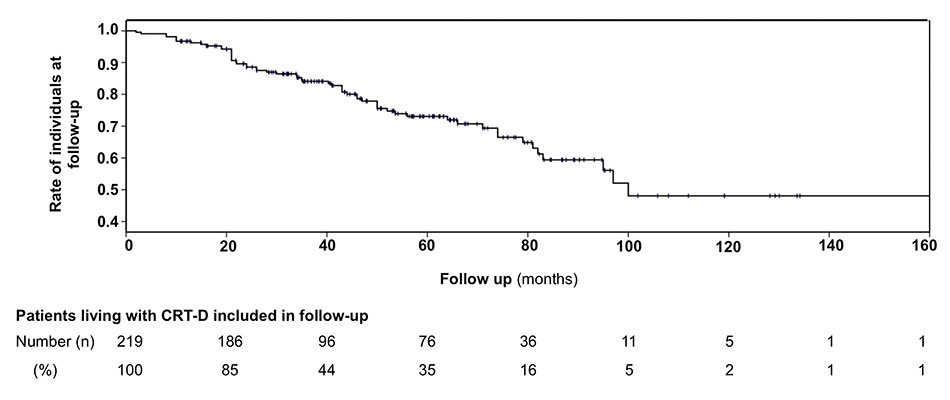
Figure 1
Kaplan-Meier-curve reflecting the mortality rate and follow-up duration of all patients included in the CRT-D registry of Basel.
CRT-D = cardiac resynchronisation therapy with defibrillator back-up
DOI: https://doi.org/10.4414/smw.2014.13938
The number of patients living with chronic heart failure is constantly growing. This is attributable to the higher life expectancy in developed countries and advancing therapeutic options [1–3]. The impact of heart failure on mortality is reflected by Swiss registry data, which showed an annual overall case fatality rate of 26%. According to these data, overall annual mortality associated with heart failure was 32% for women and 20% for men [1].
Therefore, in selected patients with drug refractory heart failure, cardiac resynchronisation therapy (CRT) with or without a defibrillator backup (CRT-D) is a widely accepted therapeutic option, which has been shown to reduce both morbidity and mortality [4–7]. Improvements in several factors such as left ventricular ejection fraction (LVEF), various echocardiographic parameters, physical and functional capacity and quality of life were shown in numerous pivotal studies [6, 8–11]. CRT is established for heart failure patients who remain in New York Heart association (NYHA) class II, III and ambulatory class IV despite optimal medical therapy, who have a LVEF <35% and a QRS width of >120ms, and are in sinus rhythm [2, 3]. Favourable results of several recently published trials [6, 8, 9, 12] in mildly symptomatic patients will lead to a further rise in implant rates. In Switzerland, the number of newly implanted CRT-pacemakers (CRT-P) and CRT-D devices constantly increased over the last decade. In 2012, 164 CRT-P and 371 CRT-D devices were implanted, according to the Swiss pacemaker registry [13].
Those few publications that exist on mid- and long-term outcome of CRT-D patients mainly focus on mortality or CRT response (improvement in NYHA class, LVEF and left ventricular endsystolic volume) [14–17]. However, in many publications “long-term” stands for a mean follow-up of 2 to 4 years. Technical factors that affect the patients probably as much are hardly mentioned. The aim of this study in patients living with CRT-D for at least 5 years was to focus on these factors and to put them into perspective with the clinical benefits.
The patients of this retrospective analysis stem from the prospective CRT-D registry of the Cardiology Department of the University of Basel Hospital in Switzerland, which has long-standing experience with this procedure [3]. The registry currently includes 219 patients, was started in 1999 and is constantly updated. Out of this registry, all 49 patients living for at least 5 years were identified. Patients received their CRT-D device between February 2000 and November 2006 and were followed-up until December 2011.
The indication for CRT-D was, as described above, primary or secondary prevention of sudden death. The CRT-D was implanted according to standard local practice with conscious sedation and mostly dual-coil/passive implantable cardioverter-defibrillator (ICD) leads. Device interrogations were performed 1, 3 and 6 months after implantation and then every 6 months.
For primary prevention, a cut-off rate for ventricular tachycardia (VT) detection of 180–185 bpm with a series of antitachycardia pacing (ATP) bursts followed by shocks was programmed. For secondary prevention, the cut-off rate was usually 20 bpm lower than the clinically observed VT. Detection of ventricular fibrillation (VF) was usually programmed to 220 bpm with ATP during charging, whenever possible followed by shock. All ICD therapies were reviewed by an electrophysiologist and defined as either appropriate or inappropriate. Left and right ventricular stimulation thresholds were defined as “high” when they exceeded 2.5 V. For the right ventricular lead a twofold safety margin of the measured threshold was programmed. The left ventricular output was set either to 2 V or 0.6 V higher than the measured threshold in cases with a threshold of >2 V.
Indications for device replacement were end of life, infections, recall and system malfunction. Events leading to surgical intervention were classified as significant complications (e.g., lead repositioning or replacement, infection etc.) or minor complications (all other events, e.g., phrenic stimulation, chronically elevated threshold values…) [10, 18]. We defined three timings of these significant complications: early (implant to 6 months); intermediate (7 to 60 months); late (>60 months). If a patient had two significant events, the first one was considered for the Kaplan-Meier curve labeled “freedom from significant events”.
It is important to note that only patients with a follow-up of at least 5 years were included, that patients who died after the time point of 5 years were not excluded and that all complications occurring during follow-up were considered.
Changes in NYHA class and LVEF were assessed. Rate of appropriate ICD-therapy and mode of death were analysed (pump failure, other cardiovascular death, noncardiac death).
Continuous data were expressed as mean values (±one standard deviation) or median. The chi-square test or Fisher’s test were used to compare categorical data (mortality in primary/secondary prevention and ischaemic/non-ischaemic cardiopathy; ICD therapies in the same subgroups and in dead/surviving patients). Comparisons of the continuous variables (LVEF; NYHA class, plus the changes over time) were calculated using a paired and an unpaired two-sided student’s t-test. Kaplan-Meier curves were constructed to give an overview on the mortality rate of all registry patients (date of last access of the database for this analysis was 1 April 2013) and show the incidence of significant problems. A p-value <0.05 was considered statistically significant for all tests. Analyses were done using IBM SPSS version 20.1.
The study cohort consisted of 49 patients. No patient was lost. The population was predominantly male (78%). Mean age was 63±10 years (range 36–80 years). Extensive baseline characteristics of all patients are shown in table 1. Mean follow up was 84±18 months (range 62–145, median 79 months). After initially surviving 5 years, 8 patients (16%, estimated annual mortality 8%) died during further follow-up. Mean age at time of death was 73±8 years. End-stage heart failure was the reason in six patients (five of them had ischaemic heart disease), laryngeal cancer and rupture of an aortic aneurysm in one patient each. Significantly more patients with a secondary prevention indication (40%) died during follow up (40% vs 10%, p = 0.04). Mortality was also higher in patients with ischaemic heart disease (33% vs 6%, p = 0.04). Overall mortality rate in all 219 registry patients is shown in figure 1.

Figure 1
Kaplan-Meier-curve reflecting the mortality rate and follow-up duration of all patients included in the CRT-D registry of Basel.
CRT-D = cardiac resynchronisation therapy with defibrillator back-up

Figure 2
Kaplan-Meier curve of the major adverse events in individuals with CRT-D during long-term follow-up
CRT-D = cardiac resynchronisation therapy with defibrillator back-up
Mean NYHA class improved from 2.7±0.6 at implant to 2.1±0.6 at last follow-up (p = <0.0001, 95% confidence interval [CI] 0.40‒0.91). No difference between ischaemic and nonischaemic cardiomyopathy was observed (p = 0.8). Table 2 shows these changes in NYHA class during follow up in detail.
LVEF improved from 23%±7% to 35%±13% (p = <0.0001, 95% CI 8%‒16%). In 23/49 patients (47%), LVEF improved >10% and in 24/49 patients (50%) to >35%. LVEF was re-evaluated after a mean follow up of 70±30 months. An improvement from 22%±8% to 36%±13% (p = <0.0001 compared with baseline) was seen in patients with nonischaemic cardiomyopathy and from 25%±6% to 34%±13% (p = 0.007 compared with baseline) in those with ischaemic cardiomyopathy. There was no difference in LVEF improvement between groups (p = 0.2). Hyperresponse, i.e., an improvement to ≥50%, was seen in 6/49 patients (16%), 5 (83%) of whom had nonischaemic cardiomyopathy.
During follow-up, 14 patients (28%) experienced appropriate ICD therapies (ATP or shock), 8/39 (21%) in primary and 6/10 (60%) in secondary prevention (p = 0.02). Arrhythmias were true VF in 2 cases (4%), VT >220 bpm in 2 cases (4%) and VT <220 bpm in 10 patients (20%). First-ever arrhythmia occurred at a median of 11 months after ICD implant (range 1–54 months). There was no difference between patients with nonischaemic cardiomyopathy (8 patients, 16%) and patients with coronary artery disease (6 patients, 12%) (p = 0.7). Arrhythmias occurred more often in patients who died during subsequent follow-up (63%) than in long-term survivors (22%, p = 0.03). Inappropriate ICD therapy was delivered in 7 patients (14%). The causes were sinus tachycardia (n = 3), noise sensing (n = 2), atrial fibrillation (n = 1) and atrioventricular-nodal re-entrant tachycardia (n = 1).
Overall, 53 devices were replaced in 45 patients; 47 (89%) for battery depletion, 2 for recalls, 2 for infections and 2 as elective device replacements during lead revision and in anticipation of imminent battery depletion. At the time point 5 years, the ICD had already been replaced in 27 patients (55%). Mean device longevity was 54±13 months (range 26–74 months).
A total of 23 technical problems were encountered in 20 patients (40%). Significant problems accounted for 15 of them, 14 resulting in surgery (61% of technical problems, 29% of all patients). A detailed overview is given in table 3. Two infections resulted in device removal (one pocket and one lead infection). Both patients presented after device replacement (26 and 52 months, respectively). All four cases of lead dislodgement happened early after implantation (mean 2.5±2.6 months). In contrast, the occurrence of lead defects (n = 7) showed a wide range from 2 to 59 months. No other adverse events requiring surgery were seen beyond year five and no patient died due to surgery. The rate of major complications was 30% (15 of 49 patients). A Kaplan-Meier curve on the incidence of significant problems is shown in figure 2.
Mean left ventricular (LV) thresholds were 1.6±1.3 mV at implant and 1.5±1.0 mV at last follow-up with an impulse duration of 0.5 to 1.0 milliseconds. The right ventricular (RV) threshold values were 0.8±0.4 mV and 1.0±0.7 mV, respectively. There was no significant change over time (p = 0.6 and 0.1). A high LV- or RV-threshold, defined as >2.5 V, was present in six patients and no patient, respectively, at implant compared with five and two, respectively, at last follow-up.
| Table 1: Baseline characteristics. | |
| Age at implant (y), mean±SD | 63±10 |
| Male gender | 38 (78%) |
| Nonischaemic cardiomyopathy | 31 (73%) |
| Primary prevention | 39 (80%) |
| Myocardial infarction | 18 (37%) |
| Bypass surgery | 8 (16%) |
| Percutaneous coronary intervention | 10 (20%) |
| COPD | 3 (6%) |
| PAD | 2 (4%) |
| Cancer | 8 (16%) |
| CKD (≥ stage 3)§ | 23 (47%) |
| Sinus rhythm | 46 (93%) |
| QRS duration (ms), mean±SD | 161±26 |
| LBBB | 46 (94%) |
| Medication | |
| ACE inhibitor / AT2 antagonist | 48 (98%) |
| Beta-blocker | 47 (96%) |
| Diuretic | 42 (86%) |
| Statin | 28 (57%) |
| Amiodarone | 12 (24%) |
| Digoxin | 8 (16%) |
| Calcium antagonist | 3 (6%) |
| ACE = angiotensin converting-enzyme; AT = angiotensin; CKD = Cchronic kidney disease, using estimated Glomerular filtration rate according to MDRD (Modification of Diet in Renal Disease) formula; ≤60 ml/min/1.73 m2; COPD =chronic obstructive pulmonary disease; LBBB = left bundle branch block; PAD = peripheral arterial disease; SD = standard deviation | |
| Table 2: Changes in New York Hear Association (NYHA) class during follow up. | |||||
| At implant | During follow-up | ||||
| NYHA IV | NYHA III | NYHA II | NYHA I | ||
| n | n (%) | n (%) | n (%) | n (%) | |
| NYHA class II | 10 | 0 (0%) | 4 (40%) | 5 (50%) | 1 (10%)+ |
| NYHA class III * | 30 | 0 (0%) | 13 (43%) | 11 (37%)+ | 5 (17%)+ |
| NYHA class IV * | 9 | 1 (11%) | 3 (33%)+ | 3 (33%)+ | 1 (11%)+ |
| * Follow-up information with regard to NYHA class of 1 patient in each group is missing + Improvement ≥1 class | |||||
| Table 3: Overview of technical problems (n = 23 in 20 patients). | ||||
| Time-point of occurrence | ||||
| Early 0–6 months | Intermediate 7–60 months | Late >60 months | ||
| Minor complications | (n = 8) | 5 | 3 | |
| PNS resolved by reprogramming | 8 | |||
| Significant complications | (n = 15) | 8 | 5 | 2 |
| Lead defects | (n = 7) | |||
| Coronary sinus lead, ring-part | 1 | 1§ | ||
| Sprint fidelis lead (right ventricular) | 3 | 3 | ||
| Sensing defect | 1 | 1+ | ||
| Shock lead | 2 | 2‡ | ||
| Lead dislodgments | (n = 4) | |||
| Coronary sinus leads | 3 | 3 | ||
| Atrial lead | 1 | 1 | ||
| PNS resulting in lead repositioning | (n = 1) | 1§ | ||
| Infections | (n = 2) | |||
| Lead infection | 1 | 1‡ | ||
| Pocket infection | 1 | 1+ | ||
| Subclavian vein thrombosis | (n = 1) | 1 | ||
| PNS = phrenic nerve stimulation +one patient had a sensing defect and a pocket infection §one patient had phrenic stimulation and a defect of the ring part of the coronary sinus lead ‡one patient had a shock lead defect and a lead infection | ||||
The very distinctive feature of this study is that all patients had a follow-up of at least 5 years, thus giving a more precise overview of problems developing in the long term than previously published studies [14–16] in which only a minority of patients had such a long-term follow-up.
Device-related problems, especially lead issues and generator replacement, have an impact on physical and psychological wellbeing of patients [19, 20]. Such adverse events are more common in CRT-D and have mostly been attributed to the left ventricular lead [21, 22]. Landolina et al. [22] reported an annual rate of major complications of 5%, half of them due to LV lead problems. However, there the limitation is a short follow-up of median 18 months. Our results extend these findings with an annual rate of 6%, one-third for LV lead problems. Several other studies [17, 18, 22–24] reported lead-associated problems, but again their main limitations are the short follow-up and the inclusion of different devices (pacemakers, ICDs and CRTs). Fifty percent of complications requiring surgery occurred within 6 months after implantation, the other 50% were seen beyond year four. Phrenic nerve stimulation, although commonly met (11/49, 22%), could be managed by reprogramming of lead configuration and rarely needed lead replacement (1/49, 2%). This is in marked contrast to a clinically significant rate of 22% and a revision rate of 7% over a mean period of 24 months in the study by Biffi et al [25].
Device longevity is a well-known problem. As a result of the high amount of pacing, battery depletion occurs earlier than in non-CRT devices. Mean longevities of 4.7 and 4 years have been published, comparable to the 4.5 years in our series [26, 27]. Different performance of manufactures and LV threshold are known to have an important influence. Of note, both our infections occurred after device replacement. LV threshold >2.5 V was present in less than 10% at last follow-up and thus cannot be an explanation for impaired device longevity [28].
Arrhythmias requiring ICD therapies occurred in 28%. Potentially fatal arrhythmias (VF and fast VT) were present in only 8%. The number of appropriate ICD therapies was thus lower than in a previous study with a shorter follow-up of mean 21 months (42%) [29]. There was a significantly higher rate of appropriate ICD therapies in patients receiving CRT-D for secondary prevention, which is congruent with previous publications [4, 29]. Our results suggest that the long-term benefit of the defibrillator in CRT therapy, at least for certain indications (primary prevention in nonischaemic heart disease), is probably overestimated [30, 31].
Clinical response, i.e., reduction in NYHA class, in this initially highly symptomatic group of patients (mean NYHA class 2.7) was marked and sustained (mean decrease 0.6±0.9). This is consistent with data of large CRT trials [4, 5, 17, 32, 33], albeit with a much longer follow-up. Findings are promising as they show a long-lasting stabilising effect of CRT on LV function (mean LVEF improvement 14%±12%) with no significant difference between the ischaemic and nonischaemic cardiomyopathy groups. More important is the sustained LVEF improvement, the determinant with regard to further need of a defibrillator back-up. Our group recently published data showing a strong correlation between LVEF improvement and ICD therapies, indicating a very low rate of ICD therapies in patients with a sustained improvement of LVEF up to >35%. From this perspective, the observation that no first-ever ICD therapy occurred beyond year five might be an argument in favor of downgrading to a CRT pacemaker in selected patients. Of course the patient number is limited, but it should stimulate the ongoing discussion regarding the dogma “once ICD, always ICD” [30].
The mortality rate in this study is definitely not comparable to other studies, as per protocol patients who died before year five (i.e., 40 of the 219 patients initially implanted) were not included the study. However, the annual mortality rate beyond year five of 8% was similar to the early mortality in other studies [4, 7, 15, 16, 24, 34, 35]. Most patients died of progressive congestive heart failure and most of them had ischaemic heart disease. Their worse outcome was possibly influenced by several factors, such as higher mean age at implant (66 years compared with 62 years in patients with nonischaemic cardiomyopathy), ischaemic cardiomyopathy and comorbidities. Furthermore, there is a trend that patients with appropriate ICD therapies were more likely to die, which is congruent with existing data [23].
This study is a retrospective analysis of a relatively small single centre population. However, no studies with comparable mean follow-up duration have been published to date. Another limitation is lack of a prespecified echocardiographic follow-up and contemporary response parameters (e.g., left ventricular end systolic and end diastolic volumes [36]), but this was not the focus of this study and has been addressed before in larger cohorts [37]. Moreover, we did not assess the number and type of “minor” lead and device associated issues, such as. intermittent variations in pacing thresholds. One benefit of CRT is a reduction in hospitalisation rates. With our study setting, this issue could not be evaluated.
Obviously many benefits of CRT exist, but they must be carefully weighed against its disadvantages, such as reoperations for battery replacement and lead problems. Further studies will be needed to determine which patients will need a CRT-D at implant or at replacement, as a considerable number of patients improve in LVEF or never experience arrhythmic events.
1 Meyer K, Murner N, Laederach-Hofmann K, Simmet A, Hess OM. Heart failure events, and case fatalities in Switzerland based on hospital statistics and cause of death statistics. Swiss Med Wkly. 2008;138(35–36):p.506–11.
2 McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):p.803–69.
3 Sticherling C, Schaer B, Coenen M, Kuhne M, Ammann P, Osswald S. Cardiac resynchronization therapy in chronic heart failure. Swiss Med Wkly. 2006;136(39–40):p.611–7.
4 Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):p.2140–50.
5 Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):p.1539–49.
6 Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361(14):p.1329–38.
7 Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):p.2385–95.
8 Linde C, Abraham WT, Gold MR, John Sutton M St, Ghio S, Daubert C, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52(23):p.1834–43.
9 Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123(10):p.1061–72.
10 Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4(2):p.136–42.
11 Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, Spertus JA, et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J Card Fail. 2012;18(2):p.94–106.
12 Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110(18):p.2864–8.
13 Babotai I. Arbeitsgruppe Herzschrittmacher und Elektrophysiologie der Schweizerischen Gesellschaft für Kardiologie (SGK), Schweizerische Statistik für Herzschrittmacher, ICD und Ablationen 2012. 2013 April 30; Available from: http://www.pacemaker.ch/de/statistik/statistik_2012
14 Stabile G, Solimene F, Bertaglia E, La Rocca V, Accogli M, Scaccia A, et al. Long-term outcomes of CRT-PM versus CRT-D recipients. Pacing Clin Electrophysiol, 2009;32(Suppl1):p.S141–5.
15 Cleland JG, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, et al. Long-term mortality with cardiac resynchronization therapy in the Cardiac Resynchronization-Heart Failure (CARE-HF) trial. Eur J Heart Fail. 2012;14(6):p.628–34.
16 Kreuz J, Horlbeck F, Linhart M, Mellert F, Fimmers R, Schrickel J, et al. Independent predictors of mortality in patients with advanced heart failure treated by cardiac resynchronization therapy. Europace. 2012.
17 Bogale N, Priori S, Cleland JG, Brugada J, Linde C, Auricchio A, et al. The European CRT Survey: 1 year (9–15 months) follow-up results. Eur J Heart Fail. 2012;14(1):p.61–73.
18 Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P, et al. Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010.55(8):p.774–82.
19 Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006.47(12):p.2493–7.
20 Brignole M. Are complications of implantable defibrillators under-estimated and benefits over-estimated? Europace. 2009.11(9):p.1129–33.
21 Duray GZ, Schmitt J, Cicek-Hartvig S, Hohnloser SH, Israel CW, Complications leading to surgical revision in implantable cardioverter defibrillator patients: comparison of patients with single-chamber, dual-chamber, and biventricular devices. Europace. 2009;11(3):p.297–302.
22 Landolina M, Gasparini M, Lunati M, Lacopino S, Boriani G, Bonanno C, et al. Long-term complications related to biventricular defibrillator implantation: rate of surgical revisions and impact on survival: insights from the Italian Clinical Service Database. Circulation. 2011;123(22):p.2526–35.
23 Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110(25):p.3760–5.
24 Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011;8(10):p.1622–8.
25 Biffi M, Moschini C, Bertini M, Saporito D, Ziacchi M, Diemberger I, et al. Phrenic stimulation: a challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2009;2(4):p.402–10.
26 Horlbeck FW, Mellert F, Kreuz J, Nickenig G, Schwab JO. Real-World Data on the Lifespan of Implantable Cardioverter-Defibrillators Depending on Manufacturers and the Amount of Ventricular Pacing. J Cardiovasc Electrophysiol. 2012.
27 Thijssen J, Borleffs CJ, van Rees JB, Man S, de Bie MK, Venlet J, et al. Implantable cardioverter-defibrillator longevity under clinical circumstances: an analysis according to device type, generation, and manufacturer. Heart Rhythm. 2012.9(4):p.513–9.
28 Knops P, Theuns DA, Res JC, Jordaens L. Analysis of implantable defibrillator longevity under clinical circumstances: implications for device selection. Pacing Clin Electrophysiol. 2009;32(10):p.1276–85.
29 Theuns DA, Thornton AS, Klootwijk AP, Scholten MF, Vantrimpont PJ, Balk AH, et al. Outcome in patients with an ICD incorporating cardiac resynchronisation therapy: differences between primary and secondary prophylaxis. Eur J Heart Fail 2005;7(6):p.1027–32.
30 Schaer B, Kuhne M, Koller MT, Sticherling C, Osswald S. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: benefits and disadvantages. Swiss Med Wkly. 2009;139(45–46):p.647–53.
31 Schaer BA, Osswald S, Di Valentino M, Soliman OI, Sticherling C, ten Cate FJ, et al. Close connection between improvement in left ventricular function by cardiac resynchronization therapy and the incidence of arrhythmias in cardiac resynchronization therapy-defibrillator patients. Eur J Heart Fail. 2010;12(12):p.1325–32.
32 Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):p.1845–53.
33 St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107(15):p.1985–90.
34 Auricchio A, Metra M, Gasparini M, Lamp B, Klersy C, Curnis A, et al. Long-term survival of patients with heart failure and ventricular conduction delay treated with cardiac resynchronization therapy. Am J Cardiol. 2007;99(2):p.232–8.
35 Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC, Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32(8):p.991–8.
36 Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121(18):p.1985–91.
37 Marsan NA, Bleeker GB, van Bommel RJ, Ypenburg C, Delgado V, Borleffs CJ, et al. Comparison of time course of response to cardiac resynchronization therapy in patients with ischemic versus nonischemic cardiomyopathy. Am J Cardiol. 2009;103(5):p.690–4.
Funding / potential competing interests: There was no funding for this study. B. Schaer has received research grants from Boston Scientific and St. Jude Medical and has a speakers’ bureau appointment with Medtronic. C. Sticherling has received research grants from Biotronik and Boston Scientific and has a speakers’ bureau appointment with Medtronic, Biotronik, Boston Scientific and Sorin. M. Kuehne has a speakers’ bureau appointment with St. Jude Medical. S. Osswald has received research grants from Boston Scientific, St. Jude Medical, Biotronik and Medtronic and has a speakers’ bureau appointment for the same companies. M. Bossard and S. Frey have nothing to declare