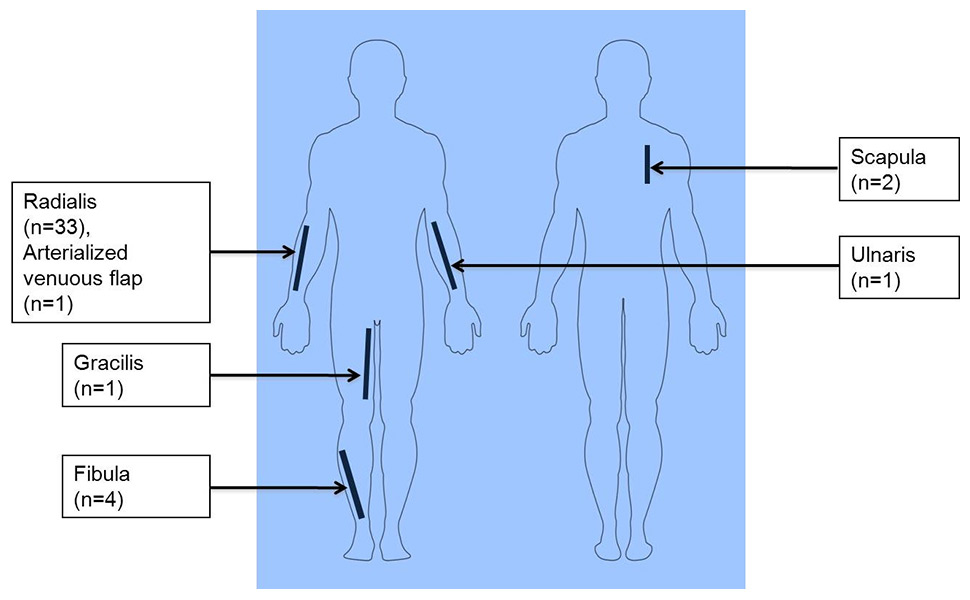
Figure 1
Distribution of flap donor sites in all reviewed patients undergoing microvascular free tissue transfer.
DOI: https://doi.org/10.4414/smw.2014.13941
Airway management in head and neck cancer (HNC) patients undergoing major surgical procedures, including microvascular free tissue transfer (MFTT), has often been routine tracheotomy. The necessity of this procedure has, however, been questioned [1, 2].
Proponents of routine tracheotomy argue that extensive HNC resection combined with MFTT is a major surgical procedure with long operative times and often performed on poly-morbid patients. Hence, the anatomical and physiological impact should not be underestimated [3]. Laryngopharyngeal oedema, posterior tongue oedema, swelling of the free flap, haemorrhage and phlegm accumulation can all potentially cause airway compromise. These concerns are particularly pressing in surgically treated oropharyngeal cancer. Furthermore, tracheotomy can help improve access to the primary tumour intraoperatively.
Proponents of not performing routine tracheotomy argue that, when performed in the appropriately experienced centres, even major HNC resection and MFTT is reliable and predictable. The perioperative mortality rate is reported to be around 1.1% and free flap failure rates are similarly low [3–5]. Even in elderly patients, MFTT is considered a safe procedure with similar complication rates to those in younger patients [4, 5]. Appropriate postoperative vigilance allows patients to avoid routine tracheotomy in the knowledge that later airway interventions can be performed if necessary.
Furthermore, it should be remembered that tracheotomy itself is not without complications, with rates as high as 4.1% to 8% in some series [6–8]. Possible complications include haemorrhage, obstruction, cannula displacement, local infection, pneumonia, fistula, tracheal stenosis and tumour recurrence due to tumour seeding [6, 9]. Also, average time to decannulation in patients with routine tracheotomy can be prolonged and the effect of tracheotomy on functional rehabilitation is difficult to quantify [10].
In an effort to reduce operative time and morbidity, and speed functional recovery, several authors have, therefore, presented evidence that routine tracheotomy is not always required [1, 2]. For example, Brickman et al. argued that maxillectomy and MFTT does not negatively impact a patient’s oropharyngeal airway and that elective tracheotomy should only be considered in patients with additional risk factors, such as cardiopulmonary diseases [2]. Indeed, outside of trauma and cancer settings, for example in bimaxillary advancement for cleft palate, routine tracheotomy is far less common.
Balancing the benefits and risks of routine tracheotomy to a specific patient is clearly no simple exercise. Recently, a tracheotomy scoring system to guide airway management after major head and neck surgery has been proposed, whereby tumour site, mandibulectomy, neck dissection and reconstruction are scoring factors [11]. However, this score requires further assessment in a prospective randomised controlled trial and a larger group of patients. In this context we present a retrospective case series analysis, evaluating both the traditional tracheotomy approach and the alternative delayed extubation approach.
A retrospective study was performed on consecutive HNC patients in the Department of Otorhinolaryngology (University Hospital, Zurich, Switzerland) between April 2011 and January 2013. All patients were discussed in our multidisciplinary tumour board meeting and staged in accordance with the latest version of the American Joint Committee on Cancer (AJCC) guidelines [12]. Inclusion criteria for this study were all HNC patients undergoing HNC resection with curative intent and reconstruction with MFTT. Primary tumours were located in the oral cavity, oropharynx, hypopharynx, larynx and salivary glands. Two patients were excluded from the study because they had pre-existing tracheostomies.
In April 2012, we changed from routine tracheotomy to prolonged intubation. Thus, the observation period was characterised by patients undergoing MFTT with (before April 2012) and without (after April 2012) routine tracheotomy. Patients with a transmandibular approach, segmental mandibular resection, maxillectomy or undergoing laryngopharyngectomy were excluded from the prolonged intubation approach.
Patient’s case records were reviewed for demographic data (gender, age at the time of operation), TNM staging, tumour histology, primary tumour site and outcome measures: overall survival (OS), disease specific survival (DSS) and any recurrence free survival (ARFS). With regard to MFTT, we analysed the type of the free tissue flap and flap complications (anastomosis revision, flap wound revision, complications in the further course of treatment, donor site complications). Furthermore, we reviewed patient data for duration of surgery, length of postoperative stay on the intensive care unit (ICU), length of stay on the intermediate care unit (IMCU), length of postoperative dependence on artificial nutrition (nasogastric tube vs percutaneous endoscopic gastrostomy vs total parenteral nutrition) and overall length of hospital stay. With regard to airway management we reviewed patient data for tracheotomy: no tracheotomy vs primary tracheotomy vs secondary tracheotomy. Duration of intubation for patients undergoing prolonged intubation without primary tracheotomy was noted.
A total of 40 patients met inclusion criteria. They were split into two groups, namely patients with no tracheotomy or secondary tracheotomy (n = 23; NO-TRACH group) and patients with primary tracheotomy (n = 17; PRIM-TRACH group).
Data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Because of the small sample size and non-normally distributed data we used nonparametric statistics (Mann-Whitney Test) to test differences between the two groups. Survival rates were estimated by the Kaplan-Meier method and compared using a log-rank test if appropriate. A p-value <0.05 was considered statistically significant.
When comparing dependence on artificial nutrition in both groups, the analysis was based on a subset of patients (n = 35) that excluded those who remained dependent on artificial nutrition until the present day or those who died in the follow-up.
In an exploratory analysis, we also investigated the difference in any complications (yes/no) with type of airway management (NO-TRACH vs PRIM-TRACH) using a regression modelling approach. The analysis was based on a subset of patients (n = 37) that excluded those with a secondary tracheotomy. To adjust for potential differences in the prognostic profile between patients receiving one of the two airway management approaches, we estimated the risk for each patient of a complication (dependent variable) based on the patient’s age, gender and tumour stage (independent variables) by means of a multivariate logistic regression analysis. In a second logistic regression analysis using complications as the dependent variable, the variable containing the prognostic probability of a complication based on these three parameters was entered along with an indicator variate for type of airway management approach. Thus, we made the two groups comparable in terms of age, gender and tumour stage. Statistical analyses were made using the Stata 11.2 statistics software package (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.).
Between April 2011 and January 2013, 42 patients HNC patients underwent HNC resection and reconstruction with MFTT at our department. Two patients were excluded from the study because they had pre-existing tracheostomies. Consequently, the total study population consisted of 40 patients: 29 male patients (72.5%) and 11 female patients (27.5%). Eighteen male patients (78.3%) and 5 female patients (21.7%) were in the NO-TRACH group, compared with 11 male patients (64.7%) and 6 female patients (35.3%) in the PRIM-TRACH group. At the time of operation, the mean age of all patients was 65.1 ± 10.8 years, with the oldest patient being 90 years old and the youngest patient being 39 years old. The mean age in the NO-TRACH group was 64.5 ± 11.0 years compared with 65.8 ± 10.9 years in the PRIM-TRACH group (p = 0.79) (table 1).

Figure 1
Distribution of flap donor sites in all reviewed patients undergoing microvascular free tissue transfer.
Histological workup of all 40 patients showed squamous cell carcinoma in 33 patients (82.5%), adenocarcinoma in 4 patients (10.0%), acinus-cell carcinoma in 1 patient (2.5%), mucoepidermoid carcinoma in 1 patient (2.5%) and spindle-cell like carcinoma in 1 patient (2.5%). Sites of primary tumours were: oral cavity (24 patients; 60.0%), oropharynx (9 patients; 22.5%), parotid gland (2 patients; 5.0%), subglottic and supraglottic larynx (2 patients; 5.0%), hard palate and maxilla (1 patient; 2.5%), floor of the nose (1 patient; 2.5%) and sublingual gland (1 patient; 2.5%). Two patients in the PRIM-TRACH group underwent neoadjuvant chemotherapy prior to surgical resection and MFTT. Seven patients of the PRIM-TRACH group and one patient of the NO-TRACH group received salvage surgery as a result of tumour persistence or tumour recurrence after a nonsurgical primary treatment approach. Table 1 provides detailed information on patient and tumour characteristics for each group separately.
| Table 1: Patient demographic data and tumour characteristics for each group. | ||
| NO-TRACH (n = 23) | PRIM-TRACH (n = 17) | |
| Demographics | ||
| Mean age ± SD (years) | 64.5 ± 11.0 | 65.8 ± 10.9 |
| Female | 5/23 (21.7%) | 6/17 (35.3%) |
| Male | 18/23 (78.3%) | 11/17 (64.7%) |
| Range of age (years) | 39–90 | 45–83 |
| Tumour site | ||
| Lateral tongue | 7/23 (30.4%) | 1/17 (5.9%) |
| Base of tongue | 2/17 (11.8%) | |
| FOM | 6/23 (26.1%) | 1/17 (5.9%) |
| Buccal mucosa | 4/23 (17.4%) | 1/17 (5.9%) |
| Soft palate | 2/23 (8.6%) | 3/17 (17.6%) |
| Hard palate | 1/17 (5.9%) | |
| Palatine tonsils | 1/17 (5.9%) | |
| Alveolar ridge | 1/17 (5.9%) | |
| Oropharynx (pharynx wall) | 1/23 (4.3%) | 1/17 (5.9%) |
| Sublingual gland | 1/23 (4.3%) | |
| Parotid gland | 2/23 (8.6%) | |
| Larynx (subglottic and supraglottic) | 2/17 (11.8%) | |
| Hard palate and maxilla | 1/17 (5.9%) | |
| Floor of the nose | 1/17 (5.9%) | |
| Lips | 1/17 (5.9%) | |
| T stage | ||
| T1 | 7/23 (30.4%) | 4/17 (23.5%) |
| T2 | 12/23 (52.2%) | 3/17 (17.6%) |
| T3 | 2/23 (8.7%) | 1/17 (5.9%) |
| T4 | 2/23 (8.7%) | 7/17 (41.2%) |
| NOS | 1/17 (5.9%) | |
| N stage | ||
| N0 | 9/23 (39.1%) | 11/17 (64.7%) |
| N1 | 7/23 (30.45%) | 1/17 (5.9%) |
| N2 | 7/23 (30.45%) | 4/17 (23.5%) |
| NOS | 1/17 (11.8%) | |
| TNM staging (I-IV) | ||
| Stage I | 4/23 (17.4%) | 3/17 (17.6%) |
| Stage II | 4/23 (17.4%) | 3/17 (17.6%) |
| Stage III | 7/23 (30.4%) | 2/17 (11.8%) |
| Stage IV | 8/23 (34.8%) | 7/17 (41.2%) |
| NOS | 2/17 (11.8%) | |
| Primary vs persistent / recurrent tumour | ||
| Primary tumour | 22/23 (95.7%) | 10/17 (58.8%) |
| Tumour persistence / recurrence | 1/23 (4.3%) | 7/17 (41.2%) |
| Histological workup | ||
| SCC | 19 (82.7%) | 14 (82.3%) |
| AC | 3 (13%) | |
| Acinus cell carcinoma | 1 (4.3%) | |
| MEC | 1 (5.9%) | |
| Spindle-cell like tumour maxilla | 1 (5.9%) | |
| Metastasis soft-tissue following adenocarcinoma | 1 (5.9%) | |
| Tracheotomy | ||
| Primary tracheotomy | 17 (100%) | |
| Secondary tracheotomy | 3 (13%) | |
| AC = adenocarcinoma; FOM = floor of the mouth; MEC = mucoepidermoid carcinoma; NO-TRACH = delayed extubation approach; NOS = not otherwise specified; PRIM-TRACH = primary tracheotomy approach; SCC = squamous cell carcinoma; SD = standard deviation | ||
| Table 2: Intraoperative and perioperative characteristics for NO-TRACH patients and PRIM-TRACH patients. | |||
| NO-TRACH | PRIM-TRACH | p-value | |
| Mean duration of surgery, SD (hours) | 8.2 ± 1.6 | 10.4 ± 2.7 | p = 0.001 |
| Median time in ICU, IQR (days) | 1.0 (1–2) | 2.0 (1–3) | p = 0.27 |
| Median time in IMCU, IQR (days) | 1.0 (1–1) | 1.0 (1–2) | p = 0.58 |
| Median duration of artificial nutrition, IQR (days) | 9.0 (8–18) | 15.0 (9–55) | p = 0.07 |
| Median duration of hospitalisation, IQR (days) | 15.0 (10–21) | 18.0 (14–22) | p = 0.14 |
| ICU = intensive care unit, IMCU = intermediate care unit; IQR = interquartile range; NO-TRACH = delayed extubation approach; PRIM-TRACH = primary tracheotomy approach; SD = standard deviation | |||
Compared with the PRIM-TRACH group, the duration of surgery in the NO-TRACH group was significantly less (8.2 ± 1.6 hours vs 10.4 ± 2.7 hours; p = 0.001) (table 2). In 20 of 23 patients in the NO-TRACH group there was no need for secondary tracheotomy (87%). In three patients (13%), secondary tracheotomy was performed, in one patient for revision of the neck due to extended cervical haematoma on the first postoperative day and in two patients owing to cardiopulmonary decompensation and the need for long-term intubation on the third and sixth postoperative day, respectively. In the PRIM-TRACH group, tracheotomy was performed at the start of the procedure in all 17 patients (table 1).
Postoperatively, 21/23 patients (91.3%) of the NO-TRACH group and 14/17 patients (82.4%) of the PRIM-TRACH group received further care on the ICU. The ICU length of stay in the NO-TRACH group was similar to the PRIM-TRACH group (median 1.0 days, IQR 1–2 vs median 2.0 days, IQR 1–3, p = 0.27). On average, delayed extubation of the NO-TRACH patients was performed after 1.1 ± 0.9 days (excluding those patients with secondary tracheotomy). Consequently, patients of both groups were transferred to the IMCU, with similar outcome in terms of IMCU stay: median 1.0 days, IQR 1–2 in the PRIM-TRACH group vs median 1.0 days, IQR 1–1 in the NO-TRACH group (p = 0.58). Two patients in the NO-TRACH and three patients of the PRIM-TRACH group proceeded directly to the IMCU on the day of operation, without any ICU stay (table 2).
In terms of postoperative nutrition, the PRIM-TRACH group showed a trend to longer dependence on artificial nutrition compared with the NO-TRACH (median 15.0 days, IQR 9–55 vs median 9.0 days, IQR 8–18; p = 0.07) (table 2). Five of the PRIM-TRACH patients remained dependent on artificial nutrition until last follow-up or until their death, whereas all patients of the NO-TRACH group were ultimately able to be orally fed.
Again our results show a (nonsignificantly) shorter median length of hospital stay in the NO-TRACH group compared with the PRIM-TRACH group (15.0 days, IQR 10–21 vs 18.0 days, IQR 14–22, p = 0.14) (table 2).
Overall, 31 patients underwent radial forearm flaps (77.5%), 3 patients had fibula flaps (7.5%), 2 patients had scapula flaps (5.0%), 1 patient received an arterialised venous flap of the forearm (2.5%) and 1 patient received a gracilis muscle flap (2.5%). One patient received both fibula and radial forearm flaps simultaneously (2.5%) and one patient received radial forearm flap and secondary ulnar forearm flap as a consequence of flap necrosis (2.5%) (fig. 1).
Anastomosis revision was performed in 3/40 patients (7.5%) because of venous thrombosis (n = 1) and venous bleeding/stasis (n = 2). Flap wound revisions were needed in two patients owing to venous bleeding/stasis, and in two patients because of partial necrosis of the flap and the need for surgical debridement (10%). Nonsalvageable flap failure occurred in one patient as a result of arterial thrombosis (2.5%), with unsuccessful revision, resulting in an overall flap survival rate of 97.5%.
In the further course, 8 of 40 patients (20.0%) developed complications including: fistula (1 patient), cervical haematoma (4 patients), flap dehiscence (2 patients) and wound healing impairment (1 patient).
Nine patients (22.5%) had flap donor site complications including wound dehiscence (3 patients), haematoma with need for vacuum-assisted closure therapy (1 patient), wound infection with the need for antibiotic therapy (2 patients), prolonged wound healing (2 patients) and seroma (1 patient).
After adjustment for potential difference in the prognostic profile between patients either receiving a tracheotomy or delayed postoperative extubation, the likelihood for complications was nearly identical in the two groups (OR [95% CI] 1.37 [0.26‒7.19], p = 0.710). Table 3 provides information on flap-associated complications for the NO-TRACH group and the PRIM-TRACH group separately, excluding those patients with secondary tracheotomy.
For the group as a whole, median follow up was 357 days (IQR 193–483) with 1-year OS, DSS and ARFS of 92.5%, 94.9% and 79.9%, respectively.
When the two groups were compared, median follow-up was 299 days (IQR 183–481) in the NO-TRACH group compared with 384 days (IQR 276–475) in the PRIM-TRACH group. One-year OS, DSS and ARFS were 95.7%, 95.7% and 89.1%, respectively, in the NO-TRACH group, and 88.2%, 93.8% and 68.2%, respectively, in the PRIM-TRACH (table 4). No significant difference between groups was found for OS or DSS (OS: p = 0.89; DSS: p = 0.79). The NO-TRACH group showed a better ARFS than the PRIM-TRACH group (p = 0.04).
| Table 3: Flap-associated complications for the NO-TRACH and the PRIM-TRACH groups. | |||
| NO-TRACH | PRIM-TRACH | p-value | |
| Flap failure | p = 0.710* | ||
| Arterial thrombosis | n = 1 | ||
| Anastomosis revision | |||
| Venous bleeding/stasis | n = 2 | ||
| Venous thrombosis | n = 1 | ||
| Flap wound revision | |||
| Partial necrosis | n = 1 | ||
| Venous bleeding/stasis | n = 1 | ||
| Complications in the further course of treatment | |||
| Cervical haematoma | n = 2 | n = 2 | |
| Flap dehiscence | n = 2 | ||
| Fistula | n = 1 | ||
| Wound healing disorder | n = 1 | ||
| Complications flap donor site | |||
| Dehiscence | n = 2 | n = 1 | |
| Infection | n = 1 | n = 1 | |
| Wound healing disorder | n = 2 | ||
| Seroma | n = 1 | ||
| Haematoma | n = 1 | ||
| NO-TRACH = delayed extubation approach; PRIM-TRACH = primary tracheotomy approach * Intergroup differences were corrected for age, gender and tumour-stage, excluding those patients with secondary tracheotomy. | |||
| Table 4: Outcome measures for each group. | ||
| NO-TRACH (n = 23) | PRIM-TRACH (n = 17) | |
| Median follow-up, IQR (days) | 299 (183–481) | 384 (276–475) |
| Number of events, 1–year overall survival (%) | 1/23 (95.7%) | 2/17 (88.2%)* |
| Number of events, 1–year disease specific survival (%) | 1/23 (95.7%) | 1/17 (93.8%)# |
| Number of events, 1–year any recurrence free survival (%) | 2/23 (89.1%) | 5/17 (68.3%)† |
| IQR = interquartile range; NO-TRACH = delayed extubation approach; PRIM-TRACH = primary tracheotomy approach * p = 0.89 vs NO-TRACH; # p = 0.79 vs NO-TRACH; † p = 0.04 vs NO-TRACH | ||
Although this was not a prospective, randomised controlled trial of tracheotomy versus no tracheotomy, we can say with some confidence that not performing routine tracheotomy in patients meeting the inclusion criteria for the delayed extubation approach was safe. Secondary tracheotomy was performed as a nonemergency procedure in three patients, two because of a prolonged intensive care stay due to cardiopulmonary problems and one whilst undergoing revision of a neck haematoma. We noted several benefits of not performing routine tracheotomy, including shorter operation time, which can partially be explained by not performing tracheotomy, and additional patient comfort in terms of postoperative swallowing rehabilitation. Furthermore, overall perioperative complications were comparable in the two groups (NO-TRACH versus PRIM-TRACH) with no perioperative airway complications occurring.
Our case series is typical for a tertiary referral centre with squamous cell cancer (82.5%) being the most common disease and the oral cavity (60.0%) the most common primary tumour site [13, 14]. Between April 2011 and January 2013, 40 HNC patients fitting the inclusion criteria for this study underwent HNC resection and reconstruction with MFTT at our department. Similar to other studies, the radial forearm flap was the most commonly used flap (77.5%), providing thin pliable skin with a highly reliable long pedicle [13–15]. This is ideal for reconstructing defects of the oral mucosa, tongue and floor of mouth [13, 16]. Our flap complication and failure rates are in line with other series, which reported flap survival rates of between 91.6% and 99.3% [13, 14, 17].
Optimal airway management in HNC patients undergoing MFTT is controversial. The traditional thinking is that the more major the procedure, the more a tracheotomy is indicated. Procedures requiring MFTT are by definition very major and long procedures, which would fall into this high risk group [11]. Laryngopharyngeal oedema, posterior tongue oedema, retained secretions or swelling of the free flap within the first postoperative days can be life threatening, and primary tracheotomy is a proven and reliable method for securing the airway. Thus, patients undergoing a transmandibular approach, segmental mandibular resection, maxillectomy or laryngopharyngectomy are treated with routine tracheotomy. In this particular subgroup of patients, either the risk of postoperative airway compromise clearly exceeds the benefit of not performing tracheotomy or the surgical procedure requires tracheotomy anyway. However, although tracheotomy usually allows reasonable functional rehabilitation with respect to speech and swallowing [10], it complicates postoperative rehabilitation, generates additional discomfort for the patient, and precludes patient independence in a subpopulation of patients. Furthermore, patients are confronted with additional risks, such as haemorrhage, obstruction, cannula displacement, local infection, pulmonary infection, fistula, tracheal stenosis and tumour seeding [6, 9].
Our current regimen is to avoid routine tracheotomy when reasonable and when meeting the above mentioned inclusion criteria. Patients with the need for MFTT to the floor of mouth or the tongue especially can often be safely managed without tracheotomy. Postoperatively, patients remain intubated until the first postoperative day and then, following extubation, proceed to the IMCU for further close monitoring. Usually 12 to 24 hours later, patients can be transferred to the regular ward and undergo routine rehabilitation. None of our patients suffered life-threatening perioperative airway complications and overall perioperative complication rates were similar between the two groups. This shows our approach to be safe. Our results also show that NO-TRACH patients remained intubated for an average of 1.1 days, which indicates our postoperative regimen of airway management to be efficient.
Lastly, our results show a decreased duration of surgery, a trend to earlier resumption of oral feeding and shortened overall hospital stay in the NO-TRACH group. Although duration of surgery, dependence on artificial nutrition and postoperative hospitalisation time are influenced by various cofactors, especially by the complexity of the surgical procedure, disease stage and comorbidities of the patients, our approach supports current efforts to concurrently optimise postoperative patient rehabilitation and clinical cost effectiveness.
Our results reflect a single-centre experience based on a small number of patients. Thus, generalisation of our findings needs to be proven in further studies. The observation period was characterised by consecutive patients undergoing MFTT with (before April 2012) and without (after April 2012) routine tracheotomy. Therefore, a certain gain of know-how and experience in the peri- and post-operative care of these patients may favour the NO-TRACH group.
We are aware of the fact that intergroup comparison (NO-TRACH group vs PRIM-TRACH group) must be interpreted with caution, because of the heterogeneous initial risk profiles of the two cohorts resulting from differences in comorbidities, distribution of primary tumour sites and, especially, TNM stage. TNM staging of the PRIM-TRACH group revealed a trend to more advanced disease with a higher rate of salvage procedures, when compared with the NO-TRACH group. This is reflected by a lower ARFS compared with the NO-TRACH group. We therefore reran our analysis excluding all the salvage patients and once again found that mean duration of surgery was significantly shorter in the NO-TRACH group and again we observed trends to shorter ICU stay and hospital length of stay. Survival analysis was similar to the results found when not excluding the salvage patients, although the small number of events in the survival analysis limits its significance. Indeed, the only difference from our original results was that the resumption of oral feeding no longer showed any difference between the two groups.
Even in patients undergoing major HNC resection and MFTT, tracheotomy should not be considered routine. We suggest that each case should be assessed on its own merits. Despite our small cohort size, we show encouraging functional results using a delayed extubation approach, without adverse perioperative or oncological impact. We emphasise, however, that NO-TRACH patients need to be closely monitored postoperatively to ensure the safety of this approach. Further work, ideally in the setting of a large prospective randomised clinical trial, is needed to confirm our findings.
1 Lin HS, Wang D, Fee WE, Goode RL, Terris DJ. Airway management after maxillectomy: routine tracheostomy is unnecessary. Laryngoscope. 2003;113(6):929–32. Epub 2003/06/05. doi: 10.1097/00005537–200306000–00002. PubMed PMID: 12782798.
2 Brickman DS, Reh DD, Schneider DS, Bush B, Rosenthal EL, Wax MK. Airway management after maxillectomy with free flap reconstruction. Head Neck. 2013;35(8):1061–5. doi: 10.1002/hed.23082. PubMed PMID: 22907774.
3 Pohlenz P, Klatt J, Schmelzle R, Li L. The importance of in-hospital mortality for patients requiring free tissue transfer for head and neck oncology. Br J Oral Maxillofac Surg. 2013;51(6):508–13. doi: 10.1016/j.bjoms.2012.10.020. PubMed PMID: 23369783.
4 Tarsitano A, Pizzigallo A, Sgarzani R, Oranges CM, Cipriani R, Marchetti C. Head and neck cancer in elderly patients: is microsurgical free-tissue transfer a safe procedure? Acta Otorhinolaryngol Ital. 2012;32(6):371–5. Epub 2013/01/26. PubMed PMID: 23349555; PubMed Central PMCID: PMC3552542.
5 Classen DA, Ward H. Complications in a consecutive series of 250 free flap operations. Ann Plast Surg. 2006;56(5):557–61. Epub 2006/04/28. doi: 10.1097/01.sap.0000205830.39108.9a00000637–200605000–00018 [pii]. PubMed PMID: 16641636.
6 Halfpenny W, McGurk M. Analysis of tracheostomy-associated morbidity after operations for head and neck cancer. Br J Oral Maxillofac Surg. 2000;38(5):509–12. Epub 2000/09/30. doi: 10.1054/bjom.2000.0310S0266–4356(00)90310–3 [pii]. PubMed PMID: 11010784.
7 Fattahi T, Vega L, Fernandes R, Goldman N, Steinberg B, Schare H. Our experience with 171 open tracheostomies. J Oral Maxillofac Surg. 2012;70(7):1699–702. Epub 2011/10/25. doi: 10.1016/j.joms.2011.07.015S0278–2391(11)01261–4 [pii]. PubMed PMID: 22018448.
8 Beausang ES, Ang EE, Lipa JE, Irish JC, Brown DH, Gullane PJ, et al. Microvascular free tissue transfer in elderly patients: the Toronto experience. Head Neck. 2003;25(7):549–53. doi: 10.1002/hed.10240. PubMed PMID: 12808658.
9 Ong SK, Morton RP, Kolbe J, Whitlock RM, McIvor NP. Pulmonary complications following major head and neck surgery with tracheostomy: a prospective, randomized, controlled trial of prophylactic antibiotics. Arch Otolaryngol Head Neck Surg. 2004;130(9):1084–7. Epub 2004/09/24. doi: 10.1001/archotol.130.9.1084130/9/1084 [pii]. PubMed PMID: 15381595.
10 Skoner JM, Andersen PE, Cohen JI, Holland JJ, Hansen E, Wax MK. Swallowing function and tracheotomy dependence after combined-modality treatment including free tissue transfer for advanced-stage oropharyngeal cancer. Laryngoscope. 2003;113(8):1294–8. Epub 2003/08/05. doi: 10.1097/00005537–200308000–00005. PubMed PMID: 12897548.
11 Cameron M, Corner A, Diba A, Hankins M. Development of a tracheostomy scoring system to guide airway management after major head and neck surgery. Int J Oral Maxillofac Surg. 2009;38(8):846–9. Epub 2009/05/09. doi: 10.1016/j.ijom.2009.03.713S0901–5027(09)00821–2 [pii]. PubMed PMID: 19423295.
12 Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM: Springer; 2010. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20180029.
13 Disa JJ, Pusic AL, Hidalgo DH, Cordeiro PG. Simplifying microvascular head and neck reconstruction: a rational approach to donor site selection. Ann Plast Surg. 2001;47(4):385–9. Epub 2001/10/17. PubMed PMID: 11601572.
14 Holom GH, Seland H, Strandenes E, Liavaag PG, Lybak S, Løes S, et al. Head and neck reconstruction using microsurgery: a 9–year retrospective study. Eur Arch Otorhinolaryngol. 2013;270(10):2737–43. doi: 10.1007/s00405–013–2390–7. PubMed PMID: 23417224.
15 Dassonville O, Poissonnet G, Chamorey E, Vallicioni J, Demard F, Santini J, et al. Head and neck reconstruction with free flaps: a report on 213 cases. Eur Arch Otorhinolaryngol. 2008;265(1):85–95. Epub 2007/08/11. doi: 10.1007/s00405–007–0410–1. PubMed PMID: 17690895.
16 Hidalgo DA, Disa JJ, Cordeiro PG, Hu QY. A review of 716 consecutive free flaps for oncologic surgical defects: refinement in donor-site selection and technique. Plast Reconstr Surg. 1998;102(3):722–32; discussion 33–4. Epub 1998/09/04. PubMed PMID: 9727437.
17 Suh JD, Sercarz JA, Abemayor E, Calcaterra TC, Rawnsley JD, Alam D, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 2004;130(8):962–6. Epub 2004/08/18. doi: 10.1001/archotol.130.8.962130/8/962 [pii]. PubMed PMID: 15313867.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.