A revival of parabiosis in biomedical research
DOI: https://doi.org/10.4414/smw.2014.13914
Alexander
Eggel, Tony
Wyss-Coray
Summary
Modern medicine wields the power to treat large numbers of diseases and injuries most of us would have died from just a hundred years ago, yet many of the most devastating diseases of our time are still untreatable. Chronic conditions of age such as cardiovascular disease, diabetes, osteoarthritis or Alzheimer’s disease turn out to be of a complexity that may require transformative ideas and paradigms to understand and treat them. Parabiosis, which is characterised by a shared blood supply between two surgically connected animals, may just provide such a transformative experimental paradigm. Although forgotten and shunned now in many countries, it has contributed to major breakthroughs in tumour biology, endocrinology and transplantation research in the past century. Interestingly, recent studies from the United States and Britain are reporting stunning advances in stem cell biology and tissue regeneration using parabiosis between young and old mice, indicating a possible revival of this paradigm. We review here briefly the history of parabiosis and discuss its utility to study physiological and pathophysiological processes. We argue that parabiosis is a technique that should enjoy wider acceptance and application, and that policies should be revisited to allow its use in biomedical research.
Parabiosis – an experimental model inspired by nature
Parabiosis (from the Greek words, para “alongside” and bios “life”) describes the union between two living organisms that share a common vascular system. Such pairings are generated experimentally by surgery, although a natural form of parabiosis can occur owing to abnormal development of embryos in monozygotic twins in some mammalian species, resulting in conjoined individuals, also known as Siamese twins in humans. The experimental technique to establish parabiosis in animals was first introduced by the French physiologist Paul Bert in the 1860s using white albino rats in an attempt to understand and facilitate organ transplantation. More than 1,700 articles related to parabiosis have been published by groups around the globe (source: http//www.gopubmed.org) since Bert’s original dissertation, with a publication peak between 1960 and 1980 (fig. 1).
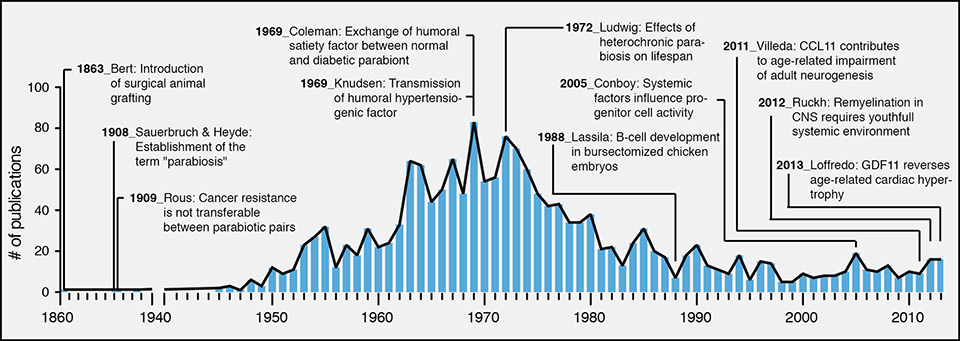
Figure 1
Parabiosis history and modern use.
The annual number of publications including parabiosis experiments is shown from 1860 to 2013. Several studies are highlighted as they provided groundbreaking findings. All values have been extracted from http://www.gopubmed.org.
CCL11 = eotaxin; CNS = central nervous system; GDF11 = growth and differentiation factor 11
Although mice are being used preferentially today, the surgical procedure still follows Bert’s initial descriptions, in general by generating skin incisions extending along the adjoining flanks of two mice and suturing adjacent skin flaps between the animals. In current protocols the incisions are longer and typically extend along the whole body flank. Moreover, the peritoneum is incised and sutured together between the animals to form a common peritoneal cavity. In some models the limbs are also sutured at the joints in order to increase stability (see Conboy et al. [1] for a detailed protocol of the procedure). As a result of revascularisation of the injured tissue, blood vessels between the two animals join to form anastomoses and establish a joint vascular system (fig. 2). By using genetically identical animals there is no “tissue” rejection, and survival rates similar to other invasive surgical procedures (>80%) can be attained in mouse parabionts. Once established, pairs of mice or rats can live together for months or years [3]. Besides mice and rats, axolotls [4] have been used for parabiosis studies and, recently, embryonic tissues in amphibians and fish have been joined to study developmental processes [5].
Early scientific breakthroughs facilitated by parabiosis
In his doctoral thesis, “la greffe animale,” Bert sutured the skin of two albino rats at their flanks and found that intravenously administered fluids passed from the circulation of one animal into the bloodstream of its adjacent partner. He therefore postulated that surgically connected animals spontaneously develop a single, shared circulatory system (fig. 2) [6]. For his pioneering work, Bert was awarded the Prize of Experimental Physiology of the French Academy of Science in the year 1866. Thereafter, very few studies followed up on his approach until the early 20th century.
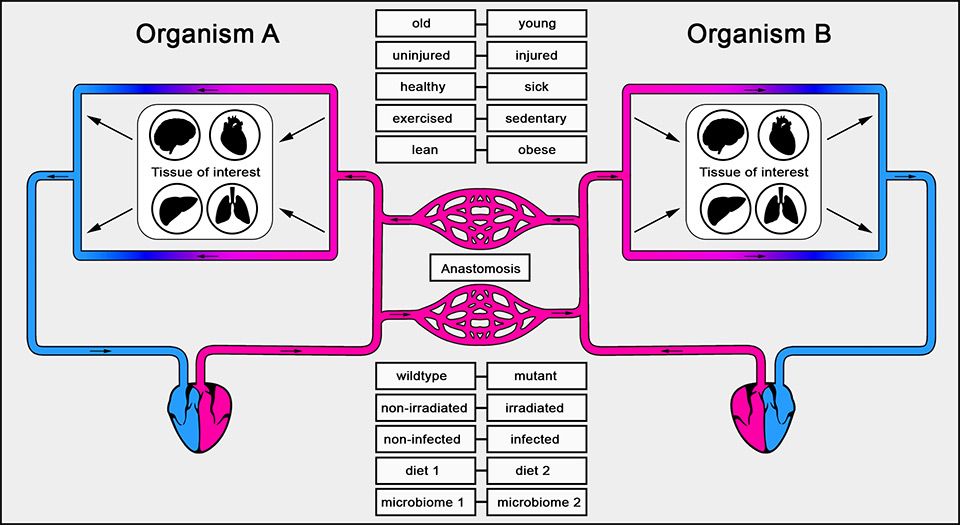
Figure 2
Circulatory system in parabiosis.
Organism A and B share a common blood supply, which spontaneously develops through anastomosis after surgery. Organisms with different physiological conditions may be used for parabiosis in order to assess the systemic effect of one organism on a particular tissue of interest in its attached partner.
In 1908, the German surgeons Sauerbruch and Heyde revived the technique and introduced the term parabiosis for the artificially established symbiosis between two animals [7]. Researchers from a variety of different fields (e.g., endocrinology, metabolism, transplantation, nephrology, radiology, allergy and immunology) started to take advantage of the parabiosis model for their own scientific investigations (table 1). A main question at the time was whether transmissible, humoral factors present in one animal have a physiological effect on its adjacent partner. Rous, who won a Noble Prize in 1966 for his discovery of tumour-inducing viruses, used parabiosis to examine whether the presence of circulating anticancer antibodies in tumour-resistant rats would affect tumour susceptibility in attached nonresistant rats. He did not succeed in identifying such protective humoral anticancer factors in these experiments [8], but parabiosis was instrumental in his early studies. By the middle of the last century, parabiosis had become a key research tool for dissecting the function of endocrine factors and growth and sex hormones, and for understanding the communication between the endocrine glands, as detailed in an extensive review on the early successes of parabiosis by Finnerty [9].
In 1969, Coleman grafted mice with the mutation diabetes (db/db), which are prone to become obese and develop type II diabetes, to inbred wild-type mice [10]. He initially hypothesised that the db/db mouse would lose weight upon exposure to a systemic environment of a non-obese mouse. Surprisingly, he observed that the wild-type mouse significantly decreased food intake while the obese mouse continued to gain body weight. Coleman concluded that there must be a satiety factor involved, to which only the wild-type but not the db/db mouse had been able to respond [11]. Almost three decades later, Friedman finally identified this satiety factor and called it leptin [12]. Today, leptin is known as one of the key hormones regulating body weight. Shortly after this remarkable discovery, Friedman and Leibel found that the db gene encodes for the leptin receptor and that mutations in this gene result in a nonfunctional molecule [13, 14]. This finding, which earned Coleman and Friedman the 2010 Lasker Award, clearly confirmed Coleman’s interpretation of his earlier experiments and underlines the importance of parabiosis models for the identification of new transmissible, humoral factors.
In 1969, another remarkable study using parabiotic pairings was performed by Lewis K. Dahl’s group [15]. They grafted wild-type rats to partners with constitutional predisposition for hypertension. As a result, they found that renoprival hypertension occurred in both rats at the same frequency. Again, this finding pointed towards a humoral factor inducing hypertension in the wild-type animal. Additionally, they described that nephrectomised rats with a predisposition to develop hypertension did not induce higher blood pressure in the wild-type parabiont, suggesting that the factor is produced in the kidney of hypertensinogenic rats. The presence of this factor has subsequently been confirmed in other studies [16] and, in 1993, Lewanczuk et al. identified it as parathyroid hypertensive factor (PHF) [17].
Parabiosis was not only helpful in the discovery and study of individual humoral factors, but it has also been useful in the assessment of the physiological consequences in an organism after exposure to the systemic environment of its attached partner. Initially, parabiotic surgeries showed highest success rates when using young, sex- and age-matched littermates. Over time, the procedure has improved and, in the early 70s, scientists started to graft animals of different ages to each other. This heterochronic parabiosis set the basis for the investigation of effects induced through exposure of an aged organism to a youthful systemic environment. In their studies, Ludwig and Elashoff particularly focused on the extension of lifespan in the old heterochronic parabiont when attached to a young counterpart. Indeed, in 1972 their results provided the first evidence that the old organism in the heterochronic pairing lived longer in response to the young environment than the age-matched isochronic control animals [18]. Later, as we will discuss, this model proved critical to study the physiology of aging and stem cells in different tissues and organ systems.
|
Table 1: Selected biological phenomena and humoral factors investigated by parabiosis. |
|
Biological phenomenon
|
Involved humoral factor(s)
|
Animal species
|
Year
|
Reference
|
| Tumour grafting |
Unidentified |
Rat |
1909 |
[8] |
| Rejection of skin grafts |
Unidentified |
Rat |
1921 |
[41] |
| Regeneration of nerves |
Unidentified |
Rat |
1923 |
[42] |
| Control of mammary growth during pregnancy |
Unidentified |
Rat |
1927 |
[43] |
| Regulation of gonadal hormone secretion |
Folliculin |
Rat |
1930 |
[44] |
| Growth stimulation in genetic dwarfism |
Pituitary growth hormones |
Mouse |
1945 |
[45] |
| Amelioration of hyperglycaemia in diabetes mellitus |
Insulin |
Rat |
1947 |
[46] |
| Mitotic activity in hepatectomised parabionts |
Unidentified |
Rat |
1951 |
[47] |
| Regulation of energy intake and expenditure |
Satiety factor (later termed leptin) |
Mouse |
1969 |
[10] |
| Regulation of blood pressure |
Rinoprival hypertension factor (later termed PHF) |
Rat |
1969 |
[15] |
| Extension of lifespan after radiation |
Multiple undefined factors |
Rat |
1972 |
[18] |
| Regeneration of muscle and liver |
Notch ligand (Delta) |
Mouse |
2005 |
[21] |
| Regulation of neurogenesis and cognitive function |
Eotaxin (CCL11) |
Mouse |
2011 |
[30] |
| Increased pancreatic β-cell replication |
Unidentified humoral factors |
Mouse |
2013 |
[48] |
| Regulation of cardiomyocyte hypertrophy |
Growth differentiation factor 11 (GDF11) |
Mouse |
2013 |
[38] |
| Remyelination of central nervous system |
Unidentified |
Mouse |
2013 |
[31] |
A revival of parabiosis for the study of stem cells and tissue regeneration
In spite of these remarkable findings by the end of the last century, parabiosis had fallen out of favour with the research community and only a handful of papers reported use of the technique (fig. 1). It was at that time when Drs Weissman, Wagers and Rando “rediscovered” parabiosis at Stanford University for the study of stem cell engraftment and trans-differentiation [19, 20], as well as tissue regeneration in the aged organism [21]. Different studies have shown that the regenerative capacities of tissues and organs are dependent on the proliferative activity of progenitor cells derived from tissue-resident stem cells [22–26]. A major hallmark of aging is that the regenerative properties significantly decline in most tissues. This has been partially attributed to impaired stem cell function [27–29]. However, whether these age-related effects were due to cell intrinsic changes or alterations in the microenvironment of stem cells required further investigation. In 2005, Conboy et al. used heterochronic parabiosis experiments to address this question. They showed that factors derived from the young systemic environment are able to activate molecular signalling pathways in hepatic or muscle stem cells of the old parabiont, leading to increased proliferation and tissue regeneration [21]. These findings strongly suggest that the age-associated impairment of stem cell function is induced to a significant extent by the molecular composition of the surrounding niche rather than by cell intrinsic changes alone.
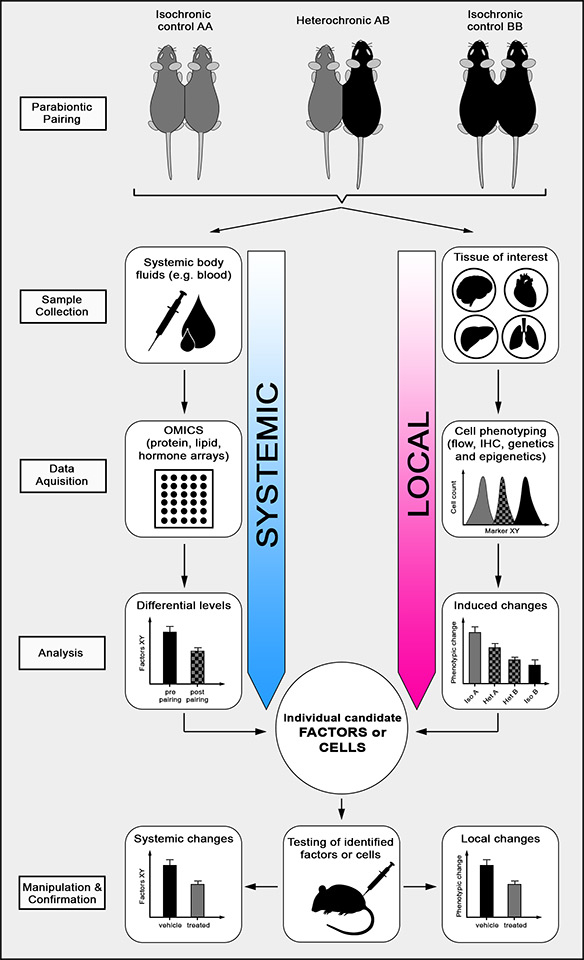
Figure 3
Heterochronic parabiosis.
Using heterochronic pairings of young (A) and old (B) mice allows assessment of the effect of a young systemic environment on a particular local tissue of interest in the aged partner and vice versa. Isochronic pairings (AA or BB) are important controls to exclude surgery-related observations and to determine age-related changes in the systemic environment. Briefly, systemic body fluids such as blood, lymph or cerebrospinal fluid are collected, assessed with omics tools such as protein, lipid or hormone arrays and analysed for differential levels of soluble factors and after parabiosis. A particular tissue of interest is isolated, phenotypically characterised by flow cytometry, immunohistochemistry (IHC) or epi-/genetic measures and analysed for parabiosis-induced phenotypic changes. The integration of these data leads to the identification of individual candidate factors or cells that can subsequently be tested in a suitable mouse model.
In line with these remarkable findings, in 2011 our group showed that an old systemic environment can be detrimental for neural stem cell function and negatively regulate adult neurogenesis in brains of young heterochronic parabionts. This led to the discovery that factors in old blood are sufficient to decrease synaptic plasticity and impair contextual fear conditioning and spatial memory. Using a systematic proteomic approach (fig. 3), we were able to identify soluble factors that were significantly increased in blood plasma of old mice and humans. One of these factors was the chemokine CCL11 (eotaxin), a chemokine known to attract eosinophils to tissues. Indeed, systemic administration of CCL11 was sufficient to induce impaired adult neurogenesis in young mice [30]. Again, these findings provide evidence that the age-related decline in stem cell function can be attributed to changes in the systemic environment. Three more recent publications using heterochronic parabiosis further support this conclusion. Ruckh et al. reported that recovery from experimentally induced demyelination in the central nervous system (CNS) is enhanced in old mice that were exposed to a young systemic environment [31]. Salpeter and colleagues showed that the decline in pancreatic β-cell proliferation in old mice can be reversed in old parabionts paired with young mice [32]. And most recently, Loffredo et al. demonstrated that age-related loss of normal cardiac function leading to diastolic heart failure is partially due to the lack of certain circulating factors in old mice. Specifically, the authors reported that cardiac hypertrophy was reversible upon exposure of an aged animal to a youthful systemic environment through heterochronic parabiosis, and that growth and differentiation factor 11 (GDF11), which is significantly reduced in the blood plasma of old mice, has a critical role in this process.
The promise of parabiosis for regenerative medicine and the study of age-related diseases
The value of parabiosis as an experimental model is most evident for physiological or pathophysiological studies that affect the organism as a whole or that induce changes in the circulatory system. Naturally, such (patho)physiological studies are most relevant to understanding the complexity of higher organisms and disease processes, but they are also the most challenging to conduct and they cannot be replaced by in-vitro experiments. Indeed, it becomes increasingly evident that many diseases and biological processes, including aging, result in organism-wide, systemic changes contributing to local tissue alterations. Thus, studying an individual organ or cell type in isolation may not lead to a holistic understanding of events. This shift in thinking has been particularly noticeable with respect to the brain, where decades of neuroncentric research has started to give way to include studies on other brain cell types as critical regulators of cognition and disease. Moreover, a growing number of studies document effects of factors outside the brain including gut microbiota, diet and other systemic changes on CNS function [33–37].
Parabiosis is an ideal tool to investigate whether alterations occurring in an organism as a consequence of disease, aging, genetic background, infection, diet, exercise, etc. might result in circulatory changes altering the status of a healthy, young, uninfected or exercised organism (fig. 3). Thus, parabiosis may help in assessing the effects of any number of functional states of one organism on a partner organism through a shared circulatory system. This is, of course, only a first step in linking particular factors or cells to a newly discovered transmissible effect. But as the above cited reports show, it has indeed been possible to identify cells that regenerate an injured brain [31] or proteins that induce satiety [11], regenerate an aging heart [38] or accelerate aspects of brain aging [30]. A generalised approach to reveal such factors or cells using heterochronic parabiosis is to analyse systemic changes and correlate them with local alterations in a particular tissue of interest (fig. 2). Whether the identified candidates are necessary or sufficient to induce pathophysiology may subsequently be assessed by exogenous application or neutralisation as well as endogenous overexpression or ablation experiments in suitable animal models.
As many of the major untreatable diseases of our time are chiefly dependent on aging, understanding them will require more insight into the systemic changes and the resulting molecular alterations occurring with age. Animal models can replicate many aspects of chronic diseases including heart disease, stroke, or neurodegeneration, yet we know very little about the contribution of the systemic environment and aging to these conditions. Parabiosis, and heterochronic parabiosis in particular, could help answer some of the fundamental questions in this regard: are circulatory factors or cells in a young organism protective against age-related disease, and vice versa, are factors or cells in the old organism predisposing or promoting disease in a younger organism? Parabiosis between mutant mice genetically manipulated to develop disease and age-matched or heterochronic, wild-type littermates or between other genetically engineered mice can help address the importance of systemic factors in the disease process. Variations of this paradigm can help elucidate pathways and mediators in many other conditions (fig. 3).
A revived future for parabiosis
Given the numerous breakthroughs, distant and very recent, it is curious then as to why this model is not being used more widely across different countries. In more recent history, most of the parabiosis studies have been conducted in the United States and in Japan, whereas only a few publications originate from Europe and only one, for example, from Switzerland (fig. 4) [39]. While there may not be laws or rules that would outlaw the procedure in certain places, it is possible that a visceral reaction towards the idea of surgically connected mice has prevented animal care committees from approving studies involving parabiosis. Based on our years of experience with this model, we have observed the well-being of paired mice far exceeds that of mice exposed to many pathogens, cancer, traumatic injuries or debilitating mutations. In fact, once they recover from surgery, mice conjoined by parabiosis regain their presurgical weights, their hormonal and endocrine profiles normalise and they start building intact nests together (our unpublished observations), an indicator of overall well-being in mice [40]. In our hands, such pairs can live together well beyond a year after the surgery although, to our knowledge, no controlled longevity studies have been done in mice. In a large rat study conducted in the mid-70s to assess the effect of whole body radiation on survival, only 1% of the pairs died as a result of the surgeries. Importantly, control pairs of nonirradiated age-matched parabionts survived on average almost 2 years following surgery and showed only a 10% reduction in lifespan compared with normal, unpaired rats [3]. This study underlines again that the procedure is very well-tolerated in rodents and has minimal impact on life expectancy. Believing strongly in the benefits careful animal experimentation can provide towards relieving human suffering, while also appreciating the importance and value of oversight in the ethical use of animals, we advocate that parabiosis should once again be considered a viable procedure for the study of some of the most devastating diseases that affect humankind.
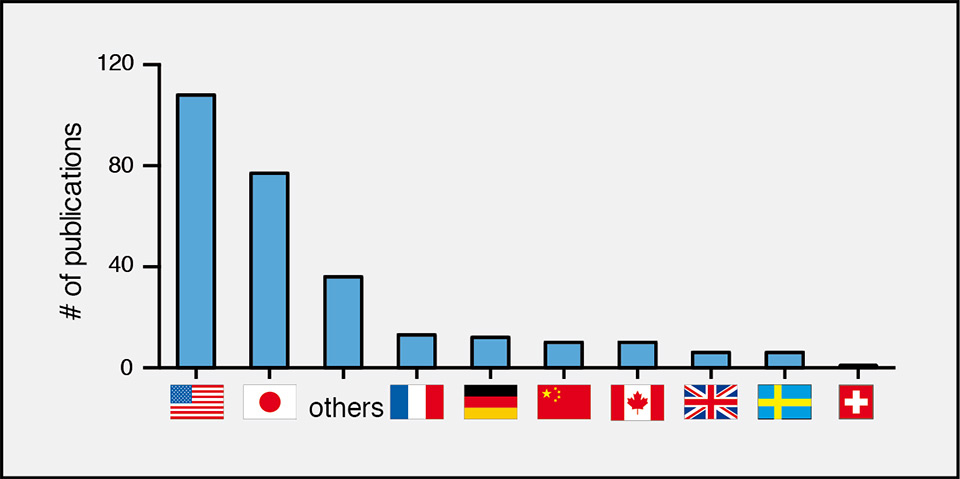
Figure 4
Parabiosis in different countries.
Publications including parabiosis experiments are listed for different countries. All values have been extracted from http://www.gopubmed.org
Acknowledgement:We thank Drs Joseph M. Castellano, Kira Mosher, and Jinte Middeldorp, for insightful comments on the manuscript.
References
1 Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12(3):525–30.
2 Kamran P, Sereti K-I, Zhao P, Ali SR, Weissman IL, Ardehali R. Parabiosis in mice: a detailed protocol. J Vis Exp. 2013;(80).
3 Warren S, Chute RN, Porter MW. The effect of parabiosis on life-span of rats stressed by radiation. J Gerontol. 1975;30(1):15–21.
4 Harris WA. Axonal pathfinding in the absence of normal pathways and impulse activity. J Neurosci. 1984;4(4):1153–62.
5 Demy DL, Ranta Z, Giorgi J-M, Gonzalez M, Herbomel P, Kissa K. Generating parabiotic zebrafish embryos for cell migration and homing studies. Nat Meth. 2013;10(3):256–8.
6 Bert P. De la greffe animale. 1863 Aug 8.
7 Sauerbruch F, Heyde M. Über Parabiose künstlich vereinigter Warmblüter. Munch. Med Wchnschr. (55):153–6. German.
8 Rous P. Parabiosis as a test for circulating anti-bodies in cancer: first paper. J Exp Med. 1909;11(6):810–4.
9 Finerty JC. Parabiosis in physiological studies. Physiol Rev. 1952;32(3):277–302.
10 Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217(5):1298–304.
11 Coleman DL. A historical perspective on leptin. Nat Med. 2010. pp. 1097–9.
12 Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32.
13 Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5.
14 Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–6.
15 Knudsen KD, Iwai J, Heine M, Leitl G, Dahl LK. Genetic influence on the development of renoprival hypertension in parabiotic rats. Evidence that a humoral hypertensinogenic factor is produced in kidney tissue of hypertension-prone rats. J Exp Med. 1969;130(6):1353–65.
16 Hirata Y, Tobian L, Simon G, Iwai J. Hypertension-producing factor in serum of hypertensive Dahl salt-sensitive rats. Hypertension. 1984;6(5):709–16.
17 Lewanczuk RZ, Pang PK. The occurrence of parathyroid hypertensive factor (PHF) in Dahl rats. Am J Hypertens. 1993;6(9):758–62.
18 Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34(7):582–7.
19 Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–9.
20 Sherwood RI, Christensen JL, Weissman IL, Wagers AJ. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells. 2004;22(7):1292–304.
21 Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4.
22 Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–5.
23 Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21(7):763–70.
24 Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–9.
25 Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6(11):1229–34.
26 Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96(25):14482–6.
27 Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–6.
28 Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192(9):1273–80.
29 Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33.
30 Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4.
31 Ruckh JM, Zhao J-W, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, et al. Rejuvenation of Regeneration in the Aging Central Nervous System. Stem Cell. 2012;10(1):96–103.
32 Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes. 2013 Apr 29.
33 Britschgi M, Wyss-Coray T. Systemic and acquired immune responses in Alzheimer’s disease. Int Rev Neurobiol. 2007;82:205–33.
34 Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122(4):1156–63.
35 Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012 Oct;13(10):701–12. Epub 2012 13 Sep.
36 Mattson MP. Energy Intake and Exercise as Determinants of Brain Health and Vulnerabilityto Injury and Disease. Cell Metab. 2012;16(6):706–22.
37 Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36(1):32–40.
38 Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, et al. Growth Differentiation Factor 11 Is a Circulating Factor that Reverses Age-Related Cardiac Hypertrophy. Cell. 2013;153(4):828–39.
39 Lassila O, Alanen A, Vainio O, Houssaint E, Pink JR, Weber WT. B cell precursors in chick embryos surgically bursectomized at 72 h of incubation. Eur J Immunol. 1988;18(11):1867–70.
40 Deacon RM. Assessing nest building in mice. Nature protocols. 2006;1(3):1117–9.
41 Mayeda T. Untersuchungen über Parabiose mit besonderer Berücksichtigung der Transplantation und Hypernephrektomie. Langenbeck’s Archives of Surgery. Springer; 1921;167(5):295–347.
42 Morpurgo B. Nervenvereinigung an Parabioseratten. J Mol Med. Springer; 1923;2(3):129–9.
43 Ernst M. Untersuchungen über hormonale Wachstumsantriebe der Brustdrüse unter Einbeziehung des Parabioseverfahrens. Langenbecks Arch Surg. 1927;202(4):231–40. German.
44 Kallas H. Zur Frage nach der Innersekretorischen Tätigkeit des infantilen Eiersockes. Klinische Wochenschrift. 1930;(29):1345–6.
45 Weitze M. The action of the pituitary gland on the growth of mice shown by parabiosis. Acta Pathol Microbiol Scand. 1945;22(2):151–8. German.
46 Shipley EG, Meyer RK. Diabetes in parabiotic rats. Am J Physiol. 1947;148(1):185–92.
47 Bucher NLR, Scott G, Aub JC. Regeneration of the liver in parabiotic rats. Cancer Res. 1951;11(6):457–65.
48 Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic Regulation of the Age-Related Decline of Pancreatic β-Cell Replication. Diabetes. 2013;62(8):2843–8.



