Figure 1
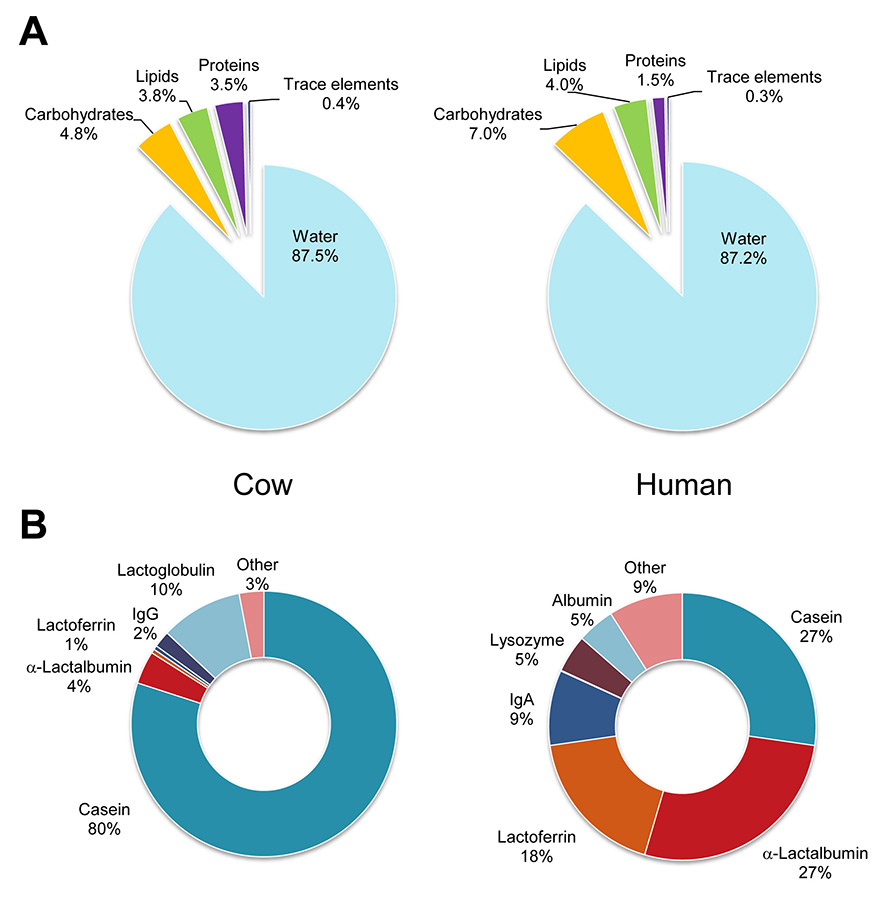
Composition of breast milk and cow’s milk. (A) General molecular classes given in percent of total mass. (B) Percentage of main types of milk proteins.
DOI: https://doi.org/10.4414/smw.2014.13927
Got breast milk? If so, was it worth it? While the first question paraphrases the tagline of a successful advertisement campaign for milk, the second one is more pertinent to the debated benefits of breast milk. The protective effect of breast milk was first recognised at the beginning of the 20th century after studies recorded that infantile mortality was seven times higher in formula-fed babies when compared with breast-fed babies [1]. Nowadays, despite the tremendous progress achieved in paediatric medicine, formula-fed infants still have a five- to ten-fold increased risk compared with breast-fed infants of developing necrotising enterocolitis [2], which affects about 5% of premature infants with a birth weight below 1,500 g [3].

Figure 1
Composition of breast milk and cow’s milk. (A) General molecular classes given in percent of total mass. (B) Percentage of main types of milk proteins.

Figure 2
Milk oligosaccharide biosynthesis. Oligosaccharides are represented by the symbolic nomenclature of the Functional Consortium for Glycomics (see www.functionalglycomics.org). The disaccharide lactose (in orange frame) is produced by the β1–4 galactosyltransferase / α-lactalbumin complex. Lactose is further modified by elongation with galactose(β1–4)N-acetylglucosamine disaccharides and addition of fucose and sialic acid.
2FL = fucosyl(α1,2)lactose; 3FL = fucosyl(α1,3)lactose; 3SL = sialyl(α2,3)lactose; 6SL = sialyl(α2,6)lactose

Figure 3
Relative amount of two complex milk oligosaccharides during lactation. The relative levels of the pentasaccharide lacto-N-fucopentaose drop rapidly in the first week of lactation, whereas the relative levels of the heptasaccharide lacto-N-fucoseptaose raise in the first 2 weeks of lactation. Oligosaccharide levels are compared with the constant levels of the milk tetrasaccharide lacto-N-tetraose. The oligosaccharide levels shown were analysed in breast milk samples collected from a single mother over 3 weeks after childbirth.

Figure 4
Structure of 3–sialyllactose and 6–sialyllactose. The three-dimensional structures point to the extended configuration of 3–sialyllactose and bent configuration of 6–sialyllactose. The positions of the third and sixth C-atoms of galactose (Gal), to which sialic acid (Sia) is attached are marked with the numbers 3 for 3–sialyllactose and 6 for 6–sialyllactose.
The composition of breast milk has been extensively investigated, thereby unravelling a tremendous structural complexity; yet the mechanisms underlying the protective effects of breast milk are still not fully elucidated. When considering the main classes of molecules, breast milk is somewhat similar to cow’s milk (fig. 1A). A closer examination, however, shows profound differences that account for the distinct digestive properties and protective functions of both fluids. For example, casein represents 80% of the protein in cow’s milk, and is acid insoluble and difficult to process by the infant digestive system [4]. In contrast, breast milk is rich in protective proteins such as immunoglobulin IgA, and the antimicrobial proteins lysozyme and lactoferrin (fig. 1B). The carbohydrate fraction also dramatically differs between breast milk and cow’s milk. Whereas cow’s milk contains about 40 g/l of lactose and only traces of larger oligosaccharides, breast milk contains 50–70 g/l of lactose plus 5–10 g/l of complex oligosaccharides [5], with oligosaccharide concentrations reaching up to 20 g/l in colostrum [6]. Approximately 200 molecular species of milk oligosaccharides have been identified, based on the extension of lactose [7], which is a disaccharide combining galactose (Gal) and glucose (Glc) and represented as Gal(β1–4)Glc. Multiple glycosyltransferase enzymes expressed in the lactating mammary gland combine the carbohydrates Gal, Glc, fucose (Fuc), sialic acid (Sia), and N-acetylglucosamine (GlcNAc) into linear and branched oligosaccharide structures (fig. 2). The same glycosyltransferase enzymes are also expressed in most cell types, where they contribute to the biosynthesis of glycolipids and glycoproteins [8]. Accordingly, milk oligosaccharides carry epitopes found also in other bodily compartments such as blood and the intestinal mucosa [9].
The structural diversity of milk oligosaccharides varies from mother to mother and even varies during lactation for each mother. The individual variability in glycosyltransferase expression affects both the quality and quantity of milk oligosaccharides. For example, fucosyltransferase genes determining blood group factors such as Lewis antigens and secretor status will also affect the presence of the corresponding fucosylated epitopes on milk oligosaccharides (fig. 2) [10]. Colostrum and early milk are richest in oligosaccharides, but the production of oligosaccharides is maintained throughout lactation [11]. The structural analysis of milk oligosaccharides across the first weeks of lactation showed that short structures such as tri- to pentasaccharides are mainly produced in the first weeks after parturition, whereas the amount of longer structures increases over time (fig. 3). In contrast to other milk constituents like fatty acids, milk oligosaccharides do not depend on the nutritional status of the lactating mother. Rather, the changes in milk oligosaccharide composition are controlled by genetic mechanisms [12].
Milk oligosaccharides have no nutritional value for the breastfed infant and these molecules tolerate the acidic conditions of the upper gastrointestinal tract. In addition, they are not cleaved in the intestine owing to the lack of locally-expressed glycosidases [13]. Only a minor fraction of milk oligosaccharides is absorbed and transferred to the bloodstream, on the basis of the recovery of approximately 1% of ingested milk oligosaccharides in the urine of suckling infants [14]. Milk oligosaccharides exert important prebiotic functions by promoting the colonisation of the intestine by commensal microbiota. Recognition of the role of milk oligosaccharides in shaping intestinal microbiota dates back to the work of Schönfeld [15], who showed that the previously described bifidus factor stimulating the growth of bifidobacteria was in fact a carbohydrate fraction specifically found in breast milk known as gynolactose [16]. Gynolactose was later identified to be a mixture of fucosylated tri- to penta-saccharides (fig. 2). Several families of bacteria such as Bifidobacteriaceae and Bacteroidaceae express glycosidase enzymes that are able to cleave milk oligosaccharides and use the resulting monosaccharides as a carbon source [17]. These released monosaccharides can also be assimilated by bacteria lacking dedicated glycosidases, as in the case of Enterobacteriaceae, thereby complicating the detection of associations between specific milk oligosaccharides and intestinal microbiota [18].
Through their structural similarity to cellular glycans, milk oligosaccharides also act as soluble receptors for several bacteria and viruses. In 1956, the biochemists Kuhn and Brossmer attributed the inhibitory effect of breast milk on influenza virus to sialylated milk oligosaccharides, which mimicked the cellular receptors for that virus [19]. Fucosylated milk oligosaccharides also bind to several strains of norovirus, thereby preventing the docking of the virus to cellular glycan receptors [20] and hence the development of acute gastroenteritis. The neutralising effect of milk oligosaccharides is not limited to viruses. Milk oligosaccharides also inhibit the adhesion of multiple bacteria such as Campylobacter jejuni [21], Listeria monocytogenes [22] and Streptococcus pneumonia [23] to the intestinal epithelium. Furthermore, milk oligosaccharides protect against bacterial enterotoxins such as cholera toxin [24] by competing with cell-bound glycan receptors.
Soluble oligosaccharides and cell-surface bound glycans also interact with leucocytes, which express carbohydrate-binding proteins called lectins. These lectins function in the context of the innate immune system by recognising foreign carbohydrate antigens, but they also regulate leucocyte activation through the binding of specific cell-surface glycans. The recognition of mycobacterial lipoarabinomannan by the dendritic cell lectin DC-SIGN is an example of an interaction with microbial carbohydrates [25]. The binding of the B-cell lectin CD22 to α2,6–linked Sia further illustrates the importance of endogenous glycans in regulating B-cell activation [26]. The oligosaccharide lacto-N-fucopentaose III has been shown to induce the proliferation of splenic B-cells and to stimulate the production of interleukin-10 (IL-10) when added directly to splenic cells in vitro [27]. The question as to whether milk oligosaccharides interact with immune cells in the intestinal lumen or in the bloodstream after absorption remains open for debate. The detection of small amounts of milk oligosaccharides in the urine of breastfed infants [14] suggests that these molecules can indeed interact with circulating leucocytes, but this assumption has yet to be proven.
Considering the tremendous structural complexity of human milk oligosaccharides, the main challenge that exists is in identifying the specific functions of individual milk oligosaccharides. The multiple potential effects of milk oligosaccharides on the intestinal microbiota and on the mucosal immune system highlight the requirement for performing in-vivo investigations. Rodents such as the mouse lack the oligosaccharide diversity of human milk [28, 29], but the availability of mouse knockout models for the genes involved in milk oligosaccharide biosynthesis enables the systematic analysis of these oligosaccharides. Accordingly, our research group has applied sialyltransferase-knockout mice to investigate the biological functions of the major milk oligosaccharides Sia(α2,3)lactose (3SL) and Sia(α2,6)lactose (6SL). The trisaccharides 3SL and 6SL are produced by α2,3 sialyltransferase and α2,6 sialyltransferase, respectively, which transfer Sia to lactose through distinct linkages yielding different conformations (fig. 4). The impact of the exposure to these oligosaccharides during lactation was addressed by feeding newborn mice with either normal milk or milk deficient in the oligosaccharides of interest. Interestingly, we found that mice fed on milk lacking the oligosaccharide 3SL were more resistant as adults to a form of chemically-induced colitis [29]. In contrast, the exposure to the structurally similar oligosaccharide 6SL did not affect the development of colitis in the applied model. The presence of 3SL in the maternal milk influenced durably the composition of intestinal microbiota in the nursed mice. For example, clostridial cluster IV bacteria of genus Ruminococcus were found only in mice exposed to 3SL during lactation. The influence of intestinal microbiota on the course of chemically induced colitis was confirmed by reconstitution of germ-free mice with the intestinal flora isolated from mice previously fed either 3SL-containing milk, or 3SL-deficient milk. Mice harbouring microbiota including increased clostridial cluster IV bacteria developed a more severe colitis than mice harbouring microbiota lacking clostridial cluster IV bacteria [29]. These experiments demonstrated that the exposure to specific milk oligosaccharides during lactation affects the composition of the intestinal flora and maintains a durable effect on the susceptibility to intestinal inflammation. The question remains as to why a naturally occurring milk oligosaccharide such as 3SL would worsen an inflammatory response. This would indicate that some milk oligosaccharides mediate proinflammatory functions, which is definitively counterintuitive. A possible explanation for this paradoxical situation emerged from our study of 3SL and 6SL in the context of the innate immune system.
When supplementing mice orally with the milk oligosaccharide 3SL in the course of our study, we observed increased activation of intestinal CD11c+ dendritic cells [30]. Dendritic cells are at the forefront of the immune system. They sense the environment for antigens and trigger both innate and adaptive immune responses. Dendritic cells express a broad range of pattern-recognition receptors such as toll-like receptors (TLR) and lectins such as DC-SIGN and sialic acid-binding immunoglobulin-type lectin-E (siglec-E). In addition to presenting antigens to T-cells, dendritic cells polarise adaptive responses through the production of the tolerogenic cytokines IL-10 and transforming growth factor-beta (TGFβ), or the proinflammatory cytokines IL-6, IL-12, and tumour necrosis factor-alpha (TNFα). Dendritic cells are known to recognise foreign bacterial carbohydrate structures such as peptidoglycan and lipopolysaccharide (LPS). In contrast, a stimulatory effect of milk oligosaccharides on dendritic cells had not been reported before. We did find that the stimulatory effect of 3SL on CD11c+ dendritic cells was dose-dependent and specific, since the structurally related oligosaccharide 6SL had no effect. The stimulation of CD11c+ dendritic cells by 3SL resulted in increased expression of the activation markers CD80, CD86 and major histocompatibility complex-II (MHC-II). The activation of intestinal dendritic cells by 3SL was observed both by directly stimulating cells in vitro and in vivo after oral supplementation of mice. The 3SL-mediated activation was abrogated when testing dendritic cells lacking the adaptor protein MyD88, which is essential for the transduction of most TLR signals. Closer examination of the TLR pathway revealed that 3SL recognition mainly involved TLR4 [30], which is also required for LPS recognition [31].
The rationale for the proinflammatory effect of the milk oligosaccharide 3SL may be related to the presence of α2,3–linked Sia at the surface of several pathogenic bacteria, such as Campylobacter jejuni, Neisseria meningitides and Haemophilus influenzae. For example, TLR4 is required on dendritic cells for the recognition of Campylobacter jejuni α2,3–sialylated lipo-oligosaccharides [32]. Accordingly, the exposure of intestinal dendritic cells to 3SL during lactation may result in a priming of the infant innate immunity, which contributes to the defence against α2,3Sia-expressing bacteria. Whereas 3SL mediates proinflammatory signals via TLR4, other milk oligosaccharides may interact with different carbohydrate-binding proteins and possibly trigger suppressive signals. The siglec family, for instance, dampens leucocyte activation upon binding to sialylated ligands [26]. The exact effect of milk oligosaccharides on immune cells can only be elucidated once pure oligosaccharides become available in large amounts. The technical complexity and considerable expenses linked to the chemical synthesis of oligosaccharides have precluded the biological study of these compounds to date. Fortunately, ingenious innovations in carbohydrate chemistry now enable the large scale production of oligosaccharides [33] and thereby their investigation in vivo.
Whereas mouse models will further contribute to the functional characterisation of milk oligosaccharides, the future definitively lies in performing human studies. Milk oligosaccharides are natural products and devoid of any toxicity, meaning that they can be tested as food additives. Supplementation studies performed to date with unnatural carbohydrates such as galacto-oligosaccharides and fructo-oligosaccharide have displayed no adverse effects [34], thus confirming the safety of oligosaccharides. The supplementation of infant formula with naturally-occurring milk oligosaccharides will certainly represent a main field of development, but milk oligosaccharides should also be considered for applications in adults. Regimens including milk oligosaccharides could contribute to the restoration of a normal intestinal flora after chemotherapy and extended antibiotic treatment. Furthermore, recent studies have shown that intestinal microbiota influence the development of obesity [35], atherosclerosis [36] and diabetes [37], among other metabolic disorders. Along this line, it will be interesting to address whether milk oligosaccharides may be applied to influence intestinal microbiota durably in adults and thereby delay or even prevent disease development.
Got breast milk? The recent discoveries regarding the complex interactions of milk oligosaccharides with intestinal microbiota and the mucosal immune system have demonstrated that breast milk provides much more than just nutrients to infants. Breast milk is definitively the gold standard for infant nutrition.
1 Stevens EE, Patrick TE, Pickler R. A History of Infant Feeding. J Perinat Educ. 2009;18:32–9.
2 Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23.
3 Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506.
4 Ziegler EE. Adverse effects of cow's milk in infants. Nestle Nutr Workshop Ser Pediatr Program 2007;60:185–96; discussion 196–89.
5 Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722.
6 Viverge D, Grimmonprez L, Cassanas G, Bardet L, Solere M. Variations in oligosaccharides and lactose in human milk during the first week of lactation. J Pediatr Gastroenterol Nutr. 1990;11:361–4.
7 Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80.
8 Kleene R, Berger EG. The molecular biology of glycosyltransferases. BiochimBiophysActa. 1993;1154:283–325.
9 Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoen N, et al. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–73.
10 Le Pendu J. Histo-blood group antigen and human milk oligosaccharides: genetic polymorphism and risk of infectious diseases. Adv Exp Med Biol. 2004;554:135–43.
11 Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, et al. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88:89–94.
12 Erney R, Hilty M, Pickering L, Ruiz-Palacios G, Prieto P. Human milk oligosaccharides: a novel method provides insight into human genetics. Adv Exp Med Biol. 2001;501:285–97.
13 Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2002;71:1589–96.
14 Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo (1)(3)C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2012;107:957–63.
15 Schönfeld H. Über die Beziehungen der einzelnen Bestandteile der Frauenmilch zur Bifidusflora. Jahrbuch der Kinderh. 1926;113:19–60. German.
16 Polonowski M, Lespagnol A. Sur deux nouveaux sucres du lait de femme, le gynolactose et l’allolactose. C R Acad Sci 1931;192. French.
17 El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11: 497–504.
18 Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–8.
19 Kuhn R, Brossmer R. Über die O-Acetyl-lactamin-säure-lactose aus Kuh Colostrum und ihre Spaltbarkeit durch Influenza-Virus. ChemBer. 1956;98:2013–35. German.
20 Jiang X, Huang P, Zhong W, Tan M, Farkas T, et al. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J Infect Dis. 2004;190:1850–9.
21 Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–120.
22 Coppa GV, Bruni S, Zampini L, Galeazzi T, Facinelli B, et al. Oligosaccharides of human milk inhibit the adhesion of Listeria monocytogenes to Caco-2 cells. Ital J Pediatr. 2003;29:61–8. Italian.
23 Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153:232–7.
24 Otnaess AB, Laegreid A, Ertresvag K. Inhibition of enterotoxin from Escherichia coliand Vibrio cholerae by gangliosides from human milk. Infect Immun. 1983;40:563–9.
25 Maeda N, Nigou J, Herrmann JL, Jackson M, Amara A, et al. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–6.
26 Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007;7:255–66.
27 Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 1994;91:18–22.
28 Prieto PA, Mukerji P, Kelder B, Erney R, Gonzalez D, et al. Remodeling of mouse milk glycoconjugates by transgenic expression of a human glycosyltransferase. J Biol Chem. 1995;270:29515–9.
29 Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–54.
30 Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci U S A. 2013;110:17444–9.
31 Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–6.
32 Kuijf ML, Samsom JN, van Rijs W, Bax M, Huizinga R, et al. TLR4–mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J Immunol. 2010;185:748–55.
33 Agoston K, Kroger L, Dekany G, Thiem J. Solid-phase random glycosylation. J Comb Chem. 2009;11:813–9.
34 Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr. 2009;34:291–5.
35 Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
36 Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85.
37 Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, et al. 8) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113.
Funding / potential competing interests: This work was supported by the Zürich Centre for Integrative Human Physiology and by the Swiss National Foundation. T.H. serves as consultant for Glycom A/S.