, Homburg, Germany. Institutional Review Board approval was obtained prior to the study from the Institutional Review Board and Ethics Committee of the University Hospital of Saarland, Saarbrücken, Germany.
We prospectively evaluated all inborn infants with a birth weight <1500 g, born between 1 January 2012 and 31 December 2012. Exclusion criteria were severe congenital malformations, most importantly cardiovascular malformations, and a positive family history for HCM. However, neonates with a foramen ovale and/or a persistent ductus arteriosus (PDA) were not excluded. The following parameters were assessed in our study:
Type of steroid used (hydrocortisone/dexamethasone, others), dosage, length of administration, number of steroid courses, time points when given were evaluated, as well as whether or not the treatment was in line with specific guidelines/recommendations. The indication for steroid use was at the discretion of the treating physician. We also assessed predictive/risk factors for steroid use using a multi-regression analysis.
The main outcome parameters were:
a) HCM was suspected on clinical findings (new heart murmur, unexplained tachycardia and arterial hypotension, poor peripheral perfusion). For echocardiographic diagnosis of HCM, we used age-dependent reference values for interventricular septum thickness (diastolic/systolic), left interventricular diameter at end diastole (end systole), and left ventricular posterior wall thickness at end diastole/systole as described by Skelton et al. [17], including measurement of intraventricular pressure gradients. HCM was diagnosed on the basis of clinical findings and confirmed by echocardiography when measured values, as detailed above, were in excess of two standard deviations (SDs) above the mean values provided by Skelton et al. [17].
b) Thrombus formation (detected by repeat ultrasound examination in at least two planes).
c) Hyperglycaemia (>180 mg/dl) [according to 18].
d) Hypernatraemia (>145 mml/l).
e) Hypokalaemia (<3.5 mmol/l).
f) Infections and catheter-related bloodstream infections as defined by the National Healthcare Safety Network DoHQP [19].
The incidences of the following parameters were also analysed:
g) Necrotising enterocolitis (NEC) using Bell’s criteria (stage ≥2a) [20] and focal intestinal perforation (FIP).
h) Bronchopulmonary dysplasia (BPD) according to Bancalari and Jobe [21].
i) Any grade of intraventricular haemorrhage (IVH) according to Papile et al. [22].
j) Retinopathy of prematurity (ROP) according to the International Committee for the Classification of Retinopathy of Prematurity [23].
k) Growth patterns according to Voigt et al. [24].
In our unit, serial echocardiography and ultrasonography studies were performed by the attending neonatologist and cardiologist. Studies were routinely performed on days 1, 2, 3, 5 and 7. Further studies were performed according to the clinical status of the neonate (at least twice weekly), and adjustments were made at the discretion of the treating consultant.
Blood pressure was measured either invasively from an indwelling umbilical arterial line or a peripheral arterial catheter, or noninvasively using weight-dependent cuff sizes. Blood pressure was considered pathologically low when more than two SDs below the mean [25]. Furthermore, initial treatment included insertion of an umbilical venous catheter (removed between days 3–6) or a peripherally inserted central line. Laboratory studies included routine daily blood gas analysis, serum chemistry and blood count at the discretion of the treating physician.
The decision to start steroid treatment (initial drug: hydrocortisone) was at the discretion of the attending physician. This decision was taken individually on the basis of important clinical and pathophysiological parameters taken at the bedside (e.g., ongoing need for ventilator support, failure to wean from the ventilator, fraction of inspired oxygen [FiO2], arterial hypotension, etc.). No formal protocol in written form for steroid use existed in our NICU. Treatment was initially started intravenously, and the largest proportion of the study drug was given intravenously. If the cumulative oral drug dose was >10% of total steroid dose, we adjusted the equivalence of oral vs intravenous hydrocortisone using a ratio of 8:5 [26].
All relevant comedication was assessed; in particular, the use of insulin and inotropes were recorded as they may contribute to the development of HCM.
Weight was measured daily or every other day depending on clinical stability and necessity; head circumference and body length were recorded weekly from birth to discharge. For comparison of head circumference, we used the percentiles published by Voigt et al. [24].
Relevant data were retrieved from an electronic hospital database (SAP, Germany) as well as from patients’ hospital charts. Data are presented as median, mean, range, and SD. For data interpretation we used frequency tables and cross-tables. For comparison of categorical variables the Pearson chi-square test was employed. The Fisher exact test was used if prerequisites for the Pearson chi-square test were not met. Continuous variables were compared with the T-test for normally distributed variables, and the Wilcoxon/Kruskal-Wallis test for variables not normally distributed. Also, logistic regression was performed. The outcome variable was administration of steroids (yes/no) and the explanatory variables were birth weight (<1,000 g vs ≥1,000–1,499 g), sex, intrauterine growth restriction (IUGR), Apgar score at 5 minutes, severity of respiratory distress syndrome (grade I/II vs grade III/IV), and use of drugs (catecholamines/inotropes).
The influence of birth weight, IUGR, Apgar score at 5 minutes, severity of RDS (grade I/II vs grade III/IV) on the dose of steroids was evaluated with multiple linear regression.
Statistical significance was assumed at p ≤0.05. All statistical analyses were performed using SPSS, 20.0, Chicago, Illinois, USA.
| Table 1:Patients' demographics (given as mean, standard deviation and range). | ||||
| Parameter | Complete cohort (n = 72) | No steroids (n = 38) | Steroids (n = 34) | Significance (p-value) |
| Antenatal steroids | 56 (77.8%) | 30 (78.9%) | 26 (76.5%) | p = 0.801 |
| Apgar (1 min) | 5.2 ± 2.2 (1‒9) | 6.3 ± 1.8 (2‒9) | 3.9 ± 1.9 (1–7) | p <0.001 |
| Apgar (5 min) | 7.5 ± 1.6 (3‒10) | 8.3 ± 1.2 (6‒10) | 6.6 ± 1.6 (3–9) | p <0.001 |
| Apgar (10 min) | 8.4 ± 1.1 (5‒10) | 8.9 ± 0.8 (7‒10) | 7.7 ± 1.1 (5–10) | p <0.001 |
| pH (umbilical artery) | 7.30 ± 0.11 (6.80–7.52) | 7.33 ± 0.07 (7.16–7.45) | 7.27 ± 0.14 (6.80–7.52) | p = 0.011 |
| Gestational age (weeks) | 28.5 ± 2.4 (23.3–33.2) | 30.6 ± 1.4 (26.3–33.2) | 26.4 ± 1.5 (23.3–30.5) | p <0.001 |
| Birth weight (grams) | 967 ± 338 (320–1,490) | 1,192 ± 221 (740–1,490) | 716 ± 263 (320–1,360) | p <0.001 |
| Hospital stay (days) | 81.6 ± 35.9 (35–200) | 62.3 ± 24.1 (35–173) | 107.9 ± 32.6 (66–200) | p <0.001 |
| Mechanical ventilation (days) | 5.8 ± 7.9 (0–39) | 2.3 ± 4.6 (0–24) | 9.7 ± 8.9 (1–39) | p <0.001 |
| Table 2:Comparison of underlying and evolving medical problems and mortality between the two groups. | ||||||
| Complications | No steroids (n = 38) | Steroids (n = 34) | p-value (chi-square or Fisher test) | Odds ratio (95% confidence interval) | ||
| n | % | n | % | |||
| IUGR | 14 | 36.8 | 16 | 47.1 | 0.38 | 1.5 (0.6–3.9) |
| PDA | 8 | 21.1 | 14 | 41.2 | 0.077 | 2.6 (0.9–7.4) |
| PVL | 2 | 5.3 | 5 | 14.7 | 0.243 | 3.1 (0.6–17.2) |
| RDS | 28 | 73.7 | 33 | 97.1 | 0.007 | 11.8 (1.4–97.8) |
| Arterial hypotension* | 20 | 52.6 | 33 | 97.1 | <0.001 | 29.7 (3.7–239.8) |
| Surfactant administration | 3 | 7.8 | 26 | 76.4 | <0.001 | 37.9 (9.2–157.0) |
| Mechanical ventilation | 11 | 28.9 | 31 | 91.2 | <0.001 | 25.4 (6.4–100.5) |
| BPD | 5 | 13.2 | 22 | 64.7 | <0.001 | 12.1 (3.7–39.2) |
| Sepsis/CRBSI | 1 | 2.6 | 8 | 23.5 | 0.011 | 11.4 (1.3–96.6) |
| NEC | NA | |||||
| FIP | 1 | 2.6 | 2 | 5.9 | 0.565 | 2,3 (0.2–26.7) |
| ROP | 11 | 29.0 | 19 | 55.9 | 0.031 | 3.1 (1.2–8.2) |
| IVH (all grades) | 4 | 10.5 | 14 | 41.2 | 0.003 | 6.0 (1.7–20.6) |
| Seizures at discharge | 3 | 8.8 | 0.061 | NA | ||
| Mortality | 1 | 2.6 | 7 | 20.5 | 0.02 | 9.6 (1.1–82.6) |
| BPD = bronchopulmonary dysplasia; CRBSI = catheter-related blood stream infections; FIP = focal intestinal perforation; IUGR = intrauterine growth retardation; IVH = intraventricular haemorrhage; NA = not applicable; NEC = necrotising enterocolitis; PDA = persistent ductus arteriosus; PVL = periventricular leukomalacia; RDS = respiratory distress syndrome; ROP = retinopathy of prematurity * mandating inotropic support (dopamine, noradrenaline or adrenaline) | ||||||
| Table 3a:Results of logistic regression analysis. | |||
| Explanatory variable | Odds ratio | 95% confidence interval | p-value |
| First step: only birth weight was statistically significant: | |||
| Birth weight | 209.0 | 15‒2,903.7 | <0.001 |
| Second step: after omission of birth weight: | |||
| Severity of RDS (grade I/II vs III/IV) | 9.9 | 2.5‒39.1 | 0.001 |
| Apgar2 | 0.3 | 0.2‒0.6 | <0.001 |
| RDS = respiratory distress syndrome | |||
Between 1 January 2012 and 31 December 2012, 72 VLBW infants (m: 34, f: 38; 44 singletons, and 28 twins and triplets) were included in this study. Patients’ demographics and details with regard to treatment modalities are depicted in tables 1 and 2. Birth weight, gestational age and Apgar scores were significantly lower in the steroid group (p <0.01). Mortality rate was significantly higher in the steroid group (7/34 in steroid treated vs nontreated 1/38; OR: 9.6; 95% CI: 1.1–82.6; p = 0.02) (table 2).

Figure 1
Mean daily hydrocortisone dosage (mg/kg/day) ± standard deviation during the first 14 days of treatment.
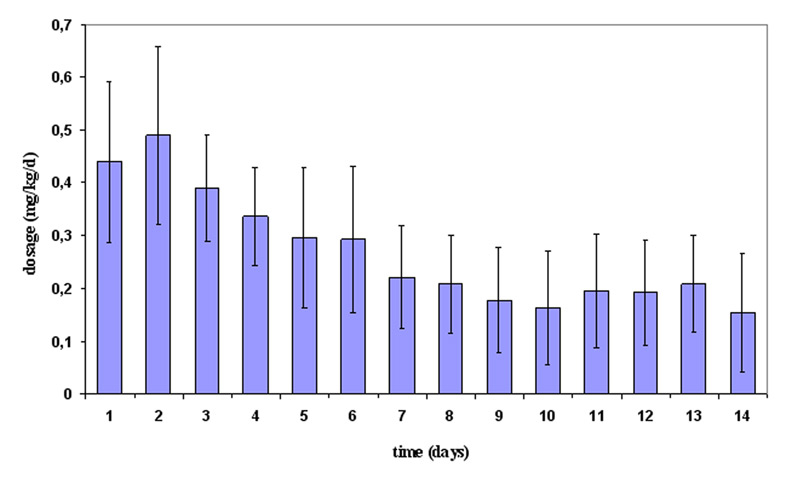
Figure 2
Mean daily dexamethasone dosage (mg/kg/day) ± standard deviation during the first 14 days of treatment.
In total, 34/72 patients were given steroids, most commonly hydrocortisone alone (n = 22); 12 neonates received additional dexamethasone. Steroids were given at an initial high dose and were subsequently tapered as shown in figures 1 and 2. The cumulative doses of steroids for hydrocortisone alone were 39.5 ± 24.6 mg/kg (95% CI: 28.6–50.4 mg/kg), and for the combination of hydrocortisone (38.0 ± 21.7 mg/kg; 95% CI: 24.2–51.7 mg/kg) and dexamethasone (4.6 ± 2.8 mg/kg; 95% CI: 2.8–6.4 mg/kg). In most cases, a single course of steroids was given with the number of steroid courses ranging from one to four (one course n = 21; two courses n = 10; three courses n = 2; four courses n = 1). Overall, mean duration of hydrocortisone administration was 23.3 ± 16.0 days (range: 2–78), mean duration of dexamethasone was 25.5 ± 18.3 days (range: 1–62). In all infants, hydrocortisone was always given as the initial drug. Hydrocortisone administration was most commonly started in the first week of life (29/34), followed by the second and third week of life (each 2), and only rarely in the fourth week of life (1).
The most common indication for use of steroids was RDS and respiratory insufficiency, including failure to wean from the ventilator (“failure to successfully extubate”) (32/34), and arterial hypotension (2/34). Birth weight (<1,000 grams) and severity of RDS (grade III/IV) were predictors of steroid use (table 3a). The multiple linear regression model demonstrated no specific predictors for steroid dose (table 3b). Adverse events that occurred significantly more often in the steroid group are depicted in (table 2 and table 4a).
HCM was first noted after a median time interval of 5.5 days (range: 1–20 days), and thrombus formation 5 days (1–36 days) after starting steroid treatment. Of note, FIP was seen in two infants with steroid treatment and in one patient without steroid administration, but no episodes of NEC occurred in either group. No significant differences were recorded in arterial blood pressure between the two groups over time. We discovered no significant differences between hydrocortisone alone and the combination of hydrocortisone with dexamethasone (table 4b).
Whereas HCM was reversible after reducing and stopping steroids in all patients, one thrombus persisted beyond discharge in one infant, and one infant died after developing massive thrombosis of the superior vena cava and right atrium. Long-term complications, including ROP, IVH, and BPD occurred significantly more often in the steroid-treated group, as detailed in table 2. Also, head circumferences at discharge (nontreated infants were discharged significantly earlier; table 2) were lower in the hydrocortisone group (34.0 ± 1.9 cm; 25th–50th percentiles) and dexamethasone group (34.9 ± 2.2 cm; 25th–50th percentiles) compared to the non-treated group (34.0 ± 3.2 cm; 50th–75th percentiles) when corrected for age.
Informed written consent to administer steroids was not documented in the patients’ charts.
| Table 3b: Result of linear regression analysis. | |
| Explanatory variable | p-value |
| Severity of RDS (grade I/II vs III/IV) | 0.764 |
| Apgar2 | 0.114 |
| Birth weight | 0.582 |
| IUGR | 0.917 |
| BPD | 0.445 |
| BPD = bronchopulmonary dysplasia; IUGR = intrauterine growth retardation; RDS = respiratory distress syndrome | |
| Table 4a: Adverse events associated with steroid treatment compared with no steroid treatment. | |||||
| Adverse events | Steroids (n = 34) | No steroids (n = 38) | p-value odds ratio (95% confidence interval) | ||
| n | % | n | % | ||
| Cardiomyopathy | 14 | 41.2 | <0.001 | ||
| Thrombosis | 8 | 23.5 | 1 | 2.6 | 0.011; 11.4 (1.3–96.6) |
| Hyperglycaemia | 27 | 79.4 | 3 | 7.9 | <0.001; 45.0 (10.6–190.4) |
| Insulin therapy | 16 | 47.1 | 2 | 5.3 | <0.001; 20.3 (4.2–97.8) |
| Hypernatraemia | 15 | 44.1 | 7 | 18.4 | 0.023; 3.5 (1.2–10.1) |
| Hypokalaemia | 7 | 31.8 | 2 | 5.3 | 0.075; 4.7 (0.9–24.3) |
| Table 4b: Adverse events associated with hydrocortisone treatment compared with dexamethasone/hydrocortisone treatment. | |||||
| Adverse events | Hydrocortisone (n = 22) | Dexamethasone/Hydrocortisone (n = 12) | p-value odds ratio (95% confidence interval) | ||
| n | % | n | % | ||
| Cardiomyopathy | 8 | 36.3 | 6 | 50 | 0.487; 1.75 (0.4–7.3) |
| Thrombosis | 6 | 27.2 | 2 | 16.7 | 0.681; 0.53 (0.1–3.2) |
| Insulin | 10 | 45.5 | 8 | 66.7 | 0.297; 2.4 (0.6–10.4) |
| Hyperglycaemia | 16 | 72.7 | 11 | 91.8 | 0.378; 4.125 (0.4–39.2) |
| Hypernatraemia | 10 | 45.5 | 6 | 50 | 1.0; 1.2 (0.3–4.9) |
| Hypokalaemia | 7 | 20.6 | 1 | 8.3 | 0.21; 0.2 (0.2–1.8) |
This is one of only a few reports that systematically and prospectively assessed the occurrence of cardiovascular and metabolic adverse events in VLBW infants receiving hydrocortisone/dexamethasone treatment. In the current medical literature, most reports have assessed the incidence of HCM in preterm neonates who received high-dose dexamethasone therapy only, but not in infants receiving hydrocortisone [16]. In our study we could demonstrate that hydrocortisone administration was associated with a high incidence of both HCM (14/34) and thrombus formation (8/34) – two significant adverse effects that may lead to severe clinical compromise. As a matter of fact, one infant died after developing massive thrombosis of the superior vena cava and right atrium. However, other relevant adverse events (hyperglycaemia, hypernatraemia, and infectious complications) also occurred significantly more often in the hydrocortisone/dexamethasone group; FIP was seen in two patients treated with steroids, and in one patient in the nonsteroid group. The two variables that were strongly associated with the administration of steroids were birth weight and severity of RDS. In accordance with previous studies, the use of steroids in our cohort was higher in smaller, less mature and sicker infants [27, 28].
Of note, the single most important reason for steroid administration was RDS and evolving BPD. There is increasing evidence from both human and animal research that has shown a strong association between BPD and NDI [29]. Conversely, PNS use significantly reduces the risk of BPD, most notably following early administration [30], which appears to be dependent both on dose and baseline BPD risk. A meta-regression of all corticosteroid trials assessing death or cerebral palsy reported this outcome to be inversely related to BPD risk at trial entry. When baseline BPD risk was below 35%, corticosteroid treatment significantly increased the risk of death or cerebral palsy [3, 31–33]), whereas when BPD risk exceeded 65% [34]), it reduced this risk. Conversely, use of even moderate doses of dexamethasone in high-risk infants was associated with regional brain volume deficits in observational studies [35]. It is unclear if this reflects dexamethasone toxicity or risks of the underlying lung disease. However, despite steroid use, the incidence of BPD in our cohort was still substantial when compared with recently published data [36] and significantly higher in the steroid group. It is also noteworthy that head growth in our study cohort was poorer in the steroid-treated group (25th–50th vs 50th–75th percentile in the nonsteroid group) – irrespective of type of steroid. However, this abnormal growth pattern may also in part be attributed to underlying intracranial pathologies (IVH, PVL) that occurred more often in the steroid group [37].
However, whereas previous reports have focussed on neurological issues (head circumference and brain volume) [34, 37, 38], the main objective of this study was to assess cardiac and metabolic adverse events. This because we had seen a number of infants who developed either HCM or thrombus formation (unpublished data) prior to this study in our NICU, and it was our aim to study the possible association between hydrocortisone/dexamethasone use and HCM and thrombus formation in more detail and more systematically. Of note, the use of steroids in our cohort was substantially higher than in previous reports [8, 28]. In a recently published report, hydrocortisone was given to 35% of ELBW infants [8]. Also, it must be mentioned that we used hydrocortisone most often within the first week of life, which is also in contrast to the study results from Fortin-Pellerin et al. [8]. Although no official recommendations exist with regard to the use and timing of hydrocortisone, national and international guidelines and meta-analyses (Cochrane) suggest using steroids (dexamethasone) – if at all ‒ between day 8 and day 21 to minimise neurological impairment while maximising pulmonary improvements [39]. Similar to the results published by Fortin-Pellerin et al. [8], informed consent for PNS in written form was not available from the patients’ charts.
Furthermore, it is noteworthy that significant differences existed in the rate of important outcome parameters (IVH, ROP, BPD, sepsis, and mortality) between the two groups. Although an association between steroid use and these morbidities was noted in our cohort, this pattern probably reflects differences in baseline characteristics and subsequent complications (birth weight, gestational age, Apgar scores, length of hospital stay, length of mechanical ventilation; see table 2) rather than a causal link. This is also reflected by significantly different mortality rates between the two groups, which are most likely attributable to underlying differences in baseline morbidity rather than the difference in steroid use.
In principle, our data are comparable to other reports that have demonstrated that steroids – most importantly dexamethasone – may induce HCM [40]. However, to the best of our knowledge, no other report so far has systematically assessed the occurrence of HCM and thrombus formation in VLBW infants while receiving steroids – most importantly hydrocortisone. In a recent report, HCM was substantially more frequent in methylprednisolone-treated than in untreated infants (40% vs 0%; p** <0.0001), but was similar in methylprednisolone- and dexamethasone-treated infants [14]. The most common location for thrombus formation in our cohort was the superior vena cava and the right atrium. Given the time course in the development of HCM and thrombus formation in some of our infants, the use of steroids must be considered one of several contributing factors; other factors that are very likely to have contributed to the development of these adverse effects include the use of inotropes and insulin [41].
Despite a considerable amount of research, the underlying mechanisms of HCM resulting from corticosteroid therapy remain by and large to be elucidated. Some experimental work in rat myocardium have suggested an important role for SGK1 gene transcription which increases cell size, protein synthesis and sarcomere organisation [42] while others have suggested transcriptional mechanisms increasing expression of α-myosin heavy chain [43]. Moreover, it is important to note that the overall number of VLBW infants included in this report was relatively small, thus possibly under- or overestimating the true incidence of HCM and thrombus formation. Moreover, it is noteworthy that the practice of steroid use in our NICU is different from other medical communities with regard to both frequency and dose [6, 28], thus limiting comparability.
We conclude that steroids (in our study most importantly hydrocortisone) should be used very prudently and should be considered only a rescue therapy in case of severe RDS / respiratory failure or profound arterial hypotension [10]. In accordance with current recommendations and published reports, the dose of hydrocortisone should be lower than in our report (approximately 1 mg/kg/day [5, 28]). In line with the results presented by Fortin-Pellerin et al. [1], it is important to assess not only mortality and long-term NDI in preterm neonates who receive PNS, but it is also mandatory to assess relevant short-term complications, including HCM and thrombus formation. When detected early, adequate measures can be initiated, most importantly a reduction and cessation of steroids, adequate fluid management, and anticoagulation as indicated. In doing so, HCM may be fully reversible while thrombus formation may be attenuated.
Acknowledgement:We thank Dagmar Sauer for linguistic help with the manuscript.
Funding / potential competing interests: The authors declare that no conflict is involved with this study. All authors have seen and approved the final version of the manuscript.
1 Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatrics. 2001;1:1.
2 O’Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics. 1999;104:15–21.
3 Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2000; 83:F177–81.
4 Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–8.
5 Watterberg KL; American Academy of Pediatrics. Committee on Fetus and NewbornPolicy statement – postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126(4):800–8. doi: 10.1542/peds.2010-1534. Epub 2010 Sep 6.
6 Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007;120(1):40–8.
7 Payne NR, Finkelstein MJ, Liu M, Kaempf JW, Sharek PJ, Olsen S. NICU Practices and Outcomes Associated With 9 Years of Quality Improvement Collaboratives. Pediatrics. 2010;125:437–46.
8 Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics. 2009;124:673–9.
9 Higgins S, Friedlich P, Seri I. Hydrocortisone for hypotension and vasopressor dependence in preterm neonates: a meta-analysis. J Perinatol. 2010;30:373–8.
10 Ibrahim H, Sinha IP, Subhedar NV. Corticosteroids for treating hypotension in preterm infants. Cochrane Database Syst Rev 2011 Dec 7; 12:CD003662.
11 Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, and the DART Study Investigators. Outcome at 2 years of age of infants from the DART Study: A Multicenter, international, randomized, controlled trial of low-dose dexamethasone. Pediatrics. 2007;119:716–21.
12 Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Impact of Postnatal Corticosteroid Use on Neurodevelopment at 18 to 22 Months’ Adjusted Age: Effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123:e430–7.
13 Finer NN, Powers RJ, Ou C-hS, Durand D, Wirtschafter D, Gould JB; California Perinatal Quality Care Collaborative Executive Committee. Prospective evaluation of postnatal steroid sdministration: A 1-Year experience from the California Perinatal Quality Care Collaborative. Pediatrics. 2006;117:704–13.
14 Dani C, Bertini G, Simone P, Rubaltelli Firmino F. Hypertrophic cardiomyopathy in preterm infants treated with methylprednisolone for bronchopulmonary dysplasia. Pediatrics. 2006;117:1866–7.
15 Khalid YA, Fadi BF, Paula H, Salman MM, Mohamad M. Transient hypertrophic cardiomyopathy in the newborn following multiple doses of antenatal corticosteroids. Am J Perinatol. 1999;16:17–21.
16 Vimala J, Prabhu A, Pavithran S, Kumar RN. Hydrocortisone induced hypertrophic cardiomyopathy. Int J Cardiol. 2011;150:e94-5
17 Skelton R, Gill AB, Parsonsb JM. Reference ranges for cardiac dimensions and blood flow velocity in preterm infants. Heart. 1998; 80:281–5.
18 Ogilvy-Stuart AL, Beardsall K. Management of hyperglycaemia in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F126–31.
19 National Healthcare Safety Network DoHQP, Centers for Disease Control and Prevention: CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/nnis/NosInfDefinitions.pdf (Accessed June 10th, 2013)
20 Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
21 Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9.
22 Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34.
23 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123(7):991–9.
24 Voigt M, Rochow N, Hesse V, Olbertz D, Schneider KT, Jorch G. Short communication about percentile values of body measures of newborn babies. Z Geburtshilfe Neonatol. 2010;214:242–29.
25 Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–79. doi: 10.1016/j.clp.2010.04.002.
26 Derendorf H, Mollmann H, Barth J, Mollmann C, Tunn S, Krieg M. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol. 1991;31:473–6.
27 Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J Pediatr. 2013;162(4):685–90.
28 Fortin-Pellerin E, Petersen C, Lefebvre F, Barrington KJ, Janvier A. Evolving neonatal steroid prescription habits and patient outcomes. Acta Paediatr. 2013;102:799–804.
29 Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88(7):509–15.
30 Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98(4):289–96.
31 Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:132.
32 Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115:655–661 33.
33 Helbock HJ, Insoft RM, Conte FA. Glucocorticoid-responsive hypotension in extremely low birth weight neonates. Pediatrics. 1993;92:715–7.
34 Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–72.
35 Stichtenoth G, Demmert M, Bohnhorst B, Stein A, Ehlers S, Heitmann F, et al. Major contributors to hospital mortality in very-low-birth-weight infants: data of the birth year 2010 cohort of the German Neonatal Network. Klin Padiatr. 2012;224:276–81.
36 Phillips JP, Montague EQ, Aragon M, Lowe JR, Schrader RM, et al. Prematurity affects cortical maturation in early childhood. Pediatr Neurol. 2011;45:213–9.
37 Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J Pediatr. 2013;162:685–90.
38 Kersbergen KJ, de Vries LS, van Kooij BJ, Išgum I, Rademaker KJ, van Bel F, et al. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J Pediatr. 2013 May 2.
39 Halliday HL, Ehrenkranz RA, Doyle LW. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2010;(1):CD001146. Review.
40 Zecca E, Papacci P, Maggio L, Gallini F, Elia S, et al. Cardiac adverse effects of early dexamethasone treatment in preterm infants: a randomized clinical trial. J Clin Pharmacol. 2001;41(10):1075–81.
41 Gill AW, Warner G, Bull L Iatrogenic neonatal hypertrophic cardiomyopathy. Pediatr Cardiol. 1996;17:335–9.
42 Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–9.
43 Muangmingsuk S, Ingram P, Gupta MP, Arcilla RA, Gupta M. Dexamethasone induced cardiac hypertrophy in newborn rats is accompanied by changes in myosin heavy chain phenotype and gene transcription. Mol Cell Biochem. 2000;209:165–74.
* Röhr SB, Sauer H, Gortner L, Gräber S, Meyer S. Cardiovascular and metabolic side effects associated with hydrocortisone and dexamethasone use in VLBW infants: a single-centre experience. Acta Paediatr 2013;10:e436.
Authors’ contribution: Sebastian Benedikt Röhr* was responsible for data compilation, data analysis and writing of the manuscript. Harald Sauer was responsible for echocardiographic assessment and writing of the manuscript. Hashim Abdul-Khaliq was responsible for echocardiographic assessment. Sven Gottschling was responsible for data analysis and writing of the manuscript. Holger Nunold was responsible for data analysis and manuscript preparation. Ludwig Gortner was responsible for data analysis and writing of the manuscript. Stefan Gräber was responsible for statistical analysis. Sascha Meyer was chief investigator. He was responsible for study/audit design, data interpretation and writing of the original and revised manuscript. Sascha Meyer* and Sebastian Benedikt Röhr* have contributed equally to this work.