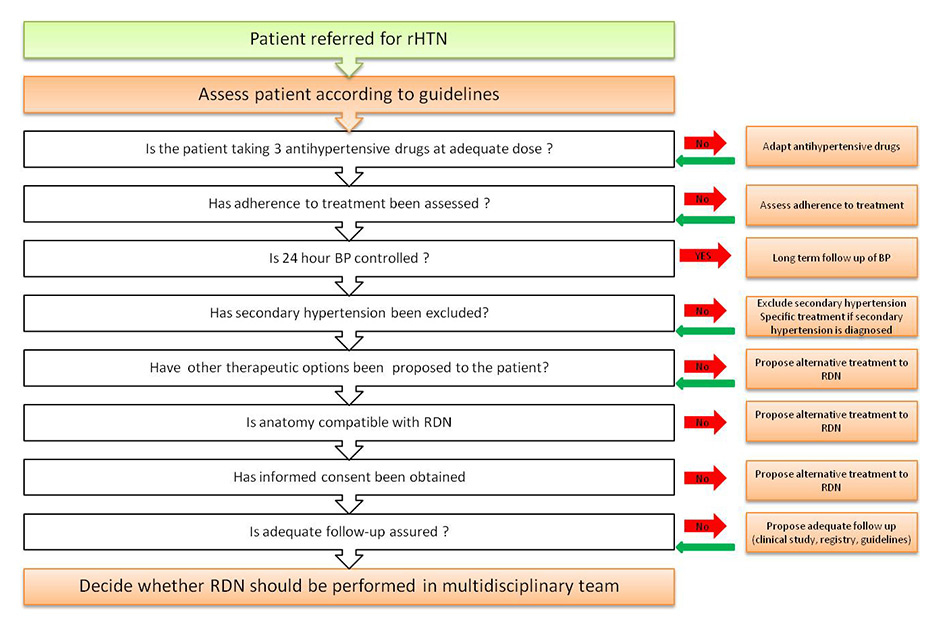
Figure 1
Suggested algorithm for the work-up before endovascular renal denervation (RDN) in resistant hypertension (rHTN).
DOI: https://doi.org/10.4414/smw.2014.13913
Arterial hypertension (HTN) is the most common cardiovascular risk factor. A recent nationwide cross-sectional study showed that the overall prevalence of HTN in adults is 26% (32% in men and 19% in women) [1]. In patients over 60 years of age the prevalence reaches 65% in men and 53% in women. In patients with hypertension, it is estimated that 10% to 30% have resistant hypertension (rHTN), defined as an uncontrolled office blood pressure (BP) – systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg – despite the use of three antihypertensive drugs, including a diuretic, at adequate doses [2, 3]. In a retrospective study, 2% of patients with a new diagnosis of HTN developed rHTN within 2 years [4]. The identification of patients with rHTN is essential, since such patients have a 50% increase in risk of cardiovascular events compared with the usual hypertensive population [4].
Transcatheter (or percutaneous) renal denervation (RDN) is a novel technique developed for the treatment of rHTN. So far, several pilot trials and one randomised controlled trial have shown a significant reduction in office BP in a selected group of patients with rHTN [5]. The technique has been available in Switzerland since 2010. The principle of the method is the thermic neurolysis of renal efferent and afferent sympathetic nerve fibres located in the adventitia of renal arteries by applying a low intensity radiofrequency within the main trunk of renal arteries.
Although expert statements of members of the European Society of Cardiology and the European Society of Hypertension have been published, no specific recommendations are currently available for the good clinical use of this technique in Switzerland [6–8]. With the introduction of renal denervation in the Swiss diagnosis related groups (DRG) catalogue of reimbursed procedures, the Swiss Society of Hypertension, the Swiss Society of Cardiology, the Swiss Society of Angiology and the Swiss Society of Interventional Radiology have decided to establish recommendations on renal denervation for practicing physicians and cardiovascular specialists based on currently available evidence.
This document summarises the current state of knowledge and proposes practical recommendations for the treatment of patients with rHTN. As with all recommendations, results from ongoing and new clinical trials and devices are likely to change these recommendations in the near future.
The kidneys play a pivotal role in the homeostasis of water and electrolytes, and hence in the long-term control of BP. They are innervated by afferent (from the kidney to the brain) and efferent sympathetic nerves (from the medulla oblongata to the kidneys) that contribute to both the development and the maintenance of HTN [9]. An increased efferent renal sympathetic nerve activity leads to: (1.) stimulation of renin release in juxtaglomerular cells thereby leading to enhanced angiotensin II production, (2.) renal vasoconstriction leading to reduced renal blood flow and (3.) increased renal tubular sodium reabsorption leading to sodium retention. Each of these renal functional alterations can decrease renal excretory function and thus affect long-term control of BP [10]. Increased renal sympathetic nerve activity is present in most forms of animal and human HTN and is, therefore, a logical target for HTN treatment [9].
The procedure is currently performed under local anaesthesia via femoral access, although radial systems are under development. Since the ablation procedure itself is associated with severe pain during the application of radiofrequency energy, systematic pain management is mandatory (usually with fentanyl derivatives). Furthermore, vital signs should be closely monitored during the procedure. Finally, aspirin (either prior chronic use of 100 mg/day or else 500 mg acetylsalicic acid i.v.) plus 5,000 IU unfractionated heparin i.v. is recommended prior to RDN as thrombus formation has been documented after the procedure [11].
Currently, more than a dozen RDN catheters and ablation systems have been developed. Novel multi-electrode systems will become available soon. In order to use a device in clinical practice, safety and efficacy of the ablation system has to be proven and the device has to be CE marked.
The devices that have obtained the CE label (conform to all applicable European Community directives) and are available in Switzerland are shown in table 1.
With any system, at the end of the procedure renal angiograms should be performed in order to detect any – albeit rare – potential procedure-related complication, such as renal artery dissection or severe spasm. Renal artery dissection can generally be treated immediately with the implantation of a stent. The puncture site must be checked for bleeding before discharge.
The procedure can be performed either during a 24-hour hospitalisation or on an outpatient basis. Indeed, as BP decreases very slowly and continuously over weeks and months, hypotension after the procedure (with the exception of vagal reactions during sheet removal) are rare. Antihypertensive treatment should not be interrupted immediately after renal denervation, since blood pressure lowering effects are delayed and the maximum effect is expected after 3 to 6 months.
| Table 1: Types of renal denervation devices. | |||||
| Device | Provider | Technology | Vascular access | Duration | |
| Symplicity | Medtronic | Single electorde, monopolar, non-irrigated | 6F introducer | 2 min / ablation point | |
| EnligHTN | St-Jude Medical | Multi-electrode basket, monopolar non-irrigated | 8F introducer | 90 sec/point | |
| Vessix | Boston Scientifc | Over the wire balloon-based irrigated catheter, bipolar | 8F introducer | 30 sec/artery | |
| Covidien ballon | Covidien | Over the wire balloon-based irrigated catheter, monopolar | 7-8F introducer | 2 min/artery | |
So far, only one multicentre randomised controlled trial (RCT) has been conducted, as well as four case control studies and a growing number of case series [3, 5, 12, 13]. The single RCT currently available included 106 patients randomised in a non-blinded fashion, 52 patients in the RDN group and 54 patients in the control group (Symplicity HTN-2). The primary efficacy endpoint was the between-group change in average office-based measurements of systolic BP from baseline to 6 months after randomisation [5]. The results showed an office-based systolic and diastolic BP reduction in the RDN group of 32/12 mm Hg (standard deviation [SD] 23/11 mm Hg, baseline BP 178/96 mm Hg, p <0.001), whereas no significant change (1/0 mm Hg) from baseline was observed in the control group (SD 21/10 mm Hg], baseline BP 178/97 mm Hg). The primary endpoint was reached as between-group differences in BP at month 6 reached 33/11 mm Hg (p <0.001). However, as with any antihypertensive intervention, when 24-hour BP was measured using ambulatory BP monitoring (ABPM), renal denervation-induced changes in BP were less pronounced (–10.2 mm Hg in systolic BP and –4.9 mm Hg in diastolic BP at 6 months) [14, 15]. The lesser effect seen in ambulatory BP compared with office BP has been confirmed in a subject-level meta-analysis [16]. Interestingly, renal denervation had no effect in patients with pseudo-resistant HTN, defined as mean ambulatory 24-hour systolic BP <130 mm Hg despite elevated office systolic BP readings [15]. Data from the Symplicity programme confirm that the reduction in blood pressure progresses further up to 24 months after RDN without any change in renal function [17, 18]. In a small randomised study using the St. Jude Basket Ablation catheter, similar reductions of BP after 6 months were found [19].
A double-blind randomised controlled trial with a sham procedure is currently ongoing in the United States and has completed recruitment of over 300 patients [20]. In this study, changes in office BP remain the primary endpoint, but the changes in ambulatory BP at 6 months have been included as a secondary endpoint.
Beyond BP lowering, exploratory studies suggest that RDN exerts a variety of potentially important protective effects, such as an improvement in insulin resistance, left ventricular hypertrophy and diastolic function of the left ventricle [21–23]. Whether or not such surrogate endpoints will translate into a reduction of major adverse cardiovascular events beyond BP reduction will have to be confirmed in large randomised clinical trials. Indeed, St. Jude has announced a prospective randomised study enrolling more than 4,000 high risk patients with HTN that will start later this year.
Bradycardia requiring atropine administration was reported in the Symplicity HTN-1 study in seven out of 52 patients [12]. Reported procedure-related and/or device-related complications are rare and include renal artery dissection (one case in the Symplicity HTN-1), pseudoaneurysm of the femoral artery at the puncture site (one case in the Symplicity HTN-1, and one in the Symplicity 2 study) [5, 12]. Late complications may include renal artery stenosis (one case observed at the 24-month follow-up of a cohort including 153 patients treated in an open-label study) [24, 25].
Recently, optical coherence tomography (OCT) was used to assess renal vascular damage [11]. Immediately after the procedure, local intimal oedema formation, de-endothelialisation, intimal detachment and thrombus formation was visible. Furthermore, upon 3D reconstruction, mean renal artery diameter is reduced by around 20% with a typical “string-of-bead” appearance reflecting diffuse spasm. Whether and to what degree such changes are reversible remains to be determined.
Overall renal function, assessed by estimated glomerular filtration rate (eGFR), remained stable in all studies. In 88 patients treated by renal denervation, no significant change in glomerular filtration rate was observed 6 months after the procedure and a decreased incidence of albuminuria was reported 6 months after the intervention [26].
No data exists regarding long-term safety (beyond 3 years), and the limited number of patients included in studies or with a systematic follow-up cannot rule out major occasional complications. Accordingly, regular clinical and imaging monitoring of patients who have had transcatheter RDN is recommended. It is also advised that any device-related complication/dysfunction should be reported to the national materiovigilance authorities (Swissmedic).
Safety issues not directly related to the procedure include the use of radiological contrast agents (i.e. contrast-induced renal dysfunction) and the use of anaesthesia. Contrast-induced kidney injury is usually transient except in patients with a very low glomerular filtration rate. Anaesthesia is required because of the severe pain transmitted centrally by the afferent renal nerves. In general a fentanyl derivative is used.
Several limitations regarding the studies published so far must be mentioned.
1. Clinical evaluation-related issues: a) Number of patients included in controlled studies is limited. b) Follow-up period is limited. c) No consistent optimal medical treatment (three drugs at full dose including a diuretic) was used. d) Office BP was used as primary endpoint.
2. Device/technology-related issues: a) Lack of any periprocedural marker that might identify good responders or success of therapy (other than changes in BP after the procedure). b) The current spot strategy application of radiofrequency energy in the renal artery is empirical as regards both the number and sites used.
So far, renal denervation is only indicated for the treatment of resistant hypertension. The rationale for a systematic work-up of candidates for the procedure (fig. 1) is to ascertain the diagnosis in order to identify patients at high cardiovascular risk and to exclude secondary causes of hypertension, which are more frequent in truly resistant hypertension and which may require specific treatment. Therefore, the initial management includes:

Figure 1
Suggested algorithm for the work-up before endovascular renal denervation (RDN) in resistant hypertension (rHTN).
1. Confirmation of true treatment resistance: a) Use of a correct BP measurement technique.
Rationale: inappropriate technique, material or setting may overestimate or underestimate BP [27]. b) Exclusion of white-coat hypertension (ABPM; home BP measurements).
Rationale: white-coat hypertension may be present in up to 30% of apparently resistant hypertensive patients [28]. c) Assessment of adherence to treatment (adherence history, pill count, follow-up of prescriptions renewal, electronic monitoring) and counselling of patients appropriately [29].
Rationale: up to 30% with apparent resistant hypertension may not have a good adherence to treatement [30]. d) Correction of lifestyle factors with negative impact on BP (high sodium diet, alcohol, physical inactivity). e) Optimal use of triple drug therapy at maximum tolerated doses (ideally a diuretic, a calcium-channel blocker and a blocker of the renin-angiotensin system).
Rationale: inadequate treatment is a frequent cause of apparent resistant hypertension [31]. f) Use of a loop diuretic in stage 4 chronic kidney disease (eGFR <30 ml/min/1.73 m2). g) Addition of a mineralocorticoid receptor or another class of antihypertensive, if tolerated, after exclusion of a secondary cause. h) Discontinuation or minimal use of interfering substances (nonsteroidal anti-inflammatory drugs, liquorice, oral contraceptives...). i) Screen for secondary cause of hypertension:
– chronic kidney disease;
– primary aldosteronism;
– sleep apnoea syndrome,
– renal artery stenosis;
– phaeochromocytoma, paraganglioma, primary hyperparathyroidism, thyroid dysfunction, Cushing’s syndrome, aortic coarctation, intracranial tumour, acromegaly (all uncommon)
Rationale: secondary causes of hypertension are more prevalent in resistant hypertension than in essential hypertension [32].
After this initial work-up, the decision to perform RDN should be discussed by a multidisciplinary team in a specialised centre after imaging of the renal arteries (computed tomography [CT] or magnetic resonance imaging) to assess their number, length and diameter. These diagnostic steps are essential since most patients are not eligible for renal denervation according to strict inclusion and exclusion criteria [33, 34]. The risk benefit ratio of the intervention should be evaluated individually for each patient, taking into account renal function and the patient’s preferences.
Based on current knowledge, the Expert Consensus panel limits the indication for transcatheter RDN to patients who have:
1. Truly resistant hypertension confirmed with systolic office BP >140 mm Hg and confirmed by daytime ABPM (or home BP measurements) >135/85 mm Hg or night time >120/70 mm Hg.
2. Intolerance to many antihypertensive drugs that impairs adequate therapy.
3. Suitable renal artery anatomy.
4. Presence of two functioning kidneys with at least 90 mm bipolar length.
5. Decision taken by a multidisciplinary team.
6. Patient informed consent obtained, after an explanation of the safety and efficacy of the procedure.
Based on current knowledge, the Expert Consensus panel recommends not performing RDN in the following situations:
1. Patient under 18 years of age.
2. Prior renal stent or renal angioplasty.
3. Significant renal artery stenosis (>70%).
4. Renal artery fibromuscular dysplasia.
5. Heavily calcified plaques in the target segment of the renal artery.
6. Extensive aortic and renal atherosclerotic disease.
7. eGFR <45 ml/min/1.73 m2.
8. Secondary forms of hypertension.
The recommendations do not apply to patients included in clinical trial with specific inclusion and exclusion criteria.
The recommended facilities should include a well-trained intervention team with at least:
1. One trained interventionist. A physician trained in RDN who has an experience track record for renal artery diagnostics and interventions, or five angioplasties of the renal artery per year during the last five years.
2. Two assistants (nurse or physician) able to handle the device and control appropriate sedation/anaesthesia. The person in charge of device handling should have received specific RDN training with practical training on the device.
3. A dedicated and modern catheterisation laboratory with: a) High-quality fluoroscopy (C-arms in an operating room are not sufficient). b) An optimal acquisition system that can analyse the angiogram at any time during the procedure, specifically for ruling out acute complications. c) Optimal radioprotection. d) Equipment for monitoring haemodynamic pressure and electrocardiographic activity. e) Available equipment to handle potential complications, such as resuscitation equipment including an external cardiac defibrillator, or dedicated equipment for percutaneous renal intervention (including balloon, conventional and “covered” stents).
RDN necessitates long-term follow-up by a hypertension specialist, which should be organised before denervation. This follow-up should include:
Renal nerve ablation might have a role in other conditions associated with increased sympathetic nerve activity. Indeed, small trials suggest that the role of this new technique may not be limited to hypertension, but might be useful also in chronic heart failure, chronic kidney disease and renal failure, polycystic ovary syndrome, sleep breathing disorders, obesity and metabolic syndrome. Future studies will have to explore these possibilities and confirm the safety and the efficacy of renal nerve ablation beyond rHTN in controlled trials.
1 Chappuis A, Bochud M, Glatz N, Vuistiner P, Paccaud F, Burnier M. Swiss survey on salt intake: main results. 2011. Available from: http://www.bag.admin.ch/themen/ernaehrung_bewegung/05190/05294/12869/index.html.
2 Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–536.
3 Gu YM, Asayama K, Liu YP, Staessen JA. Renal denervation: time to open Pandora’s box. Swiss Med Wkly. 2012;142:w13638.
4 Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–42.
5 Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9.
6 Mahfoud F, Luscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013 Apr 25.
7 Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, et al. ESH Position Paper: Renal denervation – an interventional therapy of resistant hypertension. J Hypertens. 2012;30(5):837–41.
8 Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Bohm M, Campese VM, et al. International Expert Consensus Statement: Percutaneous Transluminal Renal Denervation for the Treatment of Resistant Hypertension. J Am Coll Cardiol. 2013 Dec 3;62(22):2031-45. Epub 2013 Sep 6.
9 Krum H, Sobotka P, Mahfoud F, Bohm M, Esler M, Schlaich M. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123(2):209–15.
10 DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197.
11 Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP, et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity(R) catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34(28):2141–8. Epub 2013 Apr 25.
12 Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81.
13 Gosain P, Garimella PS, Hart PD, Agarwal R. Renal sympathetic denervation for treatment of resistant hypertension: a systematic review. J Clin Hypertens. 2013;15(1):75–84.
14 Doumas M, Anyfanti P, Bakris G. Should ambulatory blood pressure monitoring be mandatory for future studies in resistant hypertension: a perspective. J Hypertens. 2012;30(5):874–6.
15 Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128(2):132–40.
16 Persu A, Jin Y, Azizi M, Baelen M, Volz S, Elvan A, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2013.
17 Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976–82.
18 Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7.
19 Papademetriou V, Worthley S, Tsioufis C, Worthley M, Chew D, Sinhal A, et al. Catheter-Based Renal Denervation for the Treatment of Patients with Drug-Resistant Hypertension: EnligHTN I: Three-Month Data of a First in Man Study Using a Multi-Electrode Radiofrequency Ablation Catheter. Circulation. 2012;A19523(126).
20 Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clinical cardiology. 2012;35(9):528–35.
21 Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123(18):1940–6.
22 Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–9.
23 Brandt MC, Reda S, Mahfoud F, Lenski M, Bohm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60(19):1956–65.
24 Vonend O, Antoch G, Rump LC, Blondin D. Secondary rise in blood pressure after renal denervation. Lancet. 2012;380(9843):778.
25 Kaltenbach B, Id D, Franke JC, Sievert H, Hennersdorf M, Maier J, et al. Renal Artery Stenosis After Renal Sympathetic Denervation. J Am Coll Cardiol. 2012;60(25):2694–5.
26 Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60(2):419–24.
27 Dieterle T. Blood pressure measurement – an overview. Swiss Med Wkly. 2012;142:w13517.
28 de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902.
29 Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62(2):218–25.
30 Burnier M, Schneider MP, Chiolero A, Stubi CL, Brunner HR. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens. 2001;19(2):335–41.
31 Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med. 1991;151(9):1786–92.
32 Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–26.
33 Verloop WL, Vink EE, Voskuil M, Vonken EJ, Rookmaaker MB, Bots ML, et al. Eligibility for percutaneous renal denervation: the importance of a systematic screening. J Hypertens. 2013 Jun 5.
34 Savard S, Frank M, Bobrie G, Plouin PF, Sapoval M, Azizi M. Eligibility for renal denervation in patients with resistant hypertension: when enthusiasm meets reality in real-life patients. J Am Coll Cardiol. 2012;60(23):2422–4.
Funding / potential competing interests: S. Cook: Research grants from Boston Sci., SJM, Medtronic. Speaker fees from Boston Sci. and Medtronic. T. Lüscher: Research grants and honoraria from St. Jude, Medtronic, Pfizer, Merck.
Authors’ contribution: GW and OM contributed equally to the work.