Figure 1
Repartition of primary tumours by organ in WS and ES.
WS = Western Switzerland; ES = Eastern Switzerland.
DOI: https://doi.org/10.4414/smw.2014.13924
Gastro-entero-pancreatic (GEP) neuroendocrine neoplasms (NENs) are rare tumours derived from the diffuse neuroendocrine system of the gastro-intestinal tract and pancreas. Their prevalence can be estimated at 35/100’000 inhabitants and their incidence is approximately of 5 cases / 100’000 per year [1]. These figures have been increasing steadily over the last three decades, at least partially because of improvements in diagnostic procedures and better awareness among physicians [1–3].
GEP-NENs are very heterogeneous in their presentation, symptomatology and behaviour. They are mainly isolated and sporadic, but can also be multiple, especially when associated with a genetic syndrome such as type 1 multiple endocrine neoplasia (MEN-1) or von Hippel-Lindau (VHL) syndrome [3, 4]. The majority of GEP-NENs are non-functioning, mostly well-differentiated and slowly growing tumours. A subset of GEP-NENs can however produce biologically active substances such as amines or peptides that are associated with distinct clinical entities like the carcinoid syndrome, characterised by flushing and secretory diarrhoea in the case of serotonin hyper secretion. Aside from these functioning tumours, the vast majority of GEP-NENs have a relatively indolent clinical course, which can at least partially explain their delayed diagnosis [3, 4]. They are thus often discovered incidentally or when the disease is either locally advanced or widespread, and they are often metastatic when becoming symptomatic [2].
The prognosis of GEP-NENs depends on a variety of factors that include tumour localisation and size, its grading and its histopathological characteristics [1, 5]. For example, the 5-year survival for pancreatic neuroendocrine tumours varies from 30%, for non-functioning and clinically silent tumours, to 97% for benign insulinomas [3]. All these characteristics of GEP-NENs make their diagnostic and therapeutic management complex. As GEP-NENs are very heterogeneous, a universally accepted classification system will undoubtedly be of utmost importance to help both predict their biological behaviour and guide their treatment. However, the co-existence of various classification systems has generated much confusion over the years, because of the existence of discrepancies in terminology between different classifications [6]. As a result, similar tumours could be classified differently, notably with respect to their site of origin. Finally, their rarity tends to limit the evidence available in the literature.
The need for universal standards of care for patients with NENs has emerged over the last decade, and this is particularly well highlighted by recommendations from the European Neuroendocrine Tumour Society (ENETS) [7]. In the context of a rapidly evolving field, the management of patients diagnosed with NENs remains scarcely studied. In December 2005, the SwissNET society was created, regrouping all the specialists involved in the management of these patients. The main goal of this society was to establish a national registry on neuroendocrine neoplasms arising from the gastroentero-pancreatic tract and the lungs, with the purpose of elucidating the epidemiological settings of NENs in Switzerland, and unifying their management in Swiss centres.
The objectives of this work were: to describe the epidemiological and clinical characteristics of patients diagnosed with NENs and followed in the two academic centres of western Switzerland (WS), and to compare these characteristics with those of patients followed in academic centres of eastern Switzerland (ES); to compare the diagnostic and therapeutic approaches adopted in western Switzerland centres to those adopted in eastern Switzerland, and to evaluate these data with internationally accepted guidelines published by the ENETS in 2012 [7].
All the data on NEN patients originated from the SwissNET registry, a nation-wide prospective database that started prospective recruitment, on January 1st, 2008, of all the patients diagnosed with a neuroendocrine neoplasm in Switzerland. Ethical approval for SwissNet was granted by the Swiss Federal Office for Health, through its special commission “Eidgenossische Expertenkommission fur das Berufsgeheimnis in der Medizinischen Forschung”. This approval covers all centres involved in the recruitment of patients throughout Switzerland. This recruitment is now based on the individual initial histopathological report used for diagnosis, but was initially based upon functional imaging properties of tumours. An informed consent form is then sent to the patients, via the physician in charge, asking their permission to access their medical records and other sources of information concerning their NEN, for research purposes only. All patients who give their written informed consent are anonymously included in the register.
When the SwissNET database was started in 2008, GEP-NENs were initially categorised according to the WHO 2000 classification. After the 2010 revision of WHO criteria, revised criteria gradually became applied in all participating centres. To try and normalise the diagnoses for all patients, the following links were used to convert the initial classification of early patients to the newly applied system: well-differentiated neuroendocrine tumours (WDET) became identified as NET G1, well-differentiated neuroendocrine carcinomas (WDEC) became identified as NET G2, and poorly differentiated neuroendocrine carcinomas (PDEC) became identified as NEC G3.
The present study covered the period from January 1st, 2008 to January 31st, 2013. In total, 522 patients were included in total, consisting of 99 consecutive patients with a median follow-up of 242 days for WS, and 423 consecutives patients with a median follow-up of 359 days for ES. The following data were collected:
– Clinical and epidemiologic information: age, gender, origin and type of tumour, functionality of tumour, incidentally found tumour and onset of symptoms
– Information on diagnostic procedures: laboratory (measurements of serum CgA and NSE) and imagery (all modality Scintigraphy, PET/CT, MRI, CT, US) workups; and histopathological report (main diagnosis, immunohistochemistry)
– Data on treatment: first line therapy
– Information on outcome: remission status of each patient
Comparisons between WS and ES were performed with the STATA® software. For participant characteristics, means and proportions of variables were calculated. Results were expressed as either mean ± SD for normally distributed data, or median and IQR for skewed data. Characteristics were compared using Student’s t test for means and chi-square test for proportions. Statistical analyses were performed using Stata 11 (Stata Corp., College Station, Texas 77845, USA). Statistical significance was set at p = 0.05. Multiple testing was not taken into account, since this is an exploratory study. NS stands for not significant.
At the time of the present study, Swiss NEN patients had a mean age at initial diagnosis of 59.0 ± 15.7 years, with a slight male preponderance (285/522, 55% men). Only 88/522 (16.8%) of NENs were functioning (presence of a distinct clinical syndrome, table 1) and the median time elapsed since symptoms onset and diagnosis was 1.0 year with an IQR of 1.0 to 3.0 years. As the SwissNET database is relatively recent, the median time of follow-up for these patients was 318 days, ranging from 59 up to 832. There was no statistically significant difference for these parameters between WS and ES, except for incidentally found NENs representing 36/99 (36%) of all cases in WS, and only 15/423 (4%) in ES (p <0.001).

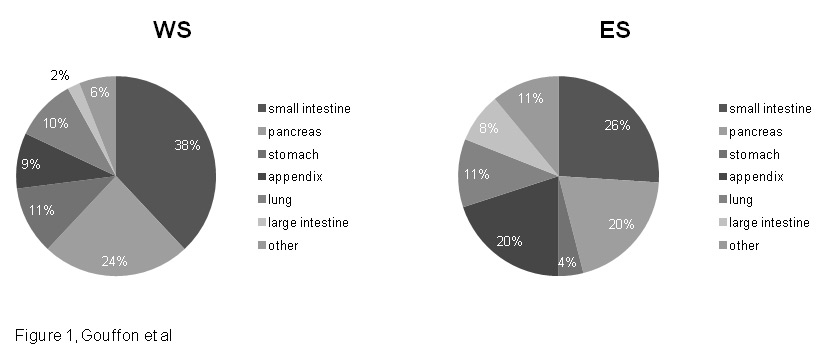
Figure 1
Repartition of primary tumours by organ in WS and ES.
WS = Western Switzerland; ES = Eastern Switzerland.
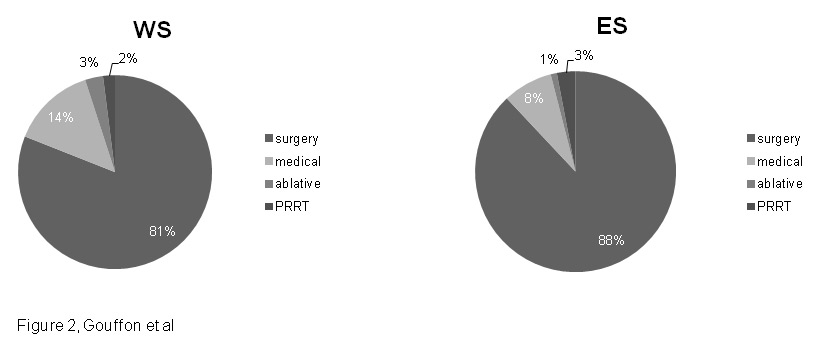
Figure 2
First line therapy in WS and ES.
WS = Western Switzerland; ES = Eastern Switzerland.
Organ repartition of NENs is displayed in (fig. 1): small intestine NENs were the predominant type, representing 38/99 (38%) of cases in WS and 111/423 (26%) of cases in ES (p = 0.019). Pancreatic NENs came in second position with 24/99 (24%) of cases in WS and 86/423 (20%) of cases in ES (p = 0.412) Then in WS, the third tumour type was gastric NENs with 11/99 (11%) of cases, whereas in ES the third most prevalent tumour type was appendiceal NENs with 84/423 (20%) of cases.
Among the 522 NENs identified in the SwissNET database, 517/522 (99%) were documented by a histopathological report, with a distribution between WS and ES of 96/99 (97%) and 421/423 (100%) respectively. The five remaining patients were diagnosed by clinical presentation and/or imaging studies. Of all patients, only 364 were diagnosed according to the WHO 2000 classification (61/96 in ES; 303/421 in WS) [8]. Using these criteria, we found 30/61 (49%) were well-differentiated neuroendocrine tumours (WDET) in WS, compared to 185/303 (61%) in ES (p = 0.046). In contrast, there were 28/61 (46%) of well-differentiated neuroendocrine carcinomas (WDEC) in WS compared to 72/303 (24%) in ES (p = 0.002). Poorly differentiated neuroendocrine carcinomas (PDEC) were the least prevalent in all regions, with 3/61 (5%) in WS and 46/303 (15%) in ES (p = 0.026). These results indicate a predominance of well-differentiated neuroendocrine carcinomas in WS, while in ES well-differentiated neuroendocrine tumours and poorly differentiated neuroendocrine carcinomas are more common.
When the 2010 WHO classification was published [9], Swiss pathologists started to use that classification as well. When available in the pathological reports, the classification became part of the data collected. Interestingly, differences described above in the classification of tumours between ES and WS disappeared when using the WHO 2010 classification (n = 389 total; WS n = 57; ES n = 332). G1 NETs were the most prevalent in both WS and ES, accounting for 36/57 (63%) and 224/332 (68%) of all cases, respectively (p = 0.14). G2 NETs followed with a rate of 17/57 (30%) in WS and 64/332 (19%) in ES (NS p = 0.182), and finally G3 NECs with a rate of 4/57 (7%) and 44/332 (13%) for WS and ES respectively (NS p = 0.146).
Chromogranin A (CgA) and synaptophysin are the two main markers used for immunohistochemical diagnosis of neuroendocrine tumours. In our series, CgA expression was initially checked in 365 samples, and was positive in 341/365 (93%) cases. Synaptophysin expression was checked in 369 samples and was positive in 358/369 (97%) cases. There was no difference in the repartition between ES and WS (table 2).
| Table 1: Repartition of functional NENs according to type and geographical region. | |||
| Name / Type | All regions N = 522 | WS N = 99 | ES N = 423 |
| Carcinoid syndrome | 49 (9.4%) | 8 (8%) | 41 (9.7%) |
| Insulinoma | 20 (3.8%) | 5 (5%) | 15 (3.5%) |
| Gastrinoma | 11 (2.1%) | 7 (7%) | 4 (0.9%) |
| Glucagonoma | 5 (1.0%) | 0 (0%) | 5 (1.2%) |
| Somatostatinoma | 2 (0.4%) | 1 (1%) | 1 (0.2%) |
| VIPoma | 1 (0.2%) | 0 (0%) | 1 (0.2%) |
| WS = Western Switzerland; ES = Eastern Switzerland. | |||
| Table 2: Immunostaining. Number of NEN histologic samples that have been stained immunologically for chromogranin A or synaptophysin for diagnostic purposes. | |||
| Marker | WS | ES | p value |
| Chromogranin A (N = 365) | N = 72 | N = 293 | |
| 66/72 positive (92%) | 275/293 positive (94%) | p = 0.872 | |
| Synaptophysin (N = 369) | N = 68 | N = 301 | |
| 66/68 positive (97%) | 292/301 positive (97%) | p = 1 | |
| WS = Western Switzerland; ES = Eastern Switzerland. | |||
We next examined how Swiss centres used the traditional somatostatin receptor scintigraphy (SRS) and the recently introduced 68Ga-DOTATOC PET in the diagnosis and management of NEN patients. Functional imaging studies include the following modalities: 111In-pentetreotide scintigraphy, 68Ga-DOTATOC PET, 18FDG-PET, 111In-DOTA-exendine-4 scintigraphy and bone scintigraphy. Overall, and when taking into account procedures performed both in the initial diagnosis and during follow-up of patients, imaging studies were ordered 139 times in WS, and 374 times in ES.
111In-pentetreotide SRS was performed 381 times in total, with a distribution of 111 exams in ES and 270 exams in WS (80% vs 72%, p = 0.08). In contrast, 20 68Ga-DOTATOC PET were performed in ES, while none were ordered in WS (where this radiopharmaceutical is not available yet).18FDG-PET was performed 85 times in total, 24 in ES and 61 in WS (p = 0.79), whereas 111In-DOTA-exendine-4 was performed only 8 times in one centre (research protocol). Finally, bone scintigraphy was obtained 4 times in all.
The total number of serum chromogranin A (CgA) and neuron-specific enolase (NSE) measurements obtained in all patients was 625 and 145, respectively. CgA was measured 63 times in WS versus 562 times in ES (p <0.001). On the other hand, NSE was measured 93 times in WS versus 52 times in ES (p <0.001).
With respect to CgA measurements, 26/99 (26%) of WS patients had its level assessed at diagnosis, and 38/99 (38%) of them had at least one assay performed during the course of the disease. In contrast, 172/423 (41%) of ES patients had CgA measured at diagnosis (p= 0.008 vs WS), and 189/423 (45%) of them had at least one measurement obtained during the course of the disease (no difference between regions, p = 0.263).
Regarding NSE measurements, 29/99 (29%) of WS patients had its level obtained at diagnosis, and 36/99 (36%) of them had at least one assay performed during the course of the disease. In sharp contrast, only 21/423 (5%) of ES patients had NSE measured at diagnosis (p <0.001 vs WS), and only 32/423 (8%) had at least one measurement obtained during the course of the disease (p <0.001 vs WS). These data are summarised in table 3.
Our data on first line therapy covered surgery as well as non-surgical ablative therapy, medical therapy, and peptide radionuclide receptor therapy (PRRT). Unfortunately, these data were only available for 467 patients in all, 85/99 from WS and 382/423 for ES (fig. 2).
Surgery was the main first line treatment for both WS and ES (p = 0.075), with 69/85 (81%) of patients from WS and 338/382 (88%) of patients from ES benefitting from this approach. Medical treatment came in second position, with 12/85 (14%) of WS patients and 30/382 (8%) of ES patients treated medically as first line therapy (p = 0.091). PRRT was performed twice in WS and 12 times in ES. Finally, non-surgical ablative therapies were performed 4 times in total. No statistically significant difference was found between WS and ES regarding first line therapy.
The different medical therapies included various somatostatin analogs (SSAs): octreotide LAR (Long Acting Release), octreotide sc, lanreotide LAR, lanreotide autogel and pasireotide LAR. In addition, a small number of patients benefitted from everolimus or sunitinib. Taken together, these various drugs were prescribed 103 times in 42 different patients. Octreotide LAR was the drug most commonly used, reaching a rate of 10/21 (48%) of all treatments prescribed in WS and 41/82 (50%) in ES (p = 1). Then came octreotide sc, with a rate of 5/21 (24%) in WS and 18/82 (22%) in ES (p = 1). Finally, everolimus represented 4/21 (19%) of all medical therapies in ES and 12/82 (15%) in WS (p = 0.736).
For the follow up data, remission status of the patient was documented in the database as complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD) or death. We could thus extract the last recorded remission status for each patient in the SwissNET database. Of the 522 patients, 348 have a known remission status, with a distribution of 63 for WS and 285 for ES (table 4).
In both regions, CR is preponderant with a rate of 29/63 (46%) in WS and 179/285 (63%) in ES. Interestingly, these figures are statistically significantly different between ES and WS (p = 0.017). For patients who did not achieve complete remission, status was different between both regions. In WS, 17/63 (27%) of these patients had stable disease, compared to 28/285 (10%) in ES (p = 0.002), whereas in ES, 60/285 (21%) of these patients were deceased, compared to 8/63 (13%) in WS (p = 0.134).
| Table 3: Laboratory workup. Number of patients who had either chromogranin A or neuron-specific enolase (NSE) levels measured at diagnosis, or at least one time during the course of the disease. | |||
| Biomarker | WS N = 99 | ES N = 423 | p value |
| Chromogranin A at diagnosis | 26 (26%) | 172 (41%) | p = 0.008 |
| Chromogranin A at least one time | 38 (38%) | 189 (45%) | p = 0.263 |
| NSE at diagnosis | 29 (29%) | 21 (5%) | p <0.001 |
| NSE at least one time | 36 (36%) | 32 (8%) | p <0.001 |
| WS = Western Switzerland; ES = Eastern Switzerland. | |||
| Table 4: Outcome of 348 patients followed for a median time of 318 days. | |||||
| Remission status | Complete Remission | Partial remission | Stable disease | Progressive disease | Death |
| All regions N = 348 | 208 (60%) | 8 (2%) | 45 (13%) | 19 (5%) | 68 (20%) |
| WS N = 63 | 29 (46%) | 2 (3%) | 17 (27%) | 7 (11%) | 8 (13%) |
| ES N = 285 | 179 (63%) | 6 (2%) | 28 (10%) | 12 (4%) | 60 (21%) |
| p-value | p = 0.017 | p = 0.65 | p = 0.002 | p = 0.066 | p = 0.134 |
| WS = Western Switzerland; ES = Eastern Switzerland. | |||||
The SwissNET database was started in order to better describe the clinical characteristics of NEN patients of Switzerland, and to improve and standardise their clinical care. Our study represents the first report on this cohort, demonstrating that the demographic characteristics of Swiss NEN patients correspond to what has been previously described in the literature [1, 3]: their mean age at diagnosis was in the late fifties, we observed an almost equal distribution between both genders, and the vast majority of them presented as a non-functioning tumour with the small bowel as the main localisation for the primary tumour.
In WS, significantly more tumours were incidentally discovered. This discrepancy may reflect a difference in the medical approach between both regions of Switzerland, suggesting that imaging studies or systematic check-ups are performed more frequently in WS. When functional, NENs mainly cause a carcinoid syndrome, with hyper production of serotonin. In our study, this syndrome was present in 9% of all patients. Very few recent data regarding functioning NENs are available in the literature, rendering the comparison with other published cohorts extremely difficult. Indeed, functioning tumours have been reported in 18% of all patients with jejuno-ileal NENs [3], and even up to 20 to 30% of all cases when considering patients with metastatic spread [10]. However, these patients represent only a subset of all NENs, a difference that could account for the discrepancy observed between these data [10] and our own results. In our study, the second and third most frequent functioning tumours were insulinomas and gastrinomas, which are the most common functional pancreatic NENs [11].
The WHO (World Health Organization) classification for NENs appeared in 2000 [8] for the gastro-intestinal tract and in 2004 [12] for the pancreas. This classification established the rational basis for the terminology and classification of GEP-NENs. However, it is not widely accepted since it is a hybrid system incorporating staging information into a grading system [9], and had limited, with questionable prognostic value in very advanced and metastatic stages of the disease [6]. Therefore a new WHO classification was proposed in 2010 [9], substantially endorsing the ENETS proposals for grading classification and site-specific staging system [6, 13].
As a result, many of the patients initially included in our database were re-classified according to the new sets of criteria. We therefore took this opportunity to compare the diagnoses obtained with the two different classifications. Using the WHO 2000 criteria, well-differentiated neuroendocrine carcinomas were relatively more frequent in WS, whereas well-differentiated neuroendocrine tumours and poorly differentiated neuroendocrine carcinomas were altogether relatively more frequent in ES. However, this difference between both regions disappeared with the use of the revised criteria. Although it is very difficult to provide a definitive explanation for this observation, we would like to speculate that WHO 2010 criteria are easier to apply, and thus possibly more accurate in characterising GEP-NENs.
These neoplasms are usually well visualised on imaging modalities using radio-labelled somatostatin analogues (SSAs) such as the traditional 111In-pentetreotide scintigraphy and the more recent 68Ga-radiolabeled somatostatin analogues PET, since most of them over express somatostatin receptors. Recommendations regarding diagnostic workup of GEP-NENs therefore comprise of functional imaging in addition to morphological studies (CT, MRI or US). Our data demonstrate the widespread use of somatostatin receptor scintigraphy (SRS) in both regions of Switzerland without any significant difference, with the number of 68Ga-radiolabeled somatostatin analogues PET increasing (presently only available only in ES). This is in line with recent data from the literature [14–17] supporting the higher sensitivity and accuracy of the latter in the detection of NENs. As a result, 68Ga-radio-labeled somatostatin analogues PET are presently becoming the preferred functional imaging modality.
Measurements of general biomarkers like chromogranin A (CgA) or neuron-specific enolase (NSE) are also recommended in the diagnosis and follow-up of NENs. The concentration of CgA in patients with NENs is linked to the tumour burden, to its differentiation, and to treatment response [18, 19]. CgA is more sensitive than NSE [20]; it is also more abundant and represents a better circulating marker than chromogranin B [21]. Very high levels of serum CgA are typical in NENs, but other neoplastic conditions are also associated with elevated CgA, like pheochromocytomas, neuroblastomas, medullary thyroid carcinomas and some pituitary tumours. In clinical practice, the most frequent causes of false (non-NEN) positive elevation of GgA are impaired renal function, treatment with proton pump inhibitors, and type A chronic atrophic gastritis (type A) [19–21]. We found that NSE is more used in WS, whereas CgA is preferred in ES. There is no clear explanation for this difference in the choice of biomarker between both regions, which could simply reflect a better availability of one or the other in the reference laboratories used. It should however be emphasised that ENETS recommends the use of CgA over NSE [20],
The present data confirm the central role played by surgery as the first line therapy in the management of these patients, in accordance with ENETS guidelines [10] recommending that every patient with a jejuno-ileal NEN is a potential candidate for curative resection of the primary tumour and lymph node metastases. In the presence of known distant metastases, the resection of the primary tumour is still recommended under certain circumstances, for example if there is a possibility of curative resection of the distant metastases, or if the primary tumour is symptomatic (haemorrhage or stenosis). Even when a curative approach is no longer possible, resection of the primary tumour can still improve the outcome [10]
For pancreatic NENs, surgery is also the recommended first line treatment for any localised neuroendocrine neoplasm, since it significantly improves survival. In a palliative setting, debulking is recommended in selected patients to avoid tumour-related complications. Finally, ENETS guidelines propose curative surgery to every patient with operable well-differentiated metastases independently from the site of origin [22]. Surgical resection of liver metastases has been demonstrated to improve overall survival and quality of life in patients with a G1 or G2 disease. In case of a NEC G3 disease, resection of liver metastases is usually not recommended.
The effects of SSA on hormone-producing NENs have long been recognised, and their use in the control of hormonal syndromes represents a classic indication for this line of treatment. Recently, the PROMID study [23] has highlighted their anti-proliferative effects in the setting of non-functioning tumours as well. As a result, the ENETS now recommends the use of SSAs, especially octreotide LAR, for anti-proliferative purposes in functioning and non-functioning midgut tumours [22]. However, our finding that SSAs are seldom used as a medical therapy in patients from SwissNET is consistent with the fact that they are not yet widely recognised for this indication in Switzerland.
Finally, our outcome data indicate that most patients are in complete remission at the time of the study. However, since the SwissNET database is relatively recent, the median follow-up of these patients is presently shorter than a year. Thus, this figure probably does not reflect the real long-term prognosis of these patients. For the same reason, it is probably impossible to draw firm conclusions from the differences observed between ES and WS on the remission status of these patients.
Overall, this study represents the first clinical description of patients diagnosed with NEN in Switzerland. From an epidemiological point of view, they are similar to what has been described in the literature. The use of general biomarkers in the diagnosis and follow-up of NEN patients show that NSE is used more frequently in WS, whereas CgA is preferred in ES. Data on functional imaging demonstrate the widespread use of somatostatin receptor scintigraphy (SRS) in both regions of Switzerland, while 68Ga-radiolabeled somatostatin analogues PET is increasing, especially in ES. In good accordance with published guidelines, data on first line therapy demonstrate the crucial role of surgery. The low incidence of biotherapy suggests that long-acting somatostatin analogues are not yet widely used for their anti-proliferative effects. The SwissNET initiative should help improve compliance with ENETS guidelines in the workup and care of NEN patients.
Acknowledgments: The authors wish to express their gratitude to all physicians involved in the care of the patients included in the SwissNet database.
1 Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008,26:3063–72.
2 Gustafsson BI, Kidd M, Modlin IM Neuroendocrine tumors of the diffuse neuroendocrine system. Current opinion in oncology. 2008,20:1–12.
3 Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. The lancet oncology. 2008,9:61–72.
4 Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World journal of gastroenterology: WJG. 2008,14:5377–84.
5 Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocrine-related cancer. 2008,15:1083–97.
6 Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010,39:707–12.
7 Salazar R, Wiedenmann B, Rindi G, Ruszniewski P. ENETS 2011 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: an update. Neuroendocrinology. 2012,95:71–3.
8 Solcia E, Klöppel G, Sobin LH. 2000 Histological typing of endocrine tumors. In: WHO international histological classification of tumors. Berlin: Springer. 56–70.
9 Rindi G, Arnold R, Bosman FT. 2010 Nomenclature and classification of neuroendocrine neoplasmas of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND eds. WHO classification of tumors of the digestive system. Lyon: IARC.
10 Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012,95:135–56.
11 Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012,95:98–119.
12 Heitz PU, Komminoth P, Perren A, Klimstra DS, Dayal Y, Bordi C, et al. 2004 Pancreatic endocrine tumours: introduction. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C eds. Pathology and genetics: tumours of endocrine organs WHO classification of tumours. Lyon: IARC Press. 177–82.
13 Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009,90:162–6.
14 Ambrosini V, Campana D, Tomassetti P, Fanti S. (6)(8)Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. European journal of nuclear medicine and molecular imaging. 2012;39(Suppl1):S52–60.
15 Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3–octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2007;48:508–18.
16 Oberg K Gallium-68 somatostatin receptor PET/CT: is it time to replace (111)Indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine. 2012;42:3–4.
17 Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010;51:875–82.
18 Kanakis G, Kaltsas G Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best practice & research Clinical gastroenterology. 2012;26:791–802.
19 Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Annals of surgical oncology. 2010,17:2427–43.
20 O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology, 2009;90:194–202.
21 Oberg K Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. Endocrine-related cancer. 2011;18(Suppl 1):S17–25.
22 Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76.
23 Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009;27:4656–63.
Funding / potential competing interest: SwissNET was created at the initiative of clinicians taking care of these patients in St Gallen and Bern, as a totally independent project. However, it is presently supported by unrestricted grants from Novartis, Ipsen and Pfizer. The manuscript was performed in the context of a medical master project by the first author (Marine Gouffon).