
Figure 1
Illustration of cochlear implant components. The external parts are shown on the left, the internal components of the implant on the right. The scheme in the middle shows the position of the cochlear implant components in situ.
DOI: https://doi.org/10.4414/smw.2014.13909
Hearing loss has a significant impact on the affected individual as well as on the society. One to two live births per 1,000 are with a permanent hearing loss, which in almost all cases is due to reduced cochlear function. Around one-third of these affected children have a profound hearing loss [1]. In addition, it is estimated that around a third of the babies born with permanent hearing loss have other neuro-developmental conditions [2]. The prevalence of permanent hearing loss in children increases with age owing to delayed onset of genetically based hearing loss, meningitis or late diagnosis [3]. At the other end of the age spectrum, more than 50% of individuals over 65 years of age suffer from some degree of hearing loss and the prevalence rises as high as 95% among individuals aged 80 years and older [4–6]. The World Health Organization estimates that 299 million men and 239 million women are affected by hearing loss [7]. The majority suffer from mild to moderate hearing loss and severe to profound hearing loss accounts for less than 10% [3].

Figure 1
Illustration of cochlear implant components. The external parts are shown on the left, the internal components of the implant on the right. The scheme in the middle shows the position of the cochlear implant components in situ.
In general, hearing loss can be split into two major categories: conductive and sensorineural. Conductive hearing loss results from diseases of the external ear canal and middle ear. Sensorineural hearing loss originates within the inner ear or along the acoustic neural pathways. Aetiologies of sensorineural hearing loss include genetic disorders, infections, ototoxicity, age or overexposure to intense sound, among others.
There is currently no primary treatment available for sensorineural hearing loss. Only prosthetic devices can help. Individuals with mild to moderate forms of hearing loss can benefit from conventional hearing aids, which amplify sound. However, the vast majority of individuals with profound hearing loss or deafness are more effectively treated with cochlear implants (CIs) than with hearing aids. As indicated in figure 1, a CI directly stimulates the spiral ganglion neurones within the inner ear using electrical pulses, thereby bypassing auditory hair cells. After adapting to the new stimulation of the auditory system through the CI, the majority of CI users are able to regain hearing and to understand speech without the need for lip-reading cues.
In the last decade, CIs have undergone impressive technological progress concerning size, microphone characteristics and stimulation strategies. Along with the availability of improved devices, indications for cochlear implantation have been extended, partially as a result of refined surgical techniques. Further, improved rehabilitation programmes have been developed. Cochlear implantation offers many more possibilities to the hearing impaired and deaf compared with 10–15 years ago. The goal of the present article is to provide an overview of the state of the art of cochlear implantation with an emphasis on the situation in Switzerland. This information can be provided because of a unique cooperative situation between Swiss CI centres. Basic information on all cochlear implantations, since the very first surgery in 1977, is centrally stored in one database for all five Swiss CI centres.
The first attempts to restore hearing by electrical stimulation of the auditory nerve were made in France in 1957. Djourno and Eriès [8] placed a wire into the peripheral vestibular system of the inner ear of a deaf patient and the patient reported useful auditory sensations. Unfortunately, this device failed after a short time. Although the recipient was unable to understand speech with the device alone, it helped lip-reading [8, 9]. In 1961 William House continued the work on single-channel intracochlear devices and his work led to the development of the first CI approved for adults by the USA Food and Drugs Administration in 1984 [10]. In Switzerland, the first custom-made CI was implanted by Professor Ugo Fisch in Zürich in 1977. In the early 1980s, multi-electrode intracochlear implants together with more efficient processing strategies were introduced. The multi-channel systems had a major impact on hearing performance and as a result cochlear implantation became the gold-standard treatment for profound hearing loss and deafness. By the end of 2012, more than 30,000 devices had been implanted worldwide and in Switzerland a total of 2,237 CIs. Since 2004, around 150 cochlear implantations per year were performed in Switzerland for a population of almost 8 million inhabitants [11]. This number is comparable to around 2,000 implantations per year for Germany with a population of 81 million. Figure 2 shows the age distribution of the patients (as percentage) implanted in Switzerland from 1996 to 2003 and 2004 to 2012. The greatest increase over the last 7 years has been in the under 3 and over 60 age groups. The greatest decrease has been the age group 3 to 18 years.
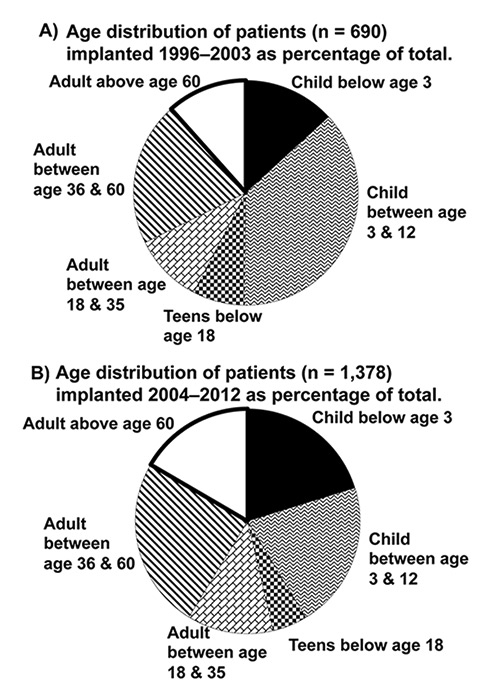
Figure 2
(A) Patients implanted in Switzerland 1999–2003 and (B) Patients implanted in Switzerland 2004–2012, divided into age groups [11].
As shown in figure 1, current CI systems can be divided into two parts – external and internal. The externally worn speech processor unit is equipped with a microphone, sound and electrical stimulation processing hard- and soft-ware, batteries and a transmitter. The implanted part consists of a receiver/stimulator unit, an electrode array with several electrode contacts for stimulation of spiral ganglion neurones in the cochlea, and a ground electrode, which is either integrated into the stimulator unit surface and/or is a separate lead from the housing, depending on the CI model. Sound (stimulation) information is processed in the speech processor and then transmitted via radio frequency through the intact skin to the implant receiver along with the electrical energy. The implant has no internal power source. All energy is provided by the batteries located in the speech processor. The transmitter coil is centred over the implant and held in place by attractive magnetic force. CI systems from four different manufacturers are currently implanted in Switzerland: Cochlear Ltd. (Australia), MEDEL (Austria), Advanced Bionics (Sonova, Switzerland) and Neurelec (France). Cochlear and MEDEL implants are the most frequently used in Switzerland [11].
Cochlear implantation has become a highly standardised and safe surgical procedure in the last decades. The surgery is performed under general anaesthesia and typically lasts around 2 hours. The surgery starts with a 5–6 cm long skin incision behind the ear. The muscle-periosteal tissue is elevated to create a pocket for the receiver/stimulator unit and a bone bed is drilled to ensure a fixed receiver location. A cortical mastoidectomy is performed and the middle ear cavity close to the round window area is entered between the facial nerve and the chorda tympany. The CI electrode array is then inserted into the scala tympani of the cochlea either directly through the round window membrane or through a nearby cochleostomy. Measurements of the electrode array impedances are taken and different intraoperative electrophysiological tests of the auditory nerve are carried out to confirm correct functional operation of the implant and provide estimates of initial speech processor fitting values. Finally, the wound is closed. The patient usually stays 2–4 days in the hospital for the procedure. Surgical complications with this procedure have become very rare. However, some risks exist such as bleeding, infection, damage to neural structures such as the chorda tympani nerve (taste sensation) and extremely rarely to the facial nerve, loss of residual hearing, vertigo and failure of implant device [12]. The need for surgical revisions or reimplantations of devices has become small. In Switzerland, the overall reimplantation rate for all CIs implanted since 1977 has been 9%. Two-thirds of these cases were due to device failure. Medical problems, accidents (head trauma) or upgrades to newer devices accounted for the remaining cases [11].
The activation of spiral ganglion neurones by electrical stimulation through cochlear implants clearly differs from the naturally occurring stimulation of these neurones through inner hair cells. For example, safety dictates that the maximum stimulation amplitude in CIs is limited. This leads to an unnaturally narrow dynamic range. The difference between acoustic threshold and maximum or uncomfortable levels can exceed 100 dB for normal hearing. In contrast, the difference between electrical threshold and the maximum tolerated electrical stimulation is typically between 5‒35 dB, depending on pulse duration and additional factors [13, 14]. This limits the ability of the device to encode intensity differences. Another major difference is in the resolution of frequency. The hair cell / auditory neurone ensemble can transmit extremely small frequency differences. In contrast, electrical stimulation is limited in distinguishing frequency differences. Empirical information suggests that performance improves only up to about eight separate electrodes (channels) for electrical stimulation [15, 16]. An additional difference is the lack of spontaneous activity in the auditory nerve after receptor hair cell loss [15]. Modulation of spontaneous activity appears to be an important mode of coding for acoustic stimuli, which is lost in CI patients.
Direct stimulation of spiral ganglion neurones differs substantially from the situation in normal hearing. Therefore coding strategies need to reflect this difference in order to mimic normal hearing. Sound detected by the speech processor microphones must be transformed into a set of stimuli that can be interpreted by the nervous system. Current CIs feature 12 to 22 physical electrodes inserted into the scala tympani and these electrodes plus those created virtually can hardly replace the function of about 4,000 auditory inner hair cells normally stimulating afferent spiral ganglion neurones. Fast microprocessors within the speech processor have enabled sophisticated signal processing of sounds that are picked up by microphones. Thus, substantial progress in the way the processors work has contributed considerably to the success of modern CI systems.
To accommodate the limited dynamic range of CI stimulation, the processor must compress the sound intensity range into the stimulation electrical range before the signals are further processed for frequency content in specific frequency bands. One of the most successful and widely used sound processing strategies used in current CIs is the “continuous interleaved sampling” (CIS) strategy introduced by Wilson et al. in 1991 [17]. CIS is normally used for 12 or fewer channels of stimulation. The advanced combination encoder (ACE) strategy chooses 8 to12 out of a number of electrodes, typically 22, to stimulate, based on amplitude criteria [18]. For both techniques, the electrical amplitude modulation is set based on a non-linear relationship to sound intensity within each frequency band. The output of each band is applied to the respective electrode. Simultaneous stimulation of more than one electrode is avoided to prevent unfavourable electrical interactions.
The last stage of the mapping required in the speech processor requires adjustment of the stimulation range to the subjective auditory perceived thresholds and maximum comfortable levels. These change over time, rapidly at first, as the central nervous system accommodates to the new signals, becoming more or less stable after 6 months. Then other speech processor parameters (external noise reduction, direction focused microphones, etc.) can be adjusted to satisfy the individual needs of the patient. Thus, frequent adjustment of the speech processor parameters is required, especially during the first 12 months after implantation. Intraoperatively obtained electrophysiological thresholds (stapedius reflex and neural response telemetry) can be used to aid this fitting process.
The following paragraphs summarise the current guidelines for cochlear implantation established by the Swiss Society of Oto-Rhino-Laryngology, Head and Neck Surgery. Moreover, the results presented here are derived from the data in the Swiss cochlear implant registry if not otherwise specified. This registry contains basic data from all five CI centres in Switzerland (Basel, Bern, Lucerne, Geneva and Zürich) about all CI surgeries performed in Switzerland since the first implantation in 1977.
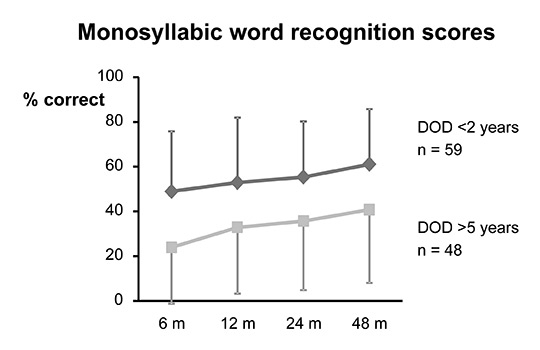
Figure 3
Speech recognition (tested at 80 dB sound pressure level) for the Freiburg monosyllabic test in 107 unilaterally implanted adults over 2 years after implantation. The patients are divided into two groups dependent on duration of deafness (DOD) prior to implantation [23]. Average (±1 standard deviation) monosyllabic word recognition scores are displayed over time after first fitting in months (m) for a group of 59 adult CI patients with a DOD <2 years (average 1.88 ± 1.24 years) and a group of 48 adult CI patients with a DOD of >5 years (16.1 ± 12 years).
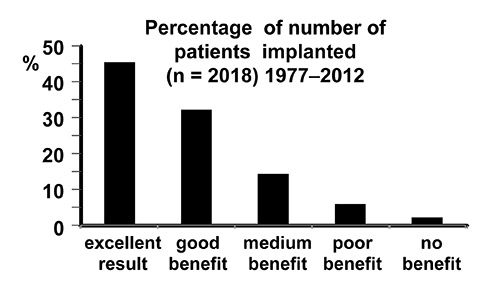
Figure 4
Subjective benefits of cochlear implant users implanted until 2012 in Switzerland. Patients with first generation single channel implants as well as patients with implant use shorter than 6 months were excluded from this summary. Patients were asked to describe their benefit on a five-point scale from “no benefit” to “excellent” [11].
Individuals with profound sensorineural hearing loss are potential candidates and should be evaluated for cochlear implantation by an experienced and specialised hospital. The evaluation includes a thorough objective and behavioural audiological assessment, imaging studies to determine the status of the inner ear and the auditory nerve, and evaluation of additional disabilities and medical conditions. Individuals with binaural hearing loss of cochlear origin, who meet the audiological criteria for implantation and cannot satisfactorily be fitted with conventional hearing aids, qualify for surgery. Preoperative evaluation and thorough counselling of candidates are crucial to ensure realistic expectations and to rule out underlying conditions potentially interfering with postoperative rehabilitation. The critical evaluation of implant candidacy and the final decision are typically the result of a team approach involving audiologists and surgeons and, for children, paediatric specialists. The goal is to enable a patient to communicate and understand speech better with their cochlear implant than with previously worn hearing aids [19, 20].
With the significant technological progress of CI systems and, thus, improving results, the guidelines for CI candidacy have broadened. In recent years, CIs have been implanted in cases with substantial residual hearing in the low frequencies (less than 1 kHz). In these cases, so-called hybrid or electro-acoustical stimulation systems are often implanted. These combine the electrical cochlear stimulation using a short CI electrode to restore high frequency loss and conventional hearing aid stimulation of the residual low frequencies. Starting in 2008, CIs have also been provided in cases of single-sided deafness and normal hearing in the opposite ear. The first results demonstrate that the brain is able to effectively integrate information from the normal hearing ear and the ear fitted with a CI [21].
In children with prelingual deafness the best results are observed if the implantation is performed early, at best before 2 years of age. Early implanted children will enter school with expressive and receptive spoken language skills similar to those of children with normal hearing [22], and the majority of these children can be enrolled in schools with normal-hearing children. In contrast, prelingually deaf children who receive the cochlear implant after 7 years of age are usually unable to obtain the same levels of spoken language skills [23]. The longer the period between the onset of deafness and cochlear implantation lasts, the greater the delay of speech development. Long periods of deafness lead to morphological changes in the auditory areas of the brain. These changes, called auditory deprivation, limit congenitally deaf individuals implanted in late childhood or adulthood from obtaining the similar benefits as individuals implanted at an early age [24].
The majority of postlingually deafened children and adults are excellent candidates for cochlear implantation. However, the time delay between onset of deafness and cochlear implantation affects speech understanding [24, 25]. For example, a study in Bern showed that a group of CI recipients with a short duration of deafness (<2 years) had a substantially better speech perception outcome compared to a group of recipients with a longer duration of deafness (>3 years) (fig. 3) [26]. In cases of meningitic deafness, progressive fibrotic changes in the cochlea mean that the earliest possible (bilateral) implantation is the best way to ensure a smooth complete insertion of the implant electrode and a satisfactory outcome [27, 28]. There is no age limit for cochlear implantation. Implantation has been performed in patients older than 80 years. However, the biological age in the context of general health, fitness for surgery, intellectual capacity and communication skills need to be considered in the CI decision process.
Bilateral CIs offer improved hearing results compared with a unilateral CI in most of bilaterally deaf implantees. Hearing with two ears or two CIs is associated with better speech perception in noise and improved localisation of sound sources [29]. Also in quiet, an improved speech perception has been observed. On average, 10% more monosyllabic words are understood in the bilateral compared with the unilateral listening situation [30]. Bilateral cochlear implantation can be performed simultaneously in a single operation or sequentially in two separate procedures. In the case of sequential implantation, the time interval between the two implantations should be as short as possible and ideally not exceed 1 year. In Switzerland, early bilateral implantation is a standard treatment for deaf children; however, some differences regarding the timing of surgery (sequential vs simultaneous implantation) can be found across the five CI centres. For this reason, among those children implanted over the past 10 years in Switzerland with an implant in the contralateral ear within 5 years of the first implant, 47.8% out of a total of 159 implants (status December 2012) received the second implant within 3 months and 34.6% simultaneously with the first operation. This resulted in a relative uniform distribution of second (bilateral) implantation times over the 5 years.
Although CIs can restore speech understanding to a high level, they cannot provide normal hearing. Sound is perceived differently with CIs. In contrast to normal hearing or hearing with conventional hearing aids, the auditory nerve is stimulated electrically and the interpretation of this new perception needs to be trained thoroughly, a process that may require several months to years. Therefore, follow-up care and guided rehabilitation is essential for recipients of CIs to ensure the best achievable outcome. The speech processors are typically fitted for the first time around 3–6 weeks after surgery. In the following months, there is a steep learning curve in auditory performance, which typically reaches a plateau after 1–2 years (fig. 3) [31].
More than 70% of the patients implanted in Switzerland describe their benefit as excellent or good [11] (fig. 4). This reflects the performance in audiological testing. More than half of the tested adults reach over 50% word recognition in the Freiburger monosyllabic test [11]. In children, more than 80% reach word discrimination scores between 80%–100% in the Monosyllabic-Trochee-Polysyllabic-Word Test and 82% score between 60%–100% in the MAIS-Test (Meaningful Auditory Integration Scale) [11].
Although the occurrence of bacterial meningitis has been reported following cochlear implantation, this medical complication is extremely rare. Nevertheless, all CI candidates need to be counselled about this rare complication, and vaccination against the most prevalent causal organism, Streptococcus pneumoniae, should be recommended to the patient [32].
Special caution is warranted if a CI user should undergo magnetic resonance imaging (MRI). If feasible, computed tomography (CT) should be used. If CT is not considered adequate, the CI manufacturer’s guidelines regarding MRI investigations need to be followed carefully. Even if an MRI is technically possible when the CI manufacturer’s guidelines are followed (use of 1.5 Tesla scanner, removal of speech processor, head bandaging to hold the implant securely) warranty issues may limit MRI investigations in some radiology institutions. Consultation with the manufacturer concerning specific recommendations according to the type of CI or at least a review of the company’s website is strongly recommended before performing MRI.
Electrosurgical instruments are capable of inducing currents that can harm the CI electronics. Therefore, monopolar electrosurgical instruments cannot be used in patients with a cochlear implant. However, bipolar electrosurgical instruments may be used. The cautery electrodes must not contact the implant.
Despite the outstanding success of cochlear implants, new innovations are possible as indicated below.
Recent publications [21, 33, 34] indicate that patients can benefit from a CI in an ear with profound hearing loss even if normal hearing is present in the opposite ear. These patients show a significantly higher speech perception in background noise and improved localisation ability compared with users of conventional CROS (contralateral routing of signal) hearing aids, BAHA (bone-anchored hearing aid) CROS or untreated patients. CROS hearing aids and BAHA simply bypass the deaf ear by routing the signals from the deaf side to the contralateral normal-hearing ear. In contrast, a CI stimulates the spiral ganglion neurones on the deaf side, thereby adding a second hearing ear to the brain. A multicentre study in Switzerland is currently aiming to further clarify the benefits of cochlear implantation for patients with single-sided deafness.
Fully implantable cochlear implants would be a significant improvement in terms of comfort and cosmetics. However, the main challenge in developing such an implant consists of creating a subcutaneous microphone which is not dominated by internal body noise. At the moment there is no approved fully implantable cochlear implant system available, but prototypes have been implanted in clinical trials outside of Switzerland [35].
The number of effective channels used in CIs is limited through overlapping electrical fields, which are a consequence of the anatomical distance between the stimulating electrodes and the spiral ganglion neurones in the cochlea. A gapless interface between the electrodes and the neurones could substantially reduce channel interaction and thereby allow a substantial increase in the number of independent channels. One way to achieve this goal would be to attract the peripheral processes of the spiral ganglion neurones towards the electrodes using growth factors released from the implant surface. If successful, such an approach may substantially improve the quality of hearing with CIs in the future [37].
Another way to increase the number of effective channels would be to change the mode of electrical stimulation so that the overlap of electrical fields is reduced. At present, monopolar stimulation is mostly used where one intracochlear electrode is stimulated versus a remote reference electrode. Using simultaneously more than one intracochlear electrode with various phase and amplitude values, so called bipolar, tripolar, quadrupolar or phase array configurations, may help to reduce current spread.
Cochlear implants have become highly successful neuroprosthetic devices in the last 20 years. In Switzerland, over 2000 CIs have been implanted since the first implantation in 1977. Current CI systems can effectively restore hearing in the majority of deaf individuals to a degree that speech can be understood, even on the telephone and without the help of visual cues. Thus, the impact on the patient’s quality of life has been exceptional. The surgical procedure can be regarded as being safe and standardized with minimum associated risks. Despite this success, limitations remain, leaving room for research developments to further improve future cochlear implants.
1 Davis AC, Davis K. Descriptive epidemiology of childhood deafness and hearing impairment. In: Seewald R, Tharpe A-M, Gravel J, eds. Comprehensive handbook of pediatric audiology. San Diego, CA: Plural Publishing 2010.
2 Van Naarden K, Decouflé P, Caldwell K. Prevalence and characteristics of children with serious hearing impairment in metropolitan Atlanta, 1991–1993. Pediatrics. 1999;10:570–5.
3 Sohn W, Jörgenshaus W. Schwerhörigkeit in Deutschland. Z Allg Med. 2001;77:143–7. German.
4 Olusanya D. “The Right Stuff”: The Global Burdon of Disease. PLoS Med. 2007;4:89.
5 Mazelova J, Popelar J, Syka J. Auditory function in presbyacusis: peripheral vs. central changes. Exp Erontol. 2003;38:87–94.
6 Stach BA, Spretnjak ML, Jerger J. The prevalence of central presbyacusi in a clinical population. J Am Acad Audiol. 1990;1:109–15.
7 Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. On behalf of the Global Burden of Disease Hearing Loss Expert Group. Global and regional impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23:146–52.
8 Djourno A, Eyries C. [Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling]. Press Med. 1957;65:1417. French.
9 Eisen MD. Djourno, Eyries, and the first implanted electrical neural stimulator to restore hearing. Otol Neurotol. 2003;24:500–6.
10 House WH. Cochlear implants. Ann Otol Rhinol Laryngol. 1976;85:3–97.
11 Dillier N. Schweizerisches Cochlear Implantat Register (CI-Datenbank). HNO-Klinik Universität Zürich. 2012 (http://www.uzh.ch/orl/links/CIREG2013.pdf). German.
12 Venail F, Sicard M, Piron JP, Levi A, Artieres F, Uziel A, et al. Raliablity and Complications of 500 consecutive cochlear implants. Arch Otolaryngol Head Neck Surg. 2008;134:1276–81.
13 Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–43.
14 Shannon RV. Quantitative comparison of electrically and acoustically evoked auditory perception: implications for the location of perceptual mechanisms. Prog Brain Res. 1993;97:261–9.
15 Fun QJ, Shannon RV. Frequency mapping in cochlear implants. Ear Hear. 2002;23:339–48.
16 Rauschecker JP, Shannon RV. Sending sound to the brain. Science. 2002;295:1025–9.
17 Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–8.
18 Taft DA, Grayden DB, Burkitt AN. Across frequency delays based on the cochlear traveling wave: enhanced speech presentation for cochlear implants. IEEE Trans Biomed Eng. 2010;57:596–606.
19 Eisenberg LS, House WF. Initial experience with the cochlear implant in children. Ann Otol Rhinol Laryngol Suppl. 1982;91:67–73.
20 Waltzman, Roland JT. Cochlear implant candidates in cochlear implants, Thieme Medical Publisher, New York, NY, USA 2006.
21 Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2010;32:39–47.
22 Mohr PE, Feldmann JJ, Dunbar JL, McConkey-Robbins A, Niparko JK, Rittenhouse RK, et al. The societal cost of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2000;16:1120–35.
23 Colletti L, Mandalà M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75:504–9.
24 Kral A, O’Donoghue GM. Profound deafness in childhood. N Engl J Med. 2010;363:1438–50.
25 Stark T, Engel A, Borkowski G. Bilateral cochlea implantation in varying duration of deafness. Laryngorhinootologie. 2004;83:20–2.
26 Sikyr S. Audiologische Resultate und subjektive Beurteilung nach Cochleaimplantation im Erwachsenenalter. Inaugural-Dissertation zur Erlangung der Doktorwürde der Humanmedizin der Medizinischen Fakultät der Universität Bern 2004. German.
27 Philippon D, Bergeron F,Ferron P, Bussières R. Cochlear implantation in postmeningitic deafness. Otol Neurotol. 2010;31:83–87.
28 Merkus P, Free RH, Mylanus EA, Stokroos R, Metselaar M, van Spronsen E, et al. Dutch cochlear implant group (CI-ON) consensus protocol on postmeningitis hearing evaluation and treatment. Otol and Neurotol. 2010;31:1281–6.
29 Ramsden JD, Gordon K, Aschendorff A, Borucki L, Bunne M, Burdo S, et al. European Bilateral Pediatric Cochlear Implant Forum consensus statement. Otol Neurotol. 2012;33:561–5.
30 Müller J, Schön F, Helms J. Speech understanding in quiet and noise in bilateral users of the MED-EL COMBI 40/40+ cochlear implant System. Ear Hearing. 2002;23:198–206.
31 Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Long-term performance of cochlear implants in postlingually deaf adults. Otolaryngol Head Neck Surg. 2012;147:112–8.
32 Lalwani AK, Cohen NL. Does meningitis after cochlear implantation remain a concern in 2011? Otol Neurotol. 2012;33:93–5.
33 Arndt S, Laszig R, Aschendorff A, Beck R, Schild C, Hassenpass F, et al. Einseitige Taubheit und Cochlearimplantatversorgung. HNO 2011:59;437–46.
34 Jacob R, Selzig Y, Nopp P, Scheich P. Audiological results with cochlear implants for single-sided deafness. HNO 2011:59;453–60.
35 Briggs RJ, Eder HC, Seligman PM, et al. Initial clinical experience with a totally implantable cochlear implant research device. Otol Neurotol. 2008;29:114–9.
36 Wilson BS, Dorman MF. Cochlear implants: current designs and future possibilities. J Rehabil Res Dev. 2008;45:695–730.
37 Wilson BS, Lawson DT, Muller JM, Tyler RS, Kiefer J. Cochlear implants: some likely next steps. Annu Rev Biomed Eng. 2003;5:207–49.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.