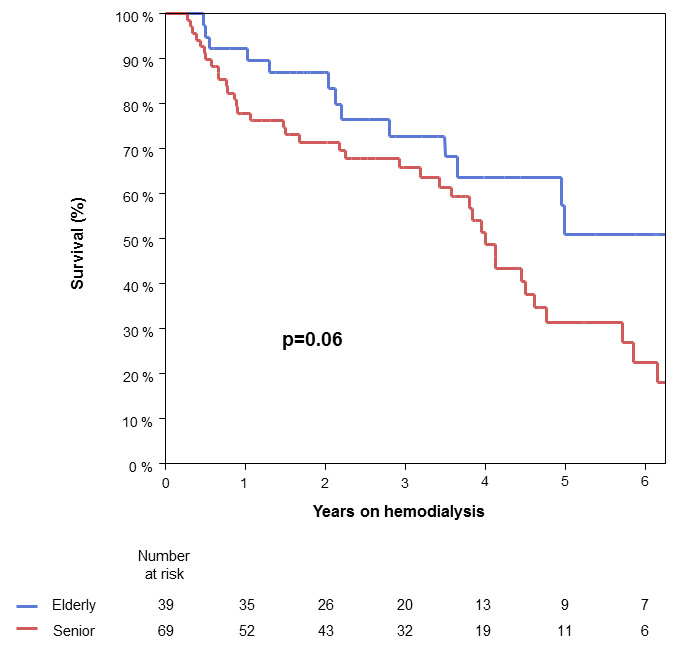
Figure 1
Patient survival
The abscissa shows years after the start of haemodialysis and the ordinate shows patient survival as a percentage.
DOI: https://doi.org/10.4414/smw.2014.13920
In past years, western society has undergone a change in its composition owing to increasing life expectancy. In nephrology, this means an increasing number of older patients with end-stage renal disease (ESRD) requiring renal replacement therapy (RRT) [1]. Age as such is seen as a good predictor of patient outcome in general. However, there is a lack of data concerning age in patients receiving RRT, namely haemodialysis. Therefore, it is important to investigate clinical outcomes in this patient population.
The number of patients being treated for ESRD was estimated to be 2,786,000 patients globally at the end of 2011 by Fresenius Medical Care, with an annual growth rate of 6%–7% [2]. Proportionately, haemodialysis is the most common RRT in patients with ESRD, with a global frequency of approximately 80%. [2]. In the United States, the incidence of ESRD grew from 366 per million population (pmp) in 2006 to 369 pmp in 2010 [3]. In patients aged 70 years and older, incidence rates of reported ESRD in the United States grew 20% in ten years to 41,825 in 2010 [4]. In Europe (30 European countries reporting data to the European Renal Association) the incidence of people having RRT grew from 118 pmp in 2006 to 122 pmp in only two years [5, 6]. In Switzerland, the number of haemodialyses performed rose from 401,458 in 2007 to 455,789 in 2011 (University Hospital Basel; personal communication).
Reflected by the demographic data, haemodialysis has become a very common therapy for patients with ESRD [7]. As the French REIN-group demonstrated in its prognostic score for older patients starting dialysis, mortality was low in patients with no significant comorbidities [6]. Aging patients become less eligible for kidney transplantation, as data from transplantation waiting lists show, and haemodialysis represents a good alternative [8]. Other benefits of haemodialysis are the monitoring of the patients during dialysis because of supervision by the medical staff and visits of a doctor.
On the other hand, haemodialysis has a significant influence on the patient’s lifestyle. It is time-consuming, patients need to have access to a medical centre performing haemodialysis, and they are often dependent on other people to bring them there three times per week. The prognostic score of the French REIN-group shows that older patients with ESRD suffering from malnutrition, cardiovascular problems and restricted mobility have an especially high mortality rate 6 months after the start of haemodialysis [6]. In general, elderly patients with severe, mainly cardiovascular, comorbidities on haemodialysis have a comparable life expectancy to those on conservative therapy. Thus, it has to be evaluated carefully, if it is worth starting dialysis or not in this patient group. Another common side effect which is worth mentioning is hypotension during haemodialysis, which for older patients can lead to more falls and increases the risk of fractures [9, 10]. Furthermore, vascular access problems are a notable challenge in those patients.
Owing to the lack of data in the literature concerning older patients on haemodialysis, their possible complications and long-term outcome, the aim of this retrospective study was to compare the outcomes of dialysis patients of 70 years of age or above and those aged 60 to 69 years.
Between 1 January 2000 and 31 December 2011, 570 patients in total started chronic haemodialysis treatment at the University Hospital Basel. All haemodialysis patients aged 70 years or above (described as “seniors”) at the time of dialysis initiation, who fulfilled to the inclusion criteria (see below), were analysed and compared with a control group of patients aged 60 to 69 years (described as “elderly”). The elderly patients were chosen out of a group of haemodialysis patients between 60 to 69 years of age, using the same inclusion criteria and with the intention of getting a homogenous population with equal distributions of gender and causes leading to ESRD between the two groups. The study was approved by the local ethics committee of the University of Basel.
Inclusion criteria for both groups were: (1.) haemodialysis as the therapy of choice for RRT, (2.) three haemodialysis sessions per week, and (3.) at least 3 months of continuous dialysis treatment. The only exclusion criterion was change to another dialysis centre, with resultant lack of accurate follow-up.
At the University Hospital Basel dialysis prescription consisted of 4-hour dialysis sessions three times a week with target blood flow >200 ml/min (>300 ml/min since 2002), and anticoagulation with low molecular weight heparins (Dalteparin, Enoxaparin). Haemodiafiltration was prescribed in >90% of patients from the beginning of the study, and since 2002 all dialysis machines have online haemodiafiltration equipment. High flux filters with 1.7 to 2.1 m² surface area from various companies were used.
Data were collected retrospectively from the patient charts and dialysis records of every patient, and further saved in a customised database. Baseline characteristics and data with a focus on haemodialysis (i.e. duration of haemodialysis, dialysis accesses, and blood pressure at start of dialysis, reasons for cessation of haemodialysis, cause of death, comorbidities, complications with outpatient treatments and complications leading to hospitalisation, length of hospital stay) were collected. The renal diseases evidently leading to ESRD or renal diseases proven by biopsy were classified; all others or patients with several differential renal diagnoses were summarised in the subgroup “other” (see results). Dialysis accesses were defined as the number of functional, temporary or permanent vascular accesses (arteriovenous shunt, arteriovenous graft, temporary or permanent catheters). The definitions of comorbidities and complications are specified below (see “analysis of comorbidities and complications”).
The study start date was the beginning of chronic haemodialysis therapy and patients were followed up until recovery of renal function, kidney transplantation, death, withdrawal of dialysis by the patient’s decision or end of the study (31December 2011).
Comorbidities of each patient were defined as every kind of disease that had been diagnosed before the start of chronic haemodialysis. The following comorbidities were specified: hypertension, heart failure, coronary heart disease (CHD), valvular heart disease, peripheral artery disease (PAD), stroke, malignancy, chronic obstructive pulmonary disease (COPD), pulmonary embolism, significant liver disease and diabetes mellitus. Patients with diabetes were split into two groups: insulin-dependent or noninsulin-dependent diabetes. Hypertension was defined as a complication if it was listed as a diagnosis in the diagnosis-list on the patient chart. Usually systolic blood pressure blood pressure above 160 mm Hg and/or diastolic blood pressure above 90 mm Hg were used as criteria.
Complications were defined as medical problems that occurred after the start of chronic haemodialysis. They were stratified into five subgroups: cardiovascular complications, fractures and osteoporosis, access-associated, infections and “other”. Infections concerning haemodialysis access were all recorded into the subgroup “access-associated”. Osteoporosis was defined as a complication if it was diagnosed during the period of haemodialysis and recorded in the patient’s chart. Further, complications were subdivided into complications leading to outpatient treatment and those needing hospitalisation. Owing to the lack of reliable records, it was not possible to analyse therapeutic consequences of complications. However, length of hospital stay due to severe complications provided enough information for further analysis.
The primary outcome was patient survival on chronic haemodialysis, stratified by age. Secondary outcomes were causes of death as well as number of dialysis accesses, elevated blood pressure at start of haemodialysis session at indicated time points, and type and frequency of complications on haemodialysis therapy, including access-related complications.
JMP software version 9.0 (SAS Institute, Cary, NC, USA) was used for statistical analysis. For categorical data, Fisher’s exact test or Pearson’s chi-square test were used. Continuous data were summarised as median and interquartile range (IQR) unless stated otherwise. Parametric continuous data were analysed with Student’s t-tests. For nonparametric continuous data, the Wilcoxon rank-sum test was used. Patient survival analysis was performed by the Kaplan-Meier method and groups compared using the log-rank test. In the survival analysis, patients were censored if they underwent renal transplantation, had recovery of renal function during the follow-up period, or were still alive and on dialysis at the end of follow-up. A two-tailed p-value <0.05 was considered to indicate statistical significance.
A total of 152 haemodialysis patients of 70 years of age or above started haemodialysis after 1January 2000. Overall, 83 of these patients could not be included in the study for the following reasons: 60 patients had a dialysis period of less than 3 months; 11 patients changed their dialysis centre; 12 patients did not provide enough reliable and useful data and therefore were also excluded. Remaining were 69 haemodialysis patients of 70 years of age or above, who fulfilled all inclusion criteria. This group of patients was compared with 39 elderly patients aged between 60 and 69 years.

Figure 1
Patient survival
The abscissa shows years after the start of haemodialysis and the ordinate shows patient survival as a percentage.
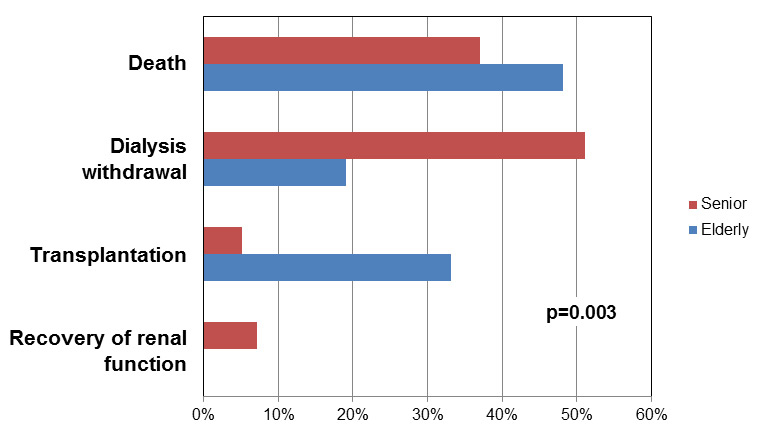
Figure 2
Reasons for cessation of haemodialysis therapy.
Baseline characteristics of the two groups are summarised in table 1. Median age at start of haemodialysis therapy of the seniors was 75 years (IQR 72–79 years) versus 65 years (IQR 62–67 years) in the elderly, respectively. There was no statistical difference regarding renal diseases leading to ESRD, gender and comorbidities (table 1). Renal diseases leading to ESRD were glomerulonephritis (n = 10), vascular nephropathy (n = 3), diabetic nephropathy (n = 16), adult polycystic kidney disease (n = 5) and other (n = 74) (table 1). The latter includes all diseases that had not been proven by biopsy and all patients with several different renal diagnoses. Therefore, the most common renal disease in both groups was “other” (67% in seniors vs 72% in elderly).
Several different comorbidities occurred in both groups, which are listed in table 1. The dominant comorbidity in both groups was hypertension (67% in seniors vs 56% in elderly, p = 0.31). Other comorbidities with high frequency were diabetes mellitus (32% in seniors vs 41% in elderly, p = 0.40), CHD (26% in seniors vs 44% in elderly, p = 0.09) and heart failure (28% in seniors vs 18% in elderly, p = 0.35).
Median date of starting haemodialysis of the entire population was in 2007 (IQR 2004‒2009) and did not differ between the two groups (i.e. median date of starting haemodialysis in seniors was in 2006 vs in 2007 in the elderly group, respectively, p = 0.65) as shown in table 2. Duration of haemodialysis therapy varied from 8 to 47 months in the senior and 13 to 38 months in the elderly group. Median duration of chronic haemodialysis was approximately the same in both groups: 18 months in seniors and 23 months in elderly (p = 0.88), see follow-up data on table 2.
Patient survival of both groups is shown in the Kaplan Meier survival curves in figure 1. Number at risk gives the quantity of patients the analysis refers to. Because less than 50% of patients were still at risk 3 years after the start of haemodialysis, the curves at later time-points have to be interpreted with caution. There is only a trend towards better survival in younger patients on haemodialysis, with a three-year survival of 66% in seniors vs 73% in elderly and five-year survival of 32% in the senior collective vs 51% in the elderly p = 0.06 (fig. 1). During the observational time, about half of the patients died: 38/69 in the senior and 14/39 in the elderly group (55% in seniors vs 36% in elderly; p = 0.07). Overall, 22/38 (58%) of the seniors and 4/14 (29%) of the elderly withdrew from haemodialysis before they died (p = 0.12).
Causes of death stratified by age are summarised in table 2. They were grouped into cardiovascular, cerebral, infection/sepsis, malignancy, multiorgan failure, sudden death and unknown. In both groups the leading reason for death is “unknown” (53% in seniors vs 36% in elderly), followed by “cardiovascular” (16% in seniors vs 21% in elderly), “infection/sepsis” (13% in seniors vs 14% in elderly) and “cerebral” (5% in seniors vs 14% elderly). This distribution of the different death events is not statistically different in the two groups (p = 0.88).
Complications are summarised in table 3. They occurred in 67/69 senior (97%) and in 37/39 elderly patients (95%); thus both groups were equally affected by complications (p = 0.62). In total there were 536 complications, 289 leading to hospitalisation and 247 complications treated on an outpatient basis. The frequency of patients who needed hospitalisation did not differ between the two groups (57/69 seniors vs 32/39 elderly, p = 1.0). It turned out that the only significant difference between the two groups was triggered by a higher frequency of complications leading to outpatient treatment in the senior group (71% vs 29% in elderly, p = 0.04). However, seniors did not have more severe complications requiring hospitalisation (p = 0.64). Nevertheless, there was a trend towards a higher frequency of complications leading to hospitalisation in seniors (62% in seniors vs 38% in elderly). Furthermore, duration of hospitalisation did not differ between both groups with a median time of 9 days in each group (IQR 5–18 in seniors vs 4–17 days in elderly, p = 0.72). The frequency of the total complications (i.e. outpatient and inpatient) as defined in the material and methods section did not differ between the two collectives (overall p = 0.87; data not shown). Numerically, the most frequent overall complication category was, in both groups, cardiovascular events (n = 99 in seniors vs n = 51 in elderly).
| Table 1:Baseline characteristics of patients stratified by age (n = 108). | |||
| Seniors (≥70 y; n = 69) | Elderly (60–69 y; n = 39) | p-value | |
| Patients | |||
| Age (y), median (IQR) | 75 (72–79) | 65 (62–67) | – |
| Females, n (%) | 25 (36) | 16 (41) | 0.68 |
| Renal disease, n (%) | |||
| Glomerulonephritis | 8 (12) | 2 (5) | |
| Vascular | 2 (3) | 1 (3) | |
| Diabetic | 10 (14) | 6 (15) | 0.87 |
| ADPKD | 3 (4) | 2 (5) | |
| Other | 46 (67) | 28 (72) | |
| Comorbidities, n (%) | |||
| Hypertension | 46 (67) | 22 (56) | 0.31 |
| Heart failure | 19 (28) | 7 (18) | 0.35 |
| CHD | 18 (26) | 17 (44) | 0.09 |
| Valvular heart disease | 14 (20) | 5 (13) | 0.43 |
| PAD | 14 (20) | 7 (18) | 1.0 |
| Stroke | 7 (10) | 9 (23) | 0.09 |
| Malignancy | 12 (17) | 5 (13) | 0.59 |
| COPD | 12 (17) | 5 (13) | 0.59 |
| Pulmonary embolism | 5 (7) | – | 0.16 |
| Liver disease | 4 (6) | 6 (15) | 0.16 |
| Diabetes mellitus | 22 (32) | 16 (41) | 0.40 |
| Insulin-dependent | 10 (45) | 8 (50) | 0.43 |
| ADPKD = adult polycystic kidney disease; CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; PAD = peripheral artery disease | |||
There was no statistical difference between the number of dialysis accesses in both groups, with a mean number of 2.2 accesses in seniors and 2.7 in elderly (p = 0.13; table 2). During follow-up, 65/108 patients in total (60%) were affected overall by 148 complications associated with dialysis accesses (i.e. shunt-complications as well as catheter-related complications): 42/69 of the seniors and 23/39 of the elderly (61% vs 59%, respectively; p = 1.0).
The number of patients with elevated blood pressure at the start of haemodialysis sessions (and corresponding frequencies) at different time points during follow-up are indicated in table 3. After 1 year on haemodialysis, blood pressure values of total n = 81 surviving patients (49 seniors, 32 elderly) were available to analyse, and after 3 years on haemodialysis they could be analysed in total n = 44 surviving patients (30 seniors, 14 elderly). The number of patients with elevated blood pressure at different time points during follow up did not differ between the two groups (table 3).
In total, 64/108 patients (n = 43 within the seniors and n = 21 within the elderly) ended their haemodialysis therapy during follow-up and there was a significant difference regarding the reasons for cessation between the two groups. The four reasons – death, kidney transplantation, dialysis withdrawal by the patient’s decision and recovery of function – for cessation of haemodialysis stratified by age are shown in figure 2 and table 2. Overall, the leading reasons were death or withdrawal of haemodialysis (both n = 26). The difference between the two groups was triggered by a higher frequency of withdrawal of haemodialysis within the seniors (n = 22/43) compared with the elderly (n = 4/21; 51% vs 19%, p = 0.02). Further, there were more transplantations in the elderly leading to cessation of haemodialysis compared with the seniors (33% in elderly vs 5% in seniors, p = 0.01).
| Table 2:Follow-up data of patients stratified by age (n = 108). | |||
| Seniors (≥70 y; n = 69) | Elderly (60–69 y; n = 39) | p-value | |
| Haemodialysis | |||
| Number of dialysis access (n), mean ± std. | 2.2 ± 1.8 | 2.7 ± 2.7 | 0.13 |
| Date of starting (y), median (IQR) | 2006 (2003–’09) | 2007 (2005–’09) | 0.65 |
| Duration* (months), median (IQR) | 18 (8–47) | 23 (13–38) | 0.88 |
| Reason for cessation, n (%#) | |||
| Death | 16 (37) | 10 (48) | 0.82 |
| Dialysis withdrawal | 22 (51) | 4 (19) | 0.02 |
| Transplantation | 2 (5) | 7 (33) | 0.01 |
| Recovery of renal function | 3 (7) | – | (0.55) |
| Exitus letalis | |||
| Overall, n (%) | 38 (55) | 14 (36) | 0.07 |
| Causes of death | |||
| Cardiovascular | 6 (16) | 3 (21) | 0.88 |
| Cerebral | 2 (5) | 2 (14) | |
| Infection/sepsis | 5 (13) | 2 (14) | |
| Malignancy | 2 (5) | 1 (7) | |
| Multiorgan failure | 1 (3) | – | |
| Sudden death | 2 (5) | 1 (7) | |
| Unknown | 20 (53) | 5 (36) | |
| *Duration of dialysis therapy. #The frequency of reason for cessation of haemodialysis refers to the total of patients who stopped haemodialysis (n = 43 within the seniors and n = 21 within the elderly). | |||
| Table 3:Complications after start of haemodialysis (in total n = 536). | |||
| Seniors (≥70 y; n = 69) | Elderly (60–69 y; n = 39) | p-value | |
| Patients with complications | |||
| Overall, n (%) | 67 (97) | 37 (95) | 0.62 |
| Inpatient treatment, n (%) | 57 (83) | 32 (82) | 1.0 |
| Access-associated | 42 (61) | 23 (59) | 1.0 |
| Elevated blood pressure§, n (IQR)# | |||
| At 1 month on haemodialysis | 14 (21) # | 7 (18) # | 1.0 |
| At 1 year on haemodialysis | 10 (20) # | 5 (16) # | 0.77 |
| At 3 years on haemodialysis | 5 (17)# | – | 0.16 |
| At last follow-up | 16 (23) # | 9 (24) # | 1.0 |
| Complications | |||
| Out-/inpatient treatment, n | 175/179 | 72/110 | 0.04 |
| Subgroups (outpatient*) | |||
| Cardiovascular | 48 (27) | 20 (28) | 0.04 |
| Fractures and osteoporosis | 24 (14) | 6 (8) | |
| Infections | 12 (7) | 10 (14) | |
| Access-associated | 61 (35) | 32 (44) | |
| Other | 30 (17) | 4 (6) | |
| Subgroups (inpatient*) | |||
| Cardiovascular | 51 (28) | 31 (28) | 0.64 |
| Fractures and osteoporosis | 17 (10) | 11 (10) | |
| Infections | 21 (12) | 11 (10) | |
| Access-associated | 38 (21) | 17 (16) | |
| Other | 52 (29) | 40 (36) | |
| § Patients with elevated blood pressure at the start of dialysis therapy session are indicated at different time points. Elevated blood pressure was defined as a systolic blood pressure above 160 mm Hg and/or diastolic blood pressure above 90 mm Hg. # Corresponding frequencies of the number of patients with elevated blood pressure refer effectively to the patients available for analysis. * The subgroups of complications refer to complications treated either outpatient or leading to hospitalisation (inpatient). | |||
In past years, a growing proportion of older patients with ESRD starting haemodialysis have been reported. However, this fact raises the question as to whether the start of haemodialysis is justified for older patients, regarding complications, survival on haemodialysis and the huge economic burden. To date, conflicting reports of the outcome of elderly patients on haemodialysis exist. Some nephrologists see advanced age as a disadvantage for dialysis [11] whereas others do not support that opinion [12, 13]. Nevertheless, the central question arises, does age limit survival on chronic haemodialysis?
To the best of our knowledge, this is the first study thoroughly comparing two groups of really old patients on chronic haemodialysis, with the focus on survival, causes of death and complications. Intriguingly, in this study there was only a trend towards a better survival in elderly patients compared with seniors, with comparable 3- and 5-year survival rates. These results concur with a study which found comparable results in patients with a median age of 75 years [14]. Another study reported similar survival rates in 65- to 74-year-old compared to 75- to 85-year old patients, also including peritoneal dialysis (PD) as RRT modality [15]. Furthermore, these results concur with the results of a cohort study, including 266 patients (median age 65 years, range 15–90), who entered the chronic haemodialysis programme of the University Hospital of Basel between 1995 and 2006 [16]. In contrast, other studies found a lower survival in dialysis patients aged 75 years and older [17, 18]. In addition, a recently published study found significantly lower survival rates in patients aged 75 years or older starting haemodialysis in the period between 2002 and 2004 compared with patients at the same age, starting haemodialysis in the period between 2005 and 2007, although there was no difference in the entire population and in patients aged below 75 years [19]. Why these conflicting results? There are several reasons which might explain this.
One of the major problems of previous studies is the different stratification of the groups by age. Many of them defined as “older” patients those above 60 to 65 years or even above 50 years of age, which nowadays might be obviously too young with respect to actual life expectancy. For example, in the Swiss population the life expectancy was 80.3 years in men and 84.7 years in women in the year 2011, and the overall median life expectancy in the United States in the year 2010 was 78.2 years [20, 21]. Thus, comparison of age as a risk factor for outcome on haemodialysis with other studies has to be done with caution. Furthermore, it seems that older patients with ESRD are referred later to a nephrologist than younger patients, which can lead to a later start of RRT and higher mortality [17, 22]. In addition, some studies also included those patients who died in the first 3 months after the start of haemodialysis, which is in our opinion not the definition of chronic haemodialysis and even implicates a bias of pre-selection that very old patients with ESRD will either refuse to start haemodialysis or withdraw it in the early phase after starting. Moreover, in general the higher number of withdrawals of haemodialysis in seniors and the relatively high number of transplantations in the elderly might influence outcomes.
During follow-up both groups had a similar frequency of deaths (i.e. 38/69 in the senior and 14/39 in the elderly group; p = 0.07). Furthermore, in both groups the leading cause of death was “unknown” (almost half of the cases). Why is this number so high? One reason could be that not every patient underwent autopsy after death. Furthermore, most of the patients who withdrew from haemodialysis may be hidden within that category of death. In literature, withdrawal of haemodialysis is a very common cause of death, especially in older patients [18, 23–25]. However, this study was not performed to investigate withdrawal of haemodialysis as a direct cause of death. The most common known cause of death in both groups was cardiovascular events, which concurs with other studies [18, 24]. However, some studies found a lower rate of cardiovascular events leading to death in younger patients [22, 26].
Concerning complications during haemodialysis, both patient groups stratified by age were affected by complications equally. Surprisingly, the only statistical difference between the two groups was the higher frequency of complications leading to an outpatient treatment within the senior group, whereas the number of patients with severe complications leading to hospitalisation was similar in both groups. This is an important finding, taking into account that older patients are often considered the biggest economic burden of the health care system. However, 83% of the seniors needed hospitalisation, which is a higher frequency than the 66% found by another study, indeed with a follow-up time of only 1 year [27]. The median hospital stay of 9 days is comparable to the USRDS Annual Report 2012 with a median of 12 days for all ages (including patients on PD; USRDS 2012, chapter G). Numerically, the leading complication category was cardiovascular events, reflecting that this is an important issue in patients on dialysis [28, 29]. Furthermore, the number of dialysis accesses and access-associated complications during follow-up were similar in both groups, supposing the conclusion that there is no need for more frequent revisions of their dialysis accesses within the senior group. These findings concur with other studies comparing different age groups with respect to their vascular accesses [26, 30]. In contrast, a meta-analysis some years ago comparing different types of fistula suggested a worse outcome in patients over 65 years of age [31]. In addition, adverse events like hypotension and hypertension during haemodialysis are suggested as risk factors for mortality in haemodialysis patients [32, 33]. However, there was no statistical difference in the frequency of patients with elevated blood pressure at any investigated timepoints during follow-up.
Another important outcome was the reason for cessation of haemodialysis. There was a statistically significant difference between the two groups regarding kidney transplantation and withdrawal of haemodialysis. Although more senior than elderly patients decided to stop haemodialysis, more elderly patients underwent kidney transplantation. Why is the withdrawal rate of haemodialysis higher in seniors than in the elderly? One reason might be specific treatment problems bothering older patients on haemodialysis, which decrease their quality of life. Further, they may suffer from a life-threatening illness, turning haemodialysis into a death prolonging therapy, which can lead to the decision to withdraw it. Interestingly, however, death as reason for cessation of haemodialysis was equally frequent in both groups (i.e. 37% in seniors and 48% in elderly, p = 0.82).
What are the limitations of the study? First, regarding the intermediate-sized patient population with total n = 108 (i.e. 69 seniors and 39 elderly), the study might be underpowered to detect potential small differences between the two groups. Another important aspect, which has to be taken into account, is that – due to the fact of the retrospective single-centre study design – only medical issues were analysed, disregarding the patient’s view of quality of life. Further, the study did not differentiate between patients with a high or low comorbidity rate in the anaylsis of survival, which could hide eventual influences on patient’s survival during haemodialysis, as other studies claim [18, 23, 34]. In addition, there might be a positive selection in the senior population due to the fact that the very ill patients with ESRD underwent conservative therapy and did not start haemodialysis. Contrary, there might be a negative selection in the elderly population, taking into account those who underwent pre-emptive transplantation, thus receiving a kidney from a living donor, before starting RRT.
In conclusion, age per se is not a good predictor for morbidity and mortality of patients of 70 years of age or above on chronic haemodialysis. Rather, attendant circumstances such as comorbidities, complications, patients’ quality of life and wishes influence whether an individual patient will benefit from starting haemodialysis or not and thus, age alone should never guide us in the decision-making process as whether to to start dialysis or not in these patients.
Acknowledgements: The authors thank the staff of the haemodialysis unit at the University Hospital of Basel for the empathic and professional survey of the haemodialysis patients during dialysis sessions, as well as helpful recording of patient`s clinical and laboratory data within their dialysis records.
1 Van de Luijtgaarden M, Noordzij M, Wanner C, Jager K. Renal replacement therapy in Europe – a summary of the 2009 ERA–EDTA Registry Annual Report. Clin Kidney J. 2012;5:109–19.
2 Fresenius Medical Care Deutschland GmbH. “ESRD Patients in 2011. A Global Perspective”. 2012 [cited 2013 5th March]; Available from: http://www.vision-fmc.com/files/download/ESRD/ESRD_Patients_in_2011.pdf
3 U S Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2012. Chapter 1, Figure 12.B, Volume 2.
4 U S Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2012. Chapter A, Table A.1, Volume 2.
5 Stel VS, Kramer A, Zoccali C, Jager KJ. The 2006 ERA-EDTA Registry annual report: a précis. J Nephrol. 2009;22:1–12.
6 Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, et al. A clinical score to predict 6–month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24(5):1553–61.
7 Burkhalter F, Steiger J, Dickenmann M. A road map for patients with imminent end-stage renal disease. Swiss Med Wkly. 2012;142:w13713
8 Raine AEG. The susceptible patient. Nephrol Dial Transplant. 1996;11(Suppl 2):6–10.
9 Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol. 2008;19:8–11.
10 Desmet C, Beguin C, Swine C, Jadoul M; Université Catholique de Louvain Collaborative Group. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45(1):148–53.
11 Callahan D. Setting Limits: Medical Goals in an Aging Society. Simon & Schuster. New York; 1987.
12 Moss AH, Stocking CB, Sachs GA, Siegler M. Variations in the attitudes of dialysis unit medical directors towards decisions to withhold and withdraw dialysis. J Am Soc Nephrol. 1993;4:229–34.
13 Holley JL. Foulks CJ, Moss AH. Nephrologists’ reported attitudes about factors influencing recommendations to initiate or withdraw dialysis. J Am Soc Nephrol. 1991;1:1284–21.
14 Ismail N. Renal replacement therapy in the elderly: an old problem with young solutions. Nephrol Dial Transplant. 1997;12(5):873–76.
15 Ismail N. Hakim RM, Oreopoulos DG, Patrikarea A. Renal replacement therapy in the elderly: Part 1. Hemodialysis and chronic peritoneal dialysis. Am J Kidney Dis. 1993;22(6):759–82.
16 Breidthardt T, Moser-Bucher CN, Praehauser C, Garzoni D, Bächler K, Steiger J, et al. Swiss Med Wkly. 2011;141:w13150
17 Letourneau I, Ouimet D, Dumont M, Pichette V, Leblanc M. Renal replacement in end-stage renal disease patients over 75 years old. Am J Nephrol. 2003;23:71–7.
18 Munshi SK, Vijayakumar N, Taub NA, Bhullar H, Lo TC, Warwick G. Outcome of renal replacement therapy in the very elderly. Nephrol Dial Transplant. 2001;16(1):128–33.
19 Glaudet F, Hottelart C, Allrad J, Allot V, Bocquentin F, Boudet R et al. BMC Nephrology. 2013;14:131(25 June 2013)
20 Schweizerische Eidgenossenschaft Federal Statistical Office, Neuchâtel 2013. Life expectancy. [cited 2013 5th March]. Available from: http://www.bfs.admin.ch/bfs/portal/en/index/themen/01/06/blank/key/04/04.html
21 The World Bank. Public data Lebenserwartung Vereinigte Staaten 2010. [cited 2013 5th March]. Available on: http://www.google.ch/publicdata/explore
22 Schwenger V, Morath C, Hofmann A, Hoffmann O, Zeier M, Ritz E. Late referral – a major cause of poor outcome in the very elderly dialysis patient. Nephrol Dial Transplant. 2006;21(4):962–67.
23 Isles C, Robertson S, Almond A, Donaldson K, Clark D. The challenges of renal replacement therapy and renal palliative care in the elderly. J R Coll Physicians Edinb. 2011;41(3):238–43.
24 Mandigers CM, de Jong W, van den Wall Bake AW, Gerlag PG. Renal replacement therapy in the elderly. Neth J Med. 1996;49(4):135–42.
25 Birmele B, Francois M, Pengloan J, Francois P, Testou D, Brillet G, et al. Death after withemodialysisrawal from dialysis: the most common cause of death in a French dialysis population. Nephrol Dial Transplant. 2004;19:686–91.
26 Latos DL. Chronic dialysis in patients over age 65. J Am Soc Nephrol. 1996;7:637–46.
27 Harris SA, Lamping DL, Brown EA, Constantinovici N. North Thames Dialysis Study (NTDS) Group. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Perit Dial Int. 2002;22(4):463–70.
28 Moldovan D, Moldovan I, Rusu C, Kacso I, Patiu IM, Gherman-Caprioara M. FGF-23, vascular calcification, and cardiovascular diseases in chronic hemodialysis patients. Int Urol Nephrol. 2013 Apr 3 [Epub ahead of print].
29 Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–76.
30 Weale R, Bevis P, Neary WD, Boyes S, Morgan JD, Lear PA, et al. Radiocephalic and brachiocephalic arteriovenous fistula outcomes in the elderly. J Vasc Surg. 2008;47:144–50.
31 Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patiens. J Vasc Surg. 2007;45:420–26.
32 Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58: 2147–54.
33 Salam MM, Bower J. Hypertension in the hemodialysis population: Any relation to ine-year survival? Am J Kidney Dis. 1996;28:737–40.
34 Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608–14.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.