Forty years of haematopoietic stem cell transplantation: a review of the Basel experience
DOI: https://doi.org/10.4414/smw.2014.13928
Alix Catherine
O'Meara, Andreas
Holbro, Sara
Meyer, Maria
Martinez, Michael
Medinger, Andreas
Buser, Joerg
Halter, Dominik
Heim, Sabine
Gerull, Christoph
Bucher, Alicia
Rovo, Thomas
Kuehne, André
Tichelli, Alois
Gratwohl, Martin
Stern, Jakob
Passweg
Summary
The purpose of this study was to examine changes in haematopoietic stem cell transplant (HSCT) characteristics and outcome in our combined paediatric and adult programme over the past four decades, since its implementation in 1973. The total number of transplant procedures rose from 109 in the first decade (1973–82) to 939 in the last decade (2003–12). Transplant characteristics changed significantly over time: patient age increased, peripheral blood largely replaced bone marrow as stem cell source, unrelated donors became an alternative to matched siblings, and patients are increasingly transplanted in more advanced disease stages.
Advances such as improved supportive care and histocompatibility typing resulted in a steady decrease of transplant-related mortality after allogeneic HSCT (43% in the first decade, 22% in the last decade). Despite this, unadjusted survival rates were stable in the last three decades for allogeneic HSCT (approximately 50% 5–year survival) and in the last two decades for autologous HSCT (approximately 60% 5–year survival). After adjustment for covariates such as donor type, age and stage, the relative risk of treatment failure continuously dropped (for allogeneic HSCT: first decade 1.0, second decade 0.58, third decade 0.51, last decade 0.41). Collectively, these data suggest that improvements in peri- and post-transplant care have allowed considerable extension of transplant indications without having a negative impact on outcome.
Introduction
Haematopoietic stem cell transplantation (HSCT) replaces diseased haematopoiesis by either the patient’s own haematopoietic stem cells (autologous), stem cells from a related or unrelated donor (allogeneic) or stem cells originating from an identical twin (syngeneic) after a course of chemotherapy and/or irradiation termed conditioning. A wide variety of haematopoietic and nonhaematopoietic pathologies, ranging from myeloid and lymphoid malignancies to acquired or congenital bone marrow failures, can be treated by HSCT [1, 2].
Since its beginnings in 1959, HSCT has seen many developments [3]. With the advent of human leucocyte antigen (HLA) typing, HSCT became a legitimate treatment option in the late 1960s and early 1970s. In the 1990s, growing unrelated donor registries started to provide donors to patients lacking a compatible family member. Additional transplant sources such as mobilised autologous and allogeneic peripheral blood stem cells (PBSCs), and umbilical cord blood (CBT), mismatched and haploidentical stem cells were successfully introduced [4, 5]. Advances in HLA-typing and enhanced registry accrual have increased donor availability and matching performance [6]. Both the prophylaxis and treatment of frequent complications of HSCT such as graft-versus-host disease (GvHD) and infections have been adapted [7, 8]. Trends to de-escalate conditioning intensity and towards novel treatments of early relapse (e.g. donor lymphocyte infusions) have been introduced [9]. HSCT may now be offered to a larger group of patients with more advanced diseases, higher age and nonpreferential donor/host constellations [10].
HSCT has become an established treatment option and in 2013 the millionth transplant worldwide was performed [11]. In Basel, HSCT was first introduced in 1973 at a time when this treatment was considered experimental by the late Bruno Speck, an early pioneer in the field [12]. The purpose of this study was to compare transplant characteristics over the last four decades since the beginnings of HSCT in our combined paediatric and adult programme. We also examined the changes in outcome parameters over the last four decades (1973–2012) and the effect of changes in disease and transplant characteristics on the outcome.
Patients and methods
The data for this analysis was taken from our unique patient number (UPN) database for the timespan November 1973 to December 2012. First autologous and first allogeneic transplants were selected, and patient, disease and transplant characteristics were compiled and compared across decades using tests for categorical or continuous data where appropriate. As patients may have received more than one transplant, such as a second HSCT in the case of failure or in the context of planned sequential HSCT, the total number of transplants exceeds the patient number as shown in tables 1 and 2. All outcome data refer to patients. The primary endpoint was overall survival (OS). Secondary endpoints consisted of progression-free survival (PFS), transplant-related mortality (TRM) and relapse incidence (RI). Both overall and progression-free survivals were estimated by Kaplan-Meier analysis. Cumulative incidences of relapse and treatment-related mortality were calculated by treating these events as competing outcomes. Variables affecting overall survival were analysed in a multivariate fashion using Cox models to compare outcome per decade. As transplant indications changed over time, diagnosis was included in multivariate models as a stratification variable. Statistical analyses were performed on SPSS version 19 (SPSS Inc, Chicago, Illinois), GraphPad Prism 5.0a (GraphPad Software, San Diego, California) and Stata/SE 12.1 (StataCorp LP, College Station, Texas).
|
Table 1: Allogeneic haematopoietic stem cell transplantation ‒ patient and first transplant characteristics per decade. |
|
|
1973–1982
|
1983–1992
|
1993–2002
|
2003–2012
|
p-value
|
| No. total transplants |
109 |
222 |
345 |
626 |
|
| No. patients |
106 |
207 |
290 |
551 |
|
| Median age (range) |
21 (5 mo.‒54 yr) |
27 (2–50 yr) |
33 (1–62 yr) |
46 (8 mo.‒70 yr) |
<0.01 |
| Male/female |
55/51 |
107/100 |
169/121 |
335/216 |
0.01 |
| Indication: |
|
|
|
|
<0.01 |
| Acute lymphoblastic leukaemia |
29 |
41 |
61 |
98 |
|
| Acute myeloid leukaemia |
32 |
65 |
73 |
190 |
|
| Myelodysplastic syndrome / myeloproliferative neoplasm |
1 |
15 |
32 |
88 |
|
| Chronic myeloid leukaemia |
11 |
57 |
65 |
25 |
|
| Lymphoid malignancies |
|
12 |
25 |
80 |
|
| Plasma cell disorder |
|
2 |
19 |
46 |
|
| Solid tumours |
|
|
|
2 |
|
| Bone marrow failure |
31 |
15 |
14 |
15 |
|
| Primary immune deficiency / autoimmune disorders |
2 |
|
1 |
7 |
|
| Donor type at first transplant: |
|
|
|
|
<0.01 |
| Identical sibling |
96 |
184 |
189 |
243 |
|
| Other relatives |
5 |
8 |
32 |
38 |
|
| Matched unrelated |
1 |
10 |
60 |
264 |
|
| Syngeneic |
4 |
5 |
9 |
6 |
|
| Cell source at first transplant: |
|
|
|
|
<0.01 |
| Bone marrow |
106 |
207 |
121 |
44 |
|
| Peripheral blood |
|
|
169 |
486 |
|
| Cord blood |
|
|
|
21 |
|
| Conditioning type at first transplant: |
|
|
|
|
<0.01 |
| Myeloablative |
106 |
207 |
244 |
435 |
|
| Reduced intensity/ nonmyeloablative |
|
|
46 |
116 |
|
| Disease stage at first transplant: |
|
|
|
|
0.001 |
| Early |
61 |
120 |
129 |
234 |
|
| Intermediate |
14 |
38 |
58 |
104 |
|
| Advanced |
20 |
45 |
103 |
213 |
|
| Missing |
11 |
4 |
|
|
|
| EBMT risk score: |
|
|
|
|
<0.01 |
| 0 (lowest risk) |
18 |
15 |
14 |
6 |
|
| 1 |
27 |
64 |
39 |
37 |
|
| 2 |
30 |
54 |
70 |
98 |
|
| 3 |
20 |
44 |
64 |
119 |
|
| 4 |
10 |
25 |
52 |
111 |
|
| 5 |
1 |
5 |
38 |
111 |
|
| 6 |
|
|
13 |
62 |
|
| 7 (highest risk) |
|
|
|
7 |
|
| EBMT = European Group for Blood and Marrow Transplantation |
|
Table 2:Autologous haematopoietic stem cell transplantation ‒ patient and first transplant characteristics per decade. |
|
|
1983–1992
|
1993–2002
|
2003–2012
|
p-value
|
| No. total transplants |
30 |
219 |
313 |
|
| No. patients |
29 |
180 |
243 |
|
| Median age in years (range) |
27 (8–61) |
47 (1–70) |
53 (1–72) |
<0.01 |
| Male/female |
18/11 |
107/73 |
151/92 |
0.72 |
| Indication: |
|
|
|
<0.01 |
| Acute lymphoblastic leukaemia |
9 |
6 |
5 |
|
| Acute myeloid leukaemia |
4 |
15 |
16 |
|
| Myelodysplastic syndrome / myeloproliferative neoplasm |
|
5 |
3 |
|
| Chronic myeloid leukaemia |
|
3 |
4 |
|
| Lymphoid malignancies |
10 |
63 |
77 |
|
| Plasma cell disorder |
1 |
35 |
107 |
|
| Solid tumours |
5 |
46 |
24 |
|
| Bone marrow failure |
|
1 |
|
|
| Primary immune deficiency / autoimmune disorders |
|
6 |
7 |
|
| Disease stage at first transplant: |
|
|
|
0.34 |
| Early |
13 |
66 |
69 |
|
| Intermediate |
6 |
69 |
111 |
|
| Advanced |
7 |
42 |
58 |
|
| Missing |
3 |
3 |
|
|
| Not applicable |
|
|
5 |
|
Results
Patient and transplant characteristics
Between 1973 and 2012, 1,154 patients (488 female, 666 male) received a first allogeneic HSCT in our centre. Indications were acute myeloid leukaemia (AML, n = 360, 31%), acute lymphoblastic leukaemia (ALL, n = 229, 20%), chronic myeloid leukaemia (CML, n = 158, 14%), myelodysplastic/myeloproliferative syndrome (MDS/MPN, n = 136, 12%), Hodgkin or non-Hodgkin lymphoma (HL/NHL, n = 117, 10%), bone marrow failure (BMF, n = 75, 7%), plasma cell disorder (PCD, n = 67, 6%), primary immune deficiency / autoimmune disorders (PID/AD, n = 10, 1%), and solid tumours (ST, n = 2, 0.2%). The stem cell source at first transplant was bone marrow in 478 (41%), mobilised peripheral blood stem cells in 655 (57%) and cord blood in 21 (2%). patientsThe most frequent stem cell donor was an identical sibling (n = 712, 62%), followed by an unrelated donor (n = 335, 29%), a mismatched relative (n = 83, 7%) and finally a syngeneic donor (n = 24, 2%). Patient and first transplant characteristics are shown in table 1.
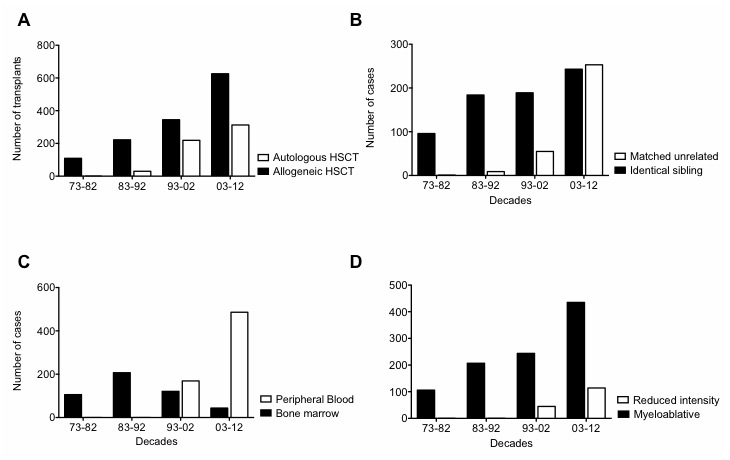
Figure 1
(A) Total allogeneic and autologous haematopoietic stem cell transplantation (HSCT) numbers over the decades. (B) Identical sibling and matched unrelated allogeneic HSCT patient case numbers over the decades. (C) Bone marrow and peripheral blood stem cell source patient case numbers in allogeneic HSCT. (D) Patient case numbers receiving myeloablative and reduced-intensity conditioning for allogeneic HSCT.
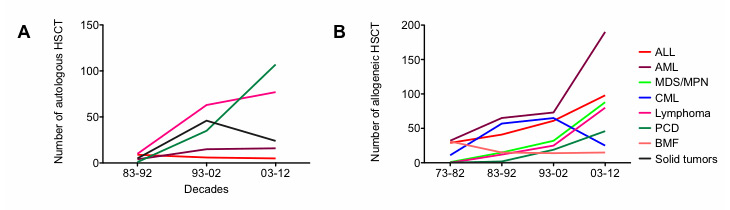
Figure 2
(A) Indications for autologous haematopoietic stem cell transplantation (HSCT) over the decades. (B) Indications for allogeneic HSCT over the decades.
ALL = acute lymphoblastic leukaemia; AML = acute myeloid leukaemia; BMF = bone marrow failure; CML = chronic myeloid leukaemia; MDS/MPN = myelodysplastic syndrome / myeloproliferative neoplasm; PCD = plasma cell disorder
When compared across decades, the total number of transplants increased significantly (no. of allogeneic HSCT performed in the first decade: 109; no. of allogeneic HSCT in the last decade: 626; fig. 1, panel A). An elevation of median age at transplantation appeared (median age first decade: 21 years; median age last decade: 46 years). Significant variations in stem cell donors as well as stem cell sources developed over the last four decades, with accrual of unrelated and peripheral stem cell donations and correspondingly fewer bone marrow donations (fig. 1, panels B and C). Furthermore, a shift in indications treated with allogeneic transplantation, away from CML and towards AML, MDS, plasma cell and lymphoid malignancies, could be observed (fig. 2, panel B). Pretransplant risk score as assessed with the European Group for Blood and Marrow Transplantation (EBMT) criteria on the basis of patient age, disease stage, lapse of time from first diagnosis to transplant and donor constellation, that is sibling versus unrelated and recipient/donor gender [13], also demonstrated a shift towards higher risk constellations (fig. 3, panel A).
A first autologous HSCT was conducted in 452 patients (276 male, 176 female) between 1986 and 2012. Indications for autologous HSCT were HL/NHL (n = 150, 33%), PCD (n = 143, 32%), ST (n = 75, 17%), AML (n = 35, 8%), ALL (n = 20, 4%), autoimmune disorders (n = 13, 3%), MDS/MPN (n = 8, 2%), CML (n = 7, 2%) and BMF (1, 0.2%). Over the decades, the total number of autologous HSCTs grew (fig. 1, panel A). A significantly higher median age at transplantation nowadays was noted (53 years in the last decade vs 27 years in the first decade). Variations in indications towards lymphoid and plasma cell neoplasms and less solid tumours was identified (fig. 2, panel A). Patient characteristics are shown in table 2.
Overall survival and progression-free survival
The Kaplan-Meier estimate of OS at 5 years was significantly lower for allogeneic HSCT performed in the period between 1973 and 1982 (34% ± 5% standard error) than all other decades (1983–1992: 51% ± 4%; 1993–2002: 52% ± 3%; 2003‒2012; p <0.01). Differences in 5–year OS between the three decades from 1983 to 2012 were nonsignificant (fig. 3, panel B, p = 0.8). Five-year PFS was significantly lower for patients receiving allogeneic HSCT between 1973 and 1982 than at any later date (31% ± 5%; p = 0.01). Allogeneic HSCT did not differ significantly in 5–year PFS over the following decades (1983–1992: 46% ± 4%; 1993–2002: 43% ± 3%; 2003–2012: 44% ± 2%; p = 0.2). In a multivariate Cox model, a graft from a mismatched related or an unrelated donor was associated with a worse OS than a graft from an HLA-identical sibling. Cord blood as a stem cell source demonstrated a trend to inferior OS. Furthermore, an intermediate or advanced disease status had a detrimental effect on survival (table 3). A trend towards OS improvement over the decades after adjusting for c-factors is suggested from the multivariate analysis.
Five-year OS was significantly inferior for autologous HSCT initiated between 1983 and 1992 versus autologous HSCT at any later date (31% ± 8%; p <0.01). The difference in 5–year OS between 1993 and 2002 (55% ± 4%) and 2003–2012 (60% ± 4%) was not significant (fig. 4; p = 0.2). Similarly, 5–year PFS for autologous HSCT between 1983 and 1992 was significantly inferior to all other subsequent decades (1983–1992: 28% ± 9%; 1993–2002: 40% ± 4%; 2003–2012: 45% ± 4%, p <0.01).
In the multivariate analysis, Cox models demonstrated an inferior survival for AML as a transplant indication as well as a more advanced disease stage at the time of autologous HSCT.
Transplant-related mortality and relapse incidence
A trend to a decrease in allogeneic TMR over the decades could be noted (1973–1982: 43% ± 5%; 1983–1992: 27% ± 3%; 1993–2002: 27% ± 3%; 2003–2012: 22% ± 2%; p <0.01; fig. 3 panel C). In contrast, relapse incidences after allogeneic HSCT remained largely stable over time (1973–1982: 37% ± 6%; 1983–1992: 28% ± 3%; 1993–2002: 30% ± 3%; 2003–2012: 35% ± 2%; p = 0.3; fig. 3, panel D).
|
Table 3: Factors affecting overall survival in the Cox model. |
|
|
Hazard ratio
|
95% confidence interval
|
p-value
|
| Donor type: |
|
|
|
| Identical sibling
Other relative
Matched unrelated
Mismatched unrelated |
1.0
1.80
1.46
2.52 |
–
1.33–2.43
1.17–1.81
1.38–4.61 |
–
<0.001
0.001
0.003 |
| Stem cell source: |
|
|
|
| Bone marrow
Peripheral blood
Umbilical cord blood |
1
0.93
1.39 |
‒
0.70–1.22
0.74–2.65 |
‒
0.59
0.30 |
| Stage: |
|
|
|
| Early
Intermediate
Advanced |
1
1.81
2.31 |
–
1.43–2.28
1.89–2.83 |
–
<0.001
<0.001 |
| Age: |
|
|
|
| Under 40 yr
40 yr and above |
1
1.49 |
‒
1.22–1.83 |
‒
<0.001 |
| Transplant decade: |
|
|
|
| 1973–1982
1983–1992
1993–2002
2003–2012 |
1
0.58
0.49
0.41 |
–
0.43–0.78
0.35–0.68
0.27–0.60 |
–
<0.001
<0.001
<0.001 |
Discussion
Stem cell transplantation, a procedure aiming to cure an array of diseases affecting haematopoiesis, was first introduced in Basel in 1973 [12]. Further milestones in our centre were the first matched unrelated HSCT in 1978, the introduction of haploidentical HSCT in 1981, the beginnings of peripheral blood stem cell donation in 1991 and the first umbilical cord blood stem cell transplant in 2003. In this study, we examined the evolution of transplant modalities, patient and disease characteristics and their influence on transplant outcome during the time span between 1973 and 2012.
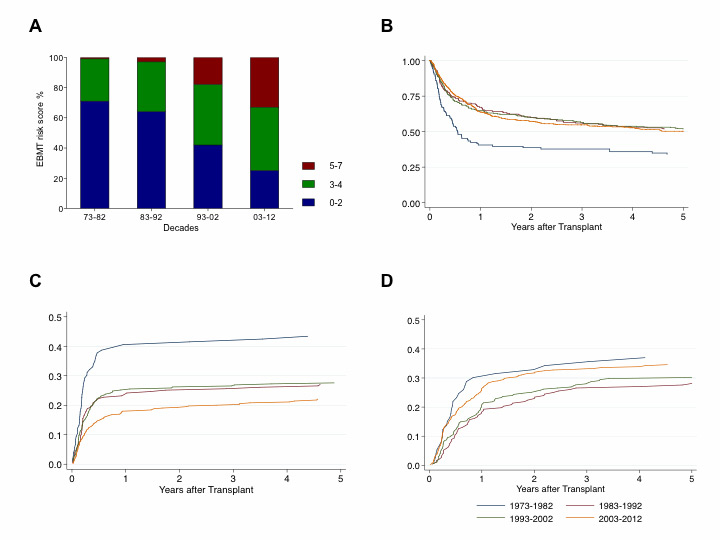
Figure 3
(A) European Group for Blood and Marrow Transplantation (EBMT) risk score distribution in % over the decades for allogeneic haematopoietic stem cell transplantation (HSCT). (B) Overall survival after allogeneic HSCT. (C) treatment-related mortality after allogeneic HSCT. (D) relapse incidence after allogeneic HSCT.
EBMT risk score: 0–2 (blue) = low; 3–4 (green) = intermediate, 5–7 (red) = high pretransplant risk constellation
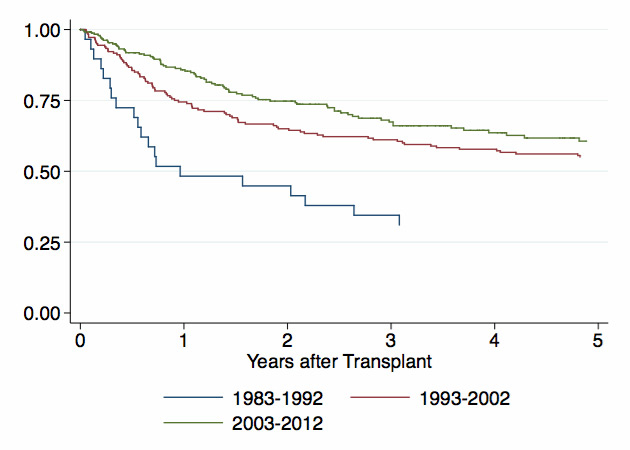
Figure 4
Overall survival after autologous haematopoietic stem cell transplantation (HSCT).
The total number of both autologous and allogeneic HSCTs increased significantly over time. The expansion of preconditions accepted for transplant is easily reflected in the significantly higher median patient age at autologous and allogeneic stem cell transplantation and higher EBMT risk scores observed in later decades. Allogeneic transplantation is today a treatment option for patients up to 70 years of age. The recognition that graft-versus-tumour effects contribute to disease control after transplantation led to the implementation of reduced-intensity and nonmyeloablative conditioning regimens, allowing transplant procedures to be carried out in elderly and comorbid patients without excess toxicity.
Major developments in medicine translated into variations in the diseases treated by HSCT in our centre. In the era of tyrosine kinase inhibitors, the group of patients needing allogeneic HSCT for CML has narrowed down to those in an accelerated phase, blast crisis, or with proof of treatment intolerance or resistance such as second-line treatment failure or carriage of the T315I mutation [14], reflected in declining numbers of patients transplanted for CML. Similarly, the addition of high-dose chemotherapy and autologous HSCT to adjuvant chemotherapy in breast cancer was shown to have no effect on survival and autologous transplantation for many solid tumours has largely been abandoned [15]. Accordingly, the numbers of patients receiving autologous HSCT for solid tumours has decreased in this survey.
HLA-matched sibling donors were the sole source of allogeneic stem cell grafts in the early 1970s. As only approximately 20%–25% of patients have an HLA-identical sibling, the majority of patients in need of HSCT could not proceed to transplantation because of the lack of a donor. Over the last two decades, registries of unrelated donors have recruited more than 20 million volunteer donors, and the safety of HSCT using “alternative donors” (i.e., haploidentical and cord blood grafts) has considerably advanced. Patients in need of an allogeneic transplant without a suitable donor are, therefore, are seen only occasionally nowadays. Similarly, the last four decades have seen a significant increase in unrelated donors and a trend to mismatched related donors. The primary stem cell source has shifted from bone marrow to peripheral blood and to a smaller degree umbilical cord blood.
In terms of allogeneic HSCT outcome, transplant-related mortality decreased dramatically between the first and second decade, with the reduction thereafter following a more decelerated pace, whereas relapse incidence remains unchanged over time. A significant improvement in 5–year OS and PFS between the first decade and second decade can be seen and at later dates to a lesser extent. The multivariate analysis of survival by decade suggests continued improvement by decade with a trend to decreased mortality risks when adjusted for factors such as donor choice and disease stage. Compatible with our observations, the trend to administer reduced-intensity conditioning prior to HSCT diminishes TRM, but further lessens disease control [16]. As in other forms of manual and intellectual work processes, a certain “learning curve” has been assumed in the context of HSCT [17, 18]. These factors may play a role in the higher survival benefit between the first and the later decades. The multitude of changes seen in the field of stem cell transplantation, such as variations in disease, patient and graft profile, as well as improved supportive measures and conditioning protocols over the decades, and their complex interplay, for instance, on the graft-versus-tumour-activity level, does not, however, permit us to point to a single most important factor explaining the improvement in survival parameters in multivariate analysis.
In conclusion, stem cell transplantation has seen a wide array of changes since its implementation in Basel. The patient group eligible for autologous and allogeneic HSCT in our centre has diversified towards more advanced patient ages and disease stages and to patients without a family donor, and this is reflected in a higher number of transplants performed over the years. Five-year overall and progression-free survival rose for allogeneic and autologous HSCT after the decade of their introduction. After adjustment for factors such as donor type and disease stage, survival improved in every decade. Further efforts in developing regimens that can accommodate the adverse circumstances of higher age, comorbidities and nonavailability of a sibling donor, while still providing better control over more advanced diseases, are warranted [10]. To this end, specific strategies are being applied at the time of transplant, such as reduction in regimen toxicity [19], elaboration of graft-versus-host- disease prophylaxis [20] and better prevention of post-transplant relapse [21].
Acknowledgement: The authors wish to express their gratitude to Mrs. Hanneke Schep and Mrs. Helen Baldomero for their invaluable help in data support and coordination.
References
1 Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26.
2 Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012;47(7):906–23.
3 Thomas ED and Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 1999;5(6):341–6.
4 Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347(8998):353–7.
5 Ballen KK, Koreth J, Chen YB, Dey BR, and Spitzer TR. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119(9):1972–80.
6 Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–83.
7 Pavletic SZ and Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Am Soc Hematol Educ Program. 2012:251–64.
8 Marr KA. Delayed opportunistic infections in hematopoietic stem cell transplantation patients: a surmountable challenge. Am Soc Hematol Educ Program. 2012:265–70.
9 Halter J, Passweg JR, and Hausermann P. Future trends in hematopoietic stem cell transplantation. Curr Probl Dermatol. 2012;43:165–70.
10 Gyurkocza B, Rezvani A, and Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–99.
11 WBMT, 1 millionth blood stem cell transplant marks major medical milestone. 2013.
12 Buckner CD. Remembering Bruno Speck. Acta Haematologica. 2000;103:5–6.
13 Gratwohl A. The EBMT risk score. Bone Marrow Transplant. 2012;47(6):749–56.
14 Jain N. and van Besien K. Chronic myelogenous leukemia: role of stem cell transplant in the imatinib era. Hematol Oncol Clin North Am. 2011;25(5):1025–48.
15 Tallman MS, Gray R, Robert NJ, LeMaistre CF, Osborne CK, Vaughan WP, et al. Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med. 2003;349(1):17–26.
16 Blaise D and Castagna L. Do different conditioning regimens really make a difference? Am Soc Hematol Educ Program. 2012;237–45.
17 Passweg JR, Baldomero H, Stern M, Bargetzi M, Ghielmini M, Leibundgut K, et al. Hematopoietic stem cell transplantation in Switzerland: a comprehensive quality control report on centre effect. Swiss Med Wkly. 2010;140(23–24):326–34.
18 Loberiza FR, Zhang MJ, Lee SJ, Klein JP, LeMaistre CF, Serna DS, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105(7):2979–87.
19 O'Meara A, Halter J, Heim D, Gerull S, Bucher C, Passweg JR, et al. Allogeneic stem cell transplantation for relapsed or refractory lymphoma after conditioning with BEAM/fludarabine/TBI. Biol Blood Marrow Transplant. 2013;19(1):82–6.
20 Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64.
21 Alyea EP, DeAngelo DJ, Moldrem J, Pagel JM, Przepiorka D, Sadelin M, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(8):1037–69.



