Serotype distribution and antimicrobial susceptibility of group B streptococci in pregnant women: results from a Swiss tertiary centre
DOI: https://doi.org/10.4414/smw.2014.13935
Simone
Fröhlicher, Gabriela
Reichen-Fahrni, Martin
Müller, Daniel
Surbek, Sara
Droz, Barbara
Spellerberg, Parham
Sendi
Summary
OBJECTIVE: To evaluate the rates of penicillin, clindamycin and erythromycin resistance and the serotype distribution among isolates of group B streptococcus (GBS) obtained from pregnant women at the University Hospital of Bern in Switzerland.
METHODS: We prospectively collected screening samples for GBS colonisation at the University Women’s Hospital Bern, Switzerland, between March 2009 and August 2010. We included 364 GBS isolates collected from vaginal, cervical or vaginal-perianal swabs at any gestation time. The minimal inhibitory concentrations for penicillin, clindamycin and erythromycin were established using Etest with 24 hours of incubation, and inducible clindamycin resistance was tested with double disk diffusion tests. Serotyping was done with a rapid latex agglutination test or, if not conclusive, with polymerase chain-reaction (PCR) testing. We looked for significant associations between resistance patterns, age groups, serotype and ethnicity.
RESULTS: All isolates were susceptible to penicillin. Resistance rates were 14.5% for erythromycin and 8.2% for clindamycin. Of 364 isolates, 5.8% were susceptible to clindamycin but not to erythromycin, although demonstrating inducible clindamycin resistance. Hence, the final reported clindamycin resistance rate was 14%. Serotype III was the most frequent serotype (29%), followed by V (25%) and Ia (19%). Serotype V was associated with erythromycin resistance (p = 0.0007). In comparison with all other ethnicities, patients from Asia showed a higher proportion of erythromycin and clindamycin resistance (p = 0.018). No significant association between resistance patterns and age groups was found.
CONCLUSION: In pregnant women with GBS colonisation, penicillin is the antibiotic of choice for intrapartum prophylaxis to prevent neonatal early-onset GBS sepsis. In women with penicillin allergy and at high risk for anaphylactic reaction, clindamycin may be an alternative. The resistance rate for clindamycin at our institution was 14%; therefore, susceptibility must be tested before administration.
Introduction
Streptococcus agalactiae, or group B streptococcus (GBS), is a common cause of neonatal sepsis and meningitis. The pathogenesis of these infections is based on GBS colonisation in the maternal genital tract and on the transmission of the microorganism from the mother to the neonate [1]. The GBS colonisation rate varies between countries, ranging from 6.5% to 36% [2]. The prevalence of anogenital GBS maternal carriage at our centre was 21% [3]. Without intrapartum antimicrobial prophylaxis, the peripartum transmission to the newborn is estimated to be 50% to 70% [2, 4], resulting in a high frequency of early-onset GBS sepsis. With the introduction of a strategy of maternal screening for GBS carriage and the practice of administering intrapartum chemoprophylaxis to colonised mothers, the incidence of neonatal early onset sepsis was significantly reduced in many countries [5–7].
GBS isolates are commonly susceptible to penicillin. Therefore, the recommendation for intrapartum prophylaxis is to administer intravenous penicillin every 4 hours until delivery [6]. However, there are some worrisome reports on reduced penicillin susceptibility in GBS [8–11].
In GBS-colonised mothers with allergy and low risk of anaphylaxis to penicillin, the use of cefazolin is recommended. In those with a high risk of anaphylaxis to penicillin, clindamycin (if the isolate is susceptible) or vancomycin (if the isolate is resistant to clindamycin) is recommended [6]. In our hospital, the risk of anaphylaxis is mainly evaluated on the patient history and the reported host reaction after a previous exposure to a penicillin-derivate. The risk is considered low if there are no signs of an immunoglobulin E (IgE) mediated reaction (e.g., exanthema, drug fever). The risk is considered high if there are indicators for an IgE-mediated reaction (e.g., anaphylactic shock, bronchospasm, angioedema, larynx oedema or Quincke’s oedema).
Published frequencies of erythromycin- and clindamycin-resistant strains range from 7% to 16% and 3% to 9%, respectively [12, 13]. There are, however, geographical variations in resistance rates [14]. In one study that tested 200 GBS isolates collected from vaginal/rectal specimens, the resistance rate was 54% for erythromycin and 33% for clindamycin [15]. The local rates of resistance to clindamycin have a significant impact on the use of antibiotics administered to women with a high risk for anaphylaxis to penicillin.
The aim of this study was to evaluate the rates of penicillin, clindamycin and erythromycin resistance among GBS isolates obtained from pregnant women at the University Hospital of Bern in Switzerland. Given the interesting reports showing an association between erythromycin resistance and serotype V [16–20], we also analysed the serotype distribution among our isolates.
Material and methods
The GBS screening was conducted at the University Women’s Hospital Bern, Switzerland. At our centre, universal screening for GBS is performed in all pregnant women between weeks 35–37 of gestation [3]. As this study focussed on the epidemiology of antimicrobial resistance patterns and serotype distribution among pregnant women, we included all GBS isolates irrespective of gestation time. The isolates were collected via a vaginal, cervical or vaginal-perianal swab. The sampling period started in March 2009 and ended in August 2010. The sampling was performed consecutively, and one isolate per patient was included in the analysis. In the case of multiple sampling from the same individual, the GBS isolate that was closest to the 35th–37th week of gestation was used for analysis. The minimal inhibitory concentrations (MICs) for penicillin, clindamycin and erythromycin were determined with Etest (bioMérieux, Marcy l’Etoile, France). Interpretation of results is based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations for broth microdilution [21]. Inducible resistance to clindamycin (i-clind-R) was assessed with a double disk diffusion test [22, 23]. Serotyping was performed by use of a rapid latex agglutination test (Strep-B-Latex kit, Statens Serum Institut, Copenhagen, Denmark) [24]. Nontypeable strains were examined by polymerase chain-reaction (PCR) analysis [25].
We looked for significant associations between resistance patterns and ethnicity, age groups and serotypes. Prior to the laboratory tests, the variables were defined. Isolates with i-clind-R were considered as clindamycin nonsusceptible. Ethnicity was categorised in patients from Western Europe, Eastern Europe, Africa, South America, Asia and Middle East. Age groups were categorised as 20–29 years, 30–39 and ≥40 years old.
GraphPad Prism 5.0 was used for statistical analysis. Differences in group proportions were assessed by contingency tables and the chi-square test, or Fisher’s exact probability test if a frequency was smaller than 5. For direct comparison a 2 x 2, and for distribution analysis a 6 x 2, 3 x 2, or 10 x 2 contingency tables, respectively, were used. A two-tailed p-value of ≤0.05 was considered significant.
Results
During the screening period, we collected 364 GBS isolates. The susceptibility patterns of the tested antibiotics are presented in figure 1. All isolates were susceptible to penicillin. The penicillin MIC of most ranged from 0.012 to 0.064 μg/ml. Twelve of the 364 isolates (3.3%) showed an MIC of 0.094 μg/ml and only one isolate (0.3%) revealed an MIC of 0.125 µg/ml. A total of 53 (14.6%) isolates were not susceptible to erythromycin, 27 of them (27/53, 50.9%) had a MIC of ≥256 µg/mL. A total of 30 (8.2%) of the 364 isolates were not susceptible to clindamycin. In addition, 21 isolates (5.8%) that were considered clindamycin susceptible in the Etest were i-clind-R when tested by the double disk diffusion test (table 1). Hence, clindamycin could not be recommended as an alternative antimicrobial regimen to penicillin in 14% of the patients (51/364) with proven GBS colonisation.
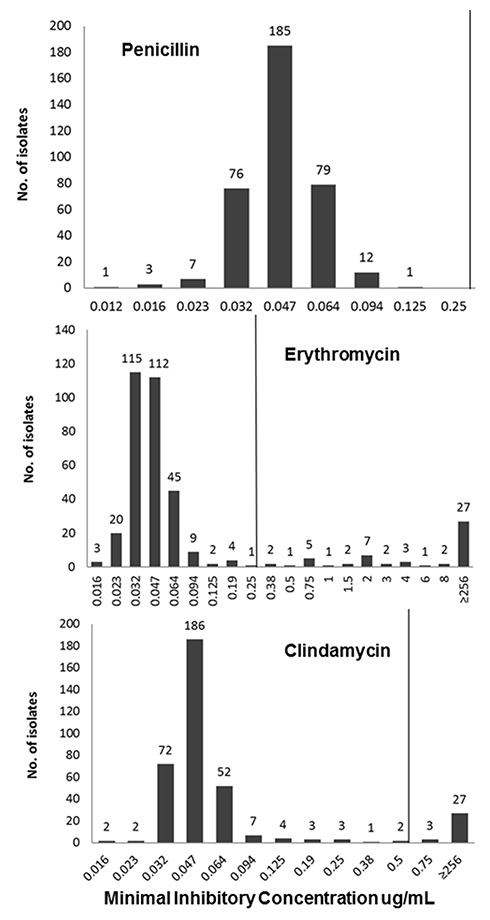
Figure 1
Minimum inhibitory concentration distributions of penicillin, clindamycin and erythromycin.
Clinical breakpoints according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) are indicated by a vertical line. Penicillin susceptible ≤0.25 µg/ml, erythromycin susceptible ≤0.25 µg/ml, clindamycin susceptible ≤0.5 µg/ml.
All GBS isolates could be designated to a serotype. Serotype III was the most frequent serotype (107/364, 29.4%), followed by V (93/364, 25.5%) and by Ia (70/364, 19.2%).
We then analysed the association between erythromycin and clindamycin resistance (table 1). A total of 85% of the isolates (309/364, 84.9%) were susceptible to both clindamycin and erythromycin. On the other hand, 7.7% (28/364) of the isolates were neither susceptible to clindamycin nor to erythromycin. Thus, there was a discrepancy between clindamycin and erythromycin susceptibility in 7.4% isolates (27/364). In 92.6% (25/27) of them, the isolates were susceptible to clindamycin, but not to erythromycin. Though, in 21 of these 25 GBS isolates, there was an i-clind-R, and hence, a typical inducible macrolide-lincosamide-streptogramin B (MLSB) phenotype. Only 4 isolates showed an M phenotype. Of note, the M phenotype is attributed to isolates that are susceptible to clindamycin but not to erythromycin and do not reveal an i-clind-R. The postulated mechanism conferring to this phenotype are active drug efflux pumps encoded by mef genes [26]. Taken together, in 92.5% ([28 + 21 = 49]/53) of the erythromycin nonsusceptible isolates, clindamycin could not be recommended.
We then analysed the association between resistance patterns and origin, age groups and serotypes (table 2). Most of our patients (238/364, 65.4%) were from western European countries. In comparison to all other ethnicities, patients from Asia showed a higher proportion of erythromycin resistance (37.5% vs 13.5%, p = 0.018). However, the overall statistical distribution among areas of origin was not significant (6 x 2 contingency table). We did not find an association between age groups and clindamycin or erythromycin resistance rates. However, the serotype distribution and the resistance to clindamycin and erythromycin showed a significant association (10 x 2 contingency table). In the univariate analysis, the strongest association was found with serotype V and erythromycin resistance (69/311, 22.2% erythromycin susceptible vs 24/53, 45.3% nonsusceptible, p = 0.0007). The associations with serotype Ia and clindamycin susceptibility (p = 0.033), and serotype II with both clindamycin and erythromycin susceptibility (p = 0.043 and 0.026, respectively), were based on small absolute numbers (n <5).
|
Table 1: Association between erythromycin and clindamycin resistance. |
|
|
Total
|
Clindamycin susceptible*
n = 313
|
Clindamycin susceptible* but i-clind-R±
n = 21
|
Clindamycin nonsusceptible*
n = 30
|
| Erythromycin susceptible |
n = 311 |
309 (99.4%1/84.9%2) |
‒ |
2 (0.6%1/0.5%2) |
| Erythromycin nonsusceptible |
n = 53 |
4§ (7.5%1/1.1%2) |
21¶ (39.6%1/5.8%2) |
28 (52.8%1/7.7%2) |
| i-clind-R = inducible clindamycin resistance.
Nonsusceptible isolates consisted of intermediate and resistant isolates.
*Susceptibility determined by Etest for 24 hours.
±Inducible clindamycin resistance was tested by the double disk diffusion test.
§ M phenotype (i.e., clindamycin susceptible, erythromycin nonsusceptible).
¶ Inducible MLSB phenotype.
1 Proportion calculated with denominator being the total number of erythromycin susceptible (311) or nonsusceptible isolates (53), respectively.
2 Proportion calculated with denominator being the total number of isolates included in the study (364). |
|
Table 2: Association between origin, age group and erythromycin/clindamycin resistance. |
|
|
Erythromycin susceptible
n (%)
|
Erythromycin nonsusceptible
n (%)
|
p-value
|
Clindamycin susceptible
n (%)
|
Clindamycinnonsusceptible*
n (%)
|
p-value
|
| Origin |
|
|
0.172±
|
|
|
0.456±
|
| Western Europe |
207 (87) |
31 (13) |
0.325 |
207 (87) |
31 (13) |
0.559 |
| Eastern Europe |
33 (84.6) |
6 (15.4) |
0.920 |
34 (87.2) |
5 (12.8) |
1 |
| Africa |
41 (85.4) |
7 (14.6) |
0.823 |
41 (85.4) |
7 (14.6) |
0.920 |
| South America |
11 (91.7) |
1 (8.3) |
0.704§
|
11 (91.7) |
1 (8.3) |
0.708§
|
| Asia |
10 (62.5) |
6 (37.5) |
0.018
|
11 (68.8) |
5 (31.2) |
0.057 |
| Middle East |
9 (81.8) |
2 (18.2) |
0.493§
|
9 (81.8) |
2 (18.2) |
0.471§
|
| Age groups |
|
|
0.224±
|
|
|
0.269±
|
| 20–29 |
121 (81.8) |
27 (18.2) |
0.134 |
122 (82.4) |
26 (17.6) |
0.143 |
| 30–39 |
168 (88.4) |
22 (11.6) |
0.124 |
168 (88.4) |
22 (11.6) |
0.213 |
| ≥40 |
22 (84.6) |
4 (15.4) |
1§
|
23 (88.5) |
3 (11.5) |
0.783§
|
| Serotype |
|
|
0.009
±
0.003
§
|
|
|
0.008
±
0.003
§
|
| Ia |
65 (92.9) |
5 (7.1) |
0.076 |
66 (94.3) |
4 (5.7) |
0.033
+
|
| Ib |
24 (96) |
1 (4) |
0.149§
|
24 (96) |
1 (4) |
0.152 |
| II |
37 (97.4) |
1 (2.6) |
0.026
§
|
37 (97.4) |
1 (2.6) |
0.043
§
|
| III |
91 (85) |
16 (15) |
1 |
91 (85) |
16 (15) |
0.862 |
| IV |
11 (78.6) |
3 (21.4) |
0.702§
|
11 (78.6) |
3 (21.4) |
0.426§
|
| V |
69 (74.2) |
24 (25.8) |
0.0007
|
70 (75.3) |
23 (24.7) |
0.0010
|
| VI |
1 (100) |
0 (0) |
1§
|
1 (100) |
0 (0) |
1§
|
| VII |
1 (50) |
1 (50) |
0.270§
|
1 (50) |
1 (50) |
0.260§
|
| VIII |
1 (100) |
0 (0) |
1§
|
1 (100) |
0 (0) |
1§
|
| IX |
11 (84.6) |
2 (15.4) |
1§
|
11 (84.6) |
2 (15.4) |
1§
|
| If not indicated otherwise, a chi-square test was performed.
* The numbers include both nonsusceptible isolates determined by Etest for 24 hours and isolates with inducible clindamycin resistance.
±Distribution analysis with 6 x 2, 3 x 2, and 10 x 2 contingency tables, respectively.
§ Fisher’s exact probability test. |
Discussion
At our institution, GBS isolates were uniformly susceptible to penicillin [6, 13, 14, 27, 28]. Hence, penicillin is the compound of choice for intrapartum prophylaxis.
The question of an alternative antimicrobial compound arises when a patient reports an allergy to penicillin. In clinical practice, it is often difficult to distinguish the extent of the allergic reaction. This may particularly be the case under stressed circumstances (e.g., delivery). Therefore, it is important to clarify the type of allergy prior to delivery.
We prospectively collected GBS from colonised women and analysed the clindamycin resistance rate. We looked for variables that were associated with resistance patterns, thereby intending to identify factors that may influence the choice of an antimicrobial compound in the case of unknown resistance patterns (e.g., late screening, preterm delivery).
We found rates of resistance to erythromycin (14.5%) and clindamycin (14.0%) comparable to those reported in other studies published in Europe [14]. However, a recent study from Geneva found higher resistance rates (clindamycin 28%, erythromycin 30%) [29]. This illustrates that there are regional differences even within the same country. In our study, cross-resistance to clindamycin (either constitutive or inducible) was found in 49 isolates (92.5%) of the erythromycin nonsusceptible strains (n = 53). Therefore, in GBS isolates that are not susceptible to erythromycin, resistance to clindamycin should be suspected until proven otherwise. If only the clindamycin Etest (incubation for 24 hours) and no double disk testing is performed, an i-clind-R can remain undetected. Clindamycin would then be reported as susceptible (which is not correct). According to the manufacturer's instructions, an i-clind-R can be detected by Etest, if results are interpreted after 48 hours of incubation. In our view, double disk testing is more established and efficient in routine laboratory work.
The clinical relevance of inducible MLSB-resistance (i-clind-R) is better known in staphylococci than in beta-haemolytic streptococci. The microorganisms show a high rate of spontaneous mutation to constitutive resistance. Conceivably, they are selected under clindamycin therapy, and hence there are reports of treatment failure [30–33]. Whether this phenotype has clinical significance in intrapartum prophylaxis for GBS-colonised women is not clear. However, guidelines recommend considering inducible MLSB phenotype of GBS to be clindamycin resistant [6, 34].
GBS can be further characterised by its serotype. Serotypes III, V and Ia were the most frequent serotypes. Interestingly, these serotypes are found in almost 70% of the invasive cases. However, the serotype distribution of both invasive and colonising strains is continuously evolving and demonstrates not only regional but also temporal variation [35]. Previous observations reported an association between erythromycin resistance and serotype V [16]. This was confirmed in our study as well.
We analysed whether or not there is an association between ethnicity and antibiotic resistance patterns. Chohan et al. [36] found that prevalence of clindamycin or erythromycin resistance was higher among Caucasian women than among African-American and Hispanic women. On the other hand, Manning et al. [18] reported that black ethnicity was associated with higher clindamycin resistance. In our population, African, South American and Asian patients represented a minority. Although patients from Asia had a higher proportion of erythromycin and clindamycin resistance, which may point towards high resistance rates in Asian countries [37], it is important to note that the absolute number was small.
In conclusion, penicillin is the antibiotic of choice for intrapartum GBS prophylaxis. Women who are allergic to penicillin should be questioned and evaluated about the extent of their allergy. In case of a relevant reaction after a previous exposure to penicillin or its derivate, they should receive either cefazolin or vancomycin depending on the risk of developing an anaphylactic reaction. If clindamycin is an option, it must be tested prior to delivery. Each institution should periodically perform resistance surveillance of their GBS isolates [38]. Susceptibility testing of GBS isolated from pregnant women must include the double disk diffusion test for the detection of i-clind-R. In our view, the resistance rates of 14.5% and 14%, respectively, do not allow administration of clindamycin empirically if GBS colonisation is proven.
References
1 Baker CJ, Barrett FF. Transmission of group B streptococci among parturient women and their neonates. The Journal of pediatrics. 1973;83:919–25.
2 Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta obstetricia et gynecologica Scandinavica. 2008;87:260–71.
3 Rausch AV, Gross A, Droz S, Bodmer T, Surbek DV. Group B Streptococcus colonization in pregnancy: prevalence and prevention strategies of neonatal sepsis. J Perinat Med. 2009;37:124–9.
4 Anthony BF. Carriage of group B streptococci during pregnancy: a puzzler. The J Infect Dis. 1982;145:789–93.
5 Renner RM, Renner A, Schmid S, Hoesli I, Nars P, Holzgreve W, et al. Efficacy of a strategy to prevent neonatal early-onset group B streptococcal (GBS) sepsis. J Perinat Med. 2006;34:32–8.
6 Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2010;59:1–36.
7 Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20.
8 Dahesh S, Hensler ME, Van Sorge NM, Gertz RE, Jr., Schrag S, Nizet V, et al. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52:2915–8.
9 Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, et al. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52:2890–7.
10 Gaudreau C, Lecours R, Ismail J, Gagnon S, Jette L, Roger M. Prosthetic hip joint infection with a Streptococcus agalactiae isolate not susceptible to penicillin G and ceftriaxone. J antimicrob Chemother. 2010;65:594–5.
11 Longtin J, Vermeiren C, Shahinas D, Tamber GS, McGeer A, Low DE, et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppressive therapy. Antimicrob Agents Chemother. 2011;55:2983–5.
12 Murdoch DR, Reller LB. Antimicrobial susceptibilities of group B streptococci isolated from patients with invasive disease: 10–year perspective. Antimicrobial agents and chemotherapy. 2001;45:3623–4.
13 Fernandez M, Hickman ME, Baker CJ. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrobial agents and chemotherapy. 1998;42:1517–9.
14 Garland SM, Cottrill E, Markowski L, Pearce C, Clifford V, Ndisang D, et al. Antimicrobial resistance in group B streptococcus: the Australian experience. J Med Microbiol. 2011;60:230–5.
15 DiPersio LP, DiPersio JR. High rates of erythromycin and clindamycin resistance among OBGYN isolates of group B Streptococcus. Diagn Microbiol Infect Dis. 2006;54:79–82.
16 von Both U, Ruess M, Mueller U, Fluegge K, Sander A, Berner R. A serotype V clone is predominant among erythromycin-resistant Streptococcus agalactiae isolates in a southwestern region of Germany. Journal of clinical microbiology. 2003;41:2166–9.
17 von Both U, Buerckstuemmer A, Fluegge K, Berner R. Heterogeneity of genotype-phenotype correlation among macrolide-resistant Streptococcus agalactiae isolates. Antimicrobial agents and chemotherapy. 2005;49:3080–2.
18 Manning SD, Foxman B, Pierson CL, Tallman P, Baker CJ, Pearlman MD. Correlates of antibiotic-resistant group B streptococcus isolated from pregnant women. Obstet Gynecol. 2003;101:74–9.
19 Lin FY, Azimi PH, Weisman LE, Philips JB, 3rd, Regan J, Clark P, et al. Antibiotic susceptibility profiles for group B streptococci isolated from neonates, 1995–1998. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;31:76–9.
20 Uh Y, Kim HY, Jang IH, Hwang GY, Yoon KJ. Correlation of serotypes and genotypes of macrolide-resistant Streptococcus agalactiae. Yonsei Med J. 2005,46:480–3.
21 EUCAST. Clinical Breakpoint Table v. 3,1. European Commitee on Antimicrobial Susceptibility Testing.
22 Woods CR. Macrolide-inducible resistance to clindamycin and the D-test. Pediatr Infect Dis J. 2009;28:1115–8.
23 EUCAST. Expert rules. European Commitee on Antimicrobial Susceptibility Testing
24 Slotved HC, Elliott J, Thompson T, Konradsen HB. Latex assay for serotyping of group B Streptococcus isolates. Journal of clinical microbiology. 2003;41:4445–7.
25 Kong F, Gowan S, Martin D, James G, Gilbert GL. Serotype identification of group B streptococci by PCR and sequencing. Journal of clinical microbiology. 2002;40:216–26.
26 Arpin C, Daube H, Tessier F, Quentin C. Presence of mefA and mefE genes in Streptococcus agalactiae. Antimicrob Agents Chemother. 1999;43:944–6.
27 Berkowitz K, Regan JA, Greenberg E. Antibiotic resistance patterns of group B streptococci in pregnant women. Journal of clinical microbiology. 1990;28:5–7.
28 de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrobial agents and chemotherapy. 2001;45:3504–8.
29 Capanna F, Emonet SP, Cherkaoui A, Irion O, Schrenzel J, Martinez de Tejada B. Antibiotic resistance patterns among group B Streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med Wkly. 2013;143:w13778.
30 Patel M, Waites KB, Moser SA, Cloud GA, Hoesley CJ. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. Journal of clinical microbiology. 2006;44:2481–4.
31 Drinkovic D, Fuller ER, Shore KP, Holland DJ, Ellis-Pegler R. Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48:315–6.
32 Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2003;37:1257–60.
33 Lewis JS, 2nd, Jorgensen JH. Inducible clindamycin resistance in Staphylococci: should clinicians and microbiologists be concerned? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40:280–5.
34 Desjardins M, Delgaty KL, Ramotar K, Seetaram C, Toye B. Prevalence and mechanisms of erythromycin resistance in group A and group B Streptococcus: implications for reporting susceptibility results. Journal of clinical microbiology. 2004;42:5620–3.
35 Sendi P, Johansson L, Norrby-Teglund A. Invasive group B Streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection. 2008;36:100–11.
36 Chohan L, Hollier LM, Bishop K, Kilpatrick CC. Patterns of antibiotic resistance among group B streptococcus isolates: 2001–2004. Infectious diseases in obstetrics and gynecology. 2006;2006:57492.
37 Wang H, Zhao CJ, He WQ, Zhang FF, Zhang LY, Cao B, et al. High Prevalence of Fluoroquinolone-Resistant Group B Streptococci among Clinical Isolates in China and Predominance of Sequence Type 19 with Serotype III. Antimicrobial Agents and Chemotherapy. 2013;57:1538–41.
38 Back EE, O'Grady EJ, Back JD. High rates of perinatal group B Streptococcus clindamycin and erythromycin resistance in an upstate New York hospital. Antimicrob Agents Chemother. 2012;56:739–42.
