Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signalling
DOI: https://doi.org/10.4414/smw.2015.14043
Summary
Novel psychoactive substances are newly used designer drugs (“internet drugs”, “research chemicals”, “legal highs”) potentially posing similar health risks to classic illicit substances. Chemically, many novel psychoactive substances can be classified as phenethylamines, amphetamines, synthetic cathinones, piperazines, pipradrols/piperidines, aminoindanes benzofurans, and tryptamines. Pharmacologically, these substances interact with various monoaminergic targets. Typically, stimulants inhibit the transport of dopamine and noradrenaline (pipradrols, pyrovalerone cathinones) or induce the release of these monoamines (amphetamines and methamphetamine-like cathinones), entactogens predominantly enhance serotonin release (phenylpiperazines, aminoindanes, para-substituted amphetamines, and MDMA-like cathinones) similar to MDMA (ecstasy), and hallucinogens (tryptamines, hallucinogenic phenethylamines) are direct agonists at serotonergic 5-HT2A receptors. Synthetic cannabinoids are another group of novel substances which all act as agonists at the cannabinoid CB1 receptor similar to THC but are chemically diverse. In particular, the relative serotonergic vs dopaminergic activity (determined by the dopamine/serotonin transporter inhibition ratioin vitro) can be helpful to predict the desired psychotropic but also the toxic effects of novel substances as well as their potential for addiction. Although the use of novel psychoactive substances mostly produces minor or moderate poisonings, serious complications occur. Serotonergic drugs (entactogens and hallucinogens) are associated with acute serotonin syndrome, hyperthermia, seizures, and hyponatremia. Dopaminergic drugs are highly addictive and acute toxicity includes prolonged stimulation, insomnia, agitation, and psychosis. Agitation, anxiety, paranoia, hypertension, and rarely myocardial infarction and renal failure are seen with synthetic cannabinoids. Treatment is supportive.
List of abbreviations
AMT alpha-methyltryptamine;
5-APB 5-(2-aminopropyl)benzofuran;
6-APB 6-(2-aminopropyl)benzofuran;
BZP benzylpiperazine;
CB cannabinoid;
2C-B 2,5-dimethoxy-4-bromophenethylamine;
2C-I 2,5-dimethoxy-4-iodophenethylamine;
m-CPP meta-chlorophenylpiperazine;
DAT dopamine transporter;
DMT dimethyltryptamine;
DOB 2,5-dimethoxy-4-bromoamphetamine;
DOI 2,5-dimethoxy-4-iodoamphetamine;
DOM 2,5-dimethoxy-4-methylamphetamine;
2-DPMP 2-diphenylmethylpiperidine;
D2PM diphenyl-2-pyrrolidinemethanol;
EMCDDA European Monitoring Centre for Drugs and Drug Addiction;
Euro-DEN European Drug Emergency Network;
3-FMC 3–fluoromethcathinone;
4-HO-MET 4-hydroxy-N-methyl-N-ethyltryptamine;
5-IAI 5-iodoaminoindane;
MDAI 5,6-methylenedioxy-2-aminoindane;
MDMA 3,4-methylenedioxymethamphetamine;
MDPV 3,4-methylenedioxypyrovalerone;
4-MEC 4-methylethylcathinone;
4-MTA 4-methylthioamphetamine;
25I-NBOMe 4-Iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine;
NET noradrenaline (norepinephrine) transporter;
PMA paramethoxyamphetamine;
PMMA paramethoxymethamphetamine;
α-PVP α-pyrrolidinopentiophenone;
SERT serotonin transporter;
TFMPP trifluoromethylphenylpiperazine;
THC tetrahydrocannabinol
Introduction
The term “novel psychoactive substances” refers to newly-misused narcotic or psychotropic drugs that may pose a threat to public health comparable to classic previously listed psychotropic substances. Typically, the novel substances or “designer drugs” are analogues or chemical derivatives of controlled substances designed to produce effects similar to the controlled substances they mimic. In contrast to classic illicit drugs (“street drugs”), novel psychoactive substances are typically sold through the Internet (“internet drugs”). The substances are misbranded as “research chemicals”, “bath salts”, “plant food”, and labelled as “not for human consumption”. The substances are typically chemically slightly different from already scheduled drugs to circumvent regulations and are therefore also termed “legal highs”. The fully synthetic drugs are mostly produced in China and South East Asia and sold world-wide by numerous Internet retail vendors often as less expensive replacements of classic stimulants or narcotics [1].
In the last years we have seen an unprecedented growth in the number of new psychoactive substances on the illicit drug market. In the European Union (EU), 41 novel psychoactive substances were identified for the first time in 2010, 49 in 2011, 73 in 2012, 81 in 2013, and 37 by April 2014 within the European Early Warning System [2]. By 2014, more than 300 novel substances have been detected since 2005. Currently, more than one new substance is identified in one of the EU countries every week. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) collects information on these substances from various national sources including police, customs, forensic laboratory networks, health care systems, event organisers, drug checks, and Internet test purchase samples. Internet snapshots are also an important method to describe trends in new substances available for online sale [3]. The EMCDDA also regularly publishes reports on the risk assessments of novel psychoactive substances (http://www.emcdda.europa.eu/activities/action-on-new-drugs). Furthermore, the European Drug Emergency Network (Euro-DEN) collects health emergency data from hospitals to look at trends in the acute harms associated with the use of novel psychoactive drugs [4]. The University Hospital of Basel participates in the Euro-DEN and we characterised several intoxications with novel drugs [5, 6] and in particular their pharmacological properties [7–9]. Data on the clinical characteristics of intoxications with novel psychoactive substances are also typically collected and reported by poison centres [10–13].
In this review I will describe the in vitro pharmacodynamics of novel psychoactive substances acting on monoamine signalling and how it relates to the clinical effects of these compounds. Several excellent publications have more comprehensively reviewed the pharmacology and toxicology of novel psychoactive substances [14, 15].
Classification of novel psychoactive substances
The new psychoactive substances can be classified based on their psychotropic effects as stimulants, empathogens/entactogens (3,4-methylenedioxymethamphetamine [MDMA, ecstasy]-like substance) or hallucinogens or according to their chemical family as phenethylamines, amphetamines, cathinones, piperazines, pipradrols/piperidines, aminoindanes, benzofurans, and tryptamines [15]. The synthetic cannabinoids include a large number of chemically diverse substances which act on the cannabinoid CB1 receptor and have mostly hallucinogenic but also some stimulant properties. Currently, the compounds are classified based on their common pharmacological action rather than similar chemical structures.
The substances exhibit a rather broad spectrum of dopaminergic, noradrenergic, or serotonergic pharmacological effects even within their chemical family [7–9, 16]. Accordingly, the psychotropic and clinical toxicological effects are also quite diverse and vary considerable from drug to drug.
Synthetic cannabinoids
Herbal mixtures (“spices”) have emerged as legal alternatives to cannabis [17]. It was initially assumed that the psychotropic effects were derived from the plants themselves. However, synthetic compounds added to the herbal blends and with action at the CB1 receptor were found to be responsible for the psychopharmacological activity [18]. “Spice” drugs have become popular alternatives to marijuana among teenagers and constitute an exceptionally large class of novel psychoactive substances [2]. Synthetic cannabinoids vary considerably in chemical structure while they uniformly act as agonists at the CB1 receptor [17]. Many of the substance names (abbreviations) typically start with the initials of the chemists (JWH or AM) followed by numbers. Most synthetic cannabinoids produce overall similar effects and toxicity to tetrahydrocannabinol (THC) [6, 17]. However, synthetic cannabinoids are thought to be associated with more severe psychosis and agitation and more sympathomimetic effects because they are more potent full receptor agonists and also lack cannabidiol, which is contained in natural THC-containing products and has anxiolytic and antipsychotic properties. In fact, agitation, aggression, paranoid thinking and anxiety are common symptoms after consumptions of synthetic cannabinoids [19, 20]. However, more recently a second generation of synthetic cannabinoids has emerged both in the US and in Europe and lead to epidemics associated with more severe toxicity including collapses, seizures, and cardiac toxicity [11]. Cases with acute kidney injury have also been described [21]. The effect of the synthetic cannabinoids is typically short-lasting compared with amphetamine-type substances [5, 20]. Short-time monitoring and supportive care is sufficient to treat most cases with intoxications. Synthetic cannabinoids are not detected by drug screening tests for THC, and highly specialised mass spectrometry techniques and time are required to document the presence of these novel compounds in biological samples [6, 22].
Phenethylamines and amphetamines
Phenethylamine is the core structure of many novel psychoactive substances (fig. 1). Amphetamines (alpha-methyl-phenyl-ethyl-amine, fig. 1) are formed by the addition of an alpha methyl group which protects against metabolism by monoamine oxidase [23]. Methylation of the terminal amine results in methamphetamine and greater CNS activity. Amphetamine and methamphetamine (fig. 1) are both classic psychostimulants which have been used clinically, recreationally and by military services since the 1930s [23]. The amphetamine-derivative MDMA (fig. 1) is not a designer drug or novel psychoactive substance as it has been used for decades. However, MDMA is still one of the most widely used recreational drugs and many novel psychoactive substances were designed to mimic its effects or as substitutes for MDMA in ecstasy pills. MDMA is the prototypical empathogen or entactogen meaning that it produces feelings of empathy or “being touched”. The drug is mostly used to enhance sociability [24]. In controlled studies, MDMA increases emotional empathy, trust, extroversion, and sociality relatively more than stimulants which mainly produce arousal and stimulation [25, 26].
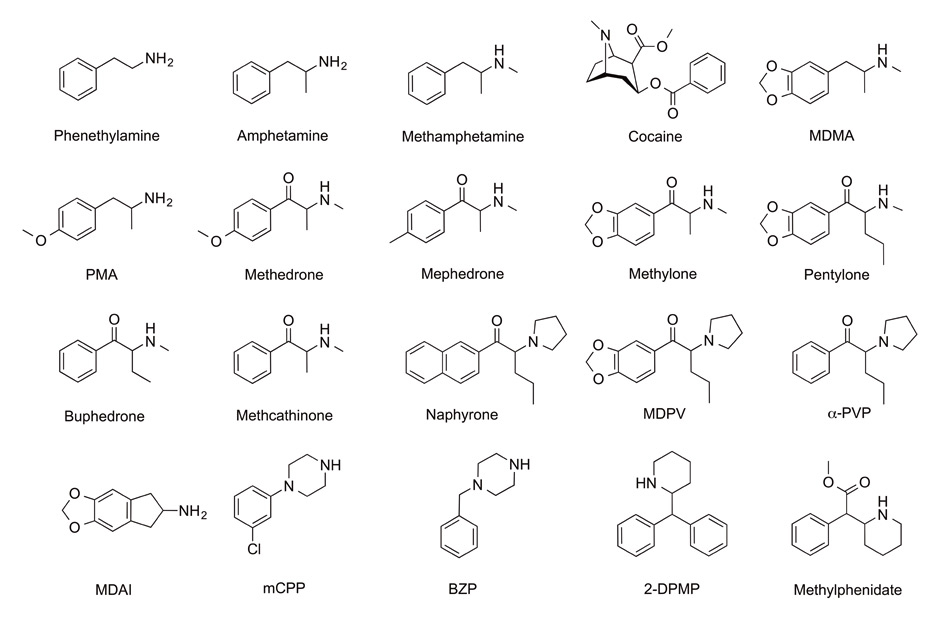
Figure 1
Structures of representative novel psychoactive substances (stimulants and empathogens). Chemically, the substances shown include amphetamines (amphetamine, methamphetamine, MDMA, PMA), cathinones (β-keto-amphetamines such as methedrone, mephedrone, methylone, pentylone, buphedrone, methcathinone, naphyrone, MDPV or α-PVP), an aminoindane (MDAI), piperazines (mCPP and BZP) and pipradrols (2-DPMP and methylphenidate). Pharmacologically, these substances are psychostimulants or empathogens and primarily interact with monoamine transporters.
Role of dopamine, serotonin and noradrenaline in the pharmacology and toxicology of novel psychoactive substances
Many novel psychoactive substances interact with biogenic amine neurotransmitter transporters. Amphetamines including methamphetamine and MDMA inhibit the dopamine, serotonin and noradrenaline (norepinephrine) transporter (DAT, SERT, NET, respectively) and also release these monoamines through the respective transporter. Methamphetamine predominantly increases dopamine and noradrenaline. MDMA mostly increases serotonin and noradrenaline. The entactogenic effects of MDMA are generally considered to depend on its serotonergic effects [27]. MDMA also increases oxytocin [25, 28] which may mediate its prosocial effects [29]. Consequently, substances which predominantly release serotonin, similar to MDMA, can be expected to produce MDMA-like entactogenic effects. MDMA and similar serotonergic substances are also associated with serotonergic toxicity including serotonin syndrome, hyponatremia, hyperthermia, and seizures [30–32]. In contrast, psychostimulants such as methamphetamine or methylphenidate are mostly enhancing dopaminergic neurotransmission [7, 33]. Dopamine mediates the reinforcing and addictive properties of drugs of abuse. In contrast, an increase in the serotonergic properties of a substance is associated with a reduced potential for addiction [34]. Consequently the relative dopaminergic to serotonergic properties in vitro (dopamine/serotonin transporter inhibition ratio) of a novel substance can be determined as a useful marker for its potential clinical psychotropic and acute toxic effects [7] (fig. 2). Serotonin release [27] and a DAT/SERT inhibition ratio of typically 0.01–0.1 are expected to result in subjective drug effects similar to those of MDMA or other empathogens [7, 8] (fig. 2). Cocaine has a DAT/SERT inhibition ratio close to unity, and methamphetamine is more selective for the DAT, with a DAT/SERT inhibition ratio >10 and mostly exerting psychostimulant effects in humans [7] (fig. 2). Compounds with a DAT/SERT inhibition ratio >1 are also associated with a high abuse potential [7]. Furthermore, all classic amphetamines and novel psychoactive substances increase the activity of the noradrenergic system [7–9], which results in the sympathomimetic cardiostimulant effects associated with all these substances. The potency of a substance to activate the noradrenergic system inversely correlates with the doses typically used recreationally [7, 35]. The profiles of novel psychoactive substances can be determined in vitro and compared with those of the classic substances where the clinical effects are known. Substances which selectively activate the catecholamine systems such as methamphetamine, or 3,4-methylenedioxypyrovalerone (MDPV) or α-pyrrolidinopentiophenone (α-PVP) [7, 36] are considered highly addictive and their acute toxicity typically includes prolonged insomnia, agitation, and psychotic symptoms [37].
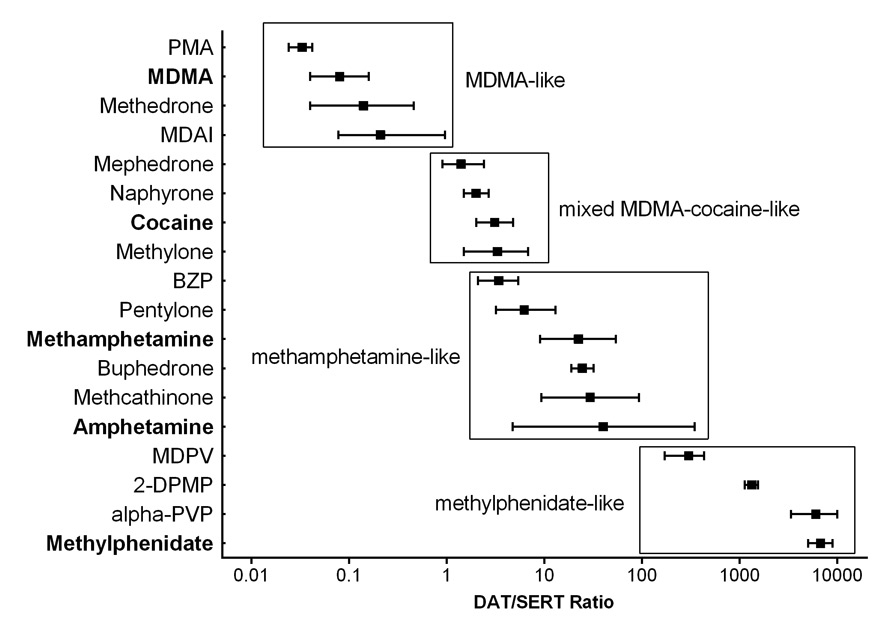
Figure 2
Relative dopamine/serotonin inhibition potencies of selected novel psychoactive substances. Dopamine to serotonin transporter (DAT/SERT) inhibition ratios (mean ± 95% confidence intervals) for novel substances are shown in comparison with those of classic empathogens/entactogens (MDMA, ecstasy) and stimulants (cocaine, amphetamine, and methamphetamine). The ratios derived from in vitro studies help to predict the typically unknown clinical toxicity of novel substances. A low DAT/SERT inhibition ratio (<0.1) indicates tenfold greater relative serotonergic vs dopaminergic activity similar to MDMA. A high DAT/SERT inhibition ratio (>10) indicates greater relative dopaminergic vs serotonergic activity similar to methamphetamine. A high DAT/SERT inhibition ratio is a pharmacological characteristic associated with more stimulant effects and with higher potential for addiction.
Para-(4)-phenyl-substituted (serotonergic) amphetamines
Paramethoxyamphetamine (PMA) and paramethoxymethamphetamine (PMMA) are typically sold as ecstasy [38]. This substitution for MDMA is unwanted because PMA and PMMA are associated with higher morbidity and mortality particularly attributable to hyperthermia [38, 39]. The para-substituted amphetamines are potent noradrenaline and serotonin transporter inhibitors and releasers of these monoamines [8]. Their hyperthermic properties are stronger than those of MDMA [40] and have been associated with serotonergic and adrenergic receptor activation [41]. Therefore, hyperthermic complications are of particular concern when these amphetamines or novel psychoactive substances with a comparable pharmacological profile are used [8]. Novel substances with a predominantly serotonergic action [8] and associated with high serotonergic toxicity are 4-methylthioamphetamine (4-MTA) [42] and methedrone (β-keto-PMMA) [43]. 4-MTA is the methylthio analog of PMA. 4-MTA produces MDMA-like effects in animals and humans and is typically used by ecstasy users [44]. Methedrone is the β-keto-substituted analog of PMMA. Methedrone is a serotonergic cathinone found in “bath salt” products.
Synthetic cathinones
Cathinones contain a ketone group at the β-position of the amphetamine (β-keto-amphetamines, fig. 1). The most commonly found synthetic cathinones are mephedrone (4-methylmethcathinone), methylone (β-keto-MDMA), 4-methylethylcathinone (4-MEC), and MDPV [45, 46]. Other abused cathinones include ethylone, methedrone, naphyrone, flephedrone, 3–fluoromethcathinone (3–FMC), pentylone, buphedrone, α-PVP, and others [7, 8, 15, 45]. The pharmacology of the various cathinones has recently been determined [7, 8, 16, 47, 48]. Cathinones can be classified based on their pharmacological profiles (mostly the DAT/SERT inhibition ratio) with relevance to their clinical toxicity [7]. The serotonergic cathinone methedrone predominantly acts on the serotonin system similar to MDMA or PMA and PMMA as noted above [8]. The cocaine-MDMA-mixed cathinons group includes substances which are roughly equipotent inhibitors of DAT and SERT similar to cocaine and release serotonin similar to MDMA. The group includes mephedrone, 4-MEC, methylone, ethylone, and butylone [7, 8]. For example, the subjective effects of mephedrone have been reported to be similar to those of cocaine [49] but also MDMA [50]. Typically, these drugs produce psychotropic effects similar to MDMA when administered orally but with enhanced psychostimulation similar to cocaine when administered intranasally. All cathinones exhibit higher dopaminergic activity when compared with their non-β-keto amphetamine analogs. This increased dopaminergic property of the cathinones suggests higher stimulant-type effects and greater risk for dependence. For example, mephedrone, which is readily self-administered by rats [51], has been reported to produce strong craving in humans and is said by users to be more addictive than cocaine [49]. In addition, substances with fast brain access have a higher risk of dependence. Naphyrone is very similar to cocaine. Both inhibit all monoamine transporters with equal potency but do not release monoamines [7]. The methamphetamine-like cathinones including cathinone, methcathinone, ethcathinone, flephedrone, and 3–FMC exhibit a DAT/SERT inhibition ratio similar to methamphetamine, with high inhibitory potencies at the DAT and low potencies at the SERT [7, 8]. These cathinones also release dopamine and noradrenaline similar to methamphetamine. Clinically, these cathinones produce similar toxicity to amphetamine, including hypertension, hyperthermia, euphoria, locomotor activation, and hallucinations following higher or repeated doses. The pyrovalerone cathinones include pyrovalerone, MDPV, α-PVP, and others. These substances are very potent and selective inhibitors of the DAT and NET, at least 10–fold more potent than cocaine or methamphetamine. The drugs are no substrates of the transporter and therefore do not release monoamines. The pyrovalerone derivatives also readily cross the blood-brain barrier because of their high lipophilicity [7]. Consistent with its high potency as a dopamine transporter inhibitor, MDPV is a potent reinforcer in rats, similar to methamphetamine [52]. Taken together these pharmacological properties predict high risks of sympathomimetic toxicity and of addiction in humans. Acute toxic effects of MDPV and α-PVP mainly include acute psychotic symptoms with agitation and hallucinations [12, 37]. Of note, the pharmacology of MDPV is strikingly different from that of MDMA, although MDPV and MDMA both share the characteristic 3,4-methylenedioxy-phenyl group. In fact, caution is needed when making predictions about the pharmacological activity of new phenetylamine-type psychoactive substances based only on the chemical structures. Pharmacological data are needed for each novel designer drug [53].
Ring substituted phenethylamines and amphetamines (2-C and 2-D series)
Addition of methoxy-groups at the 2- and 5-positions with any hydrophobic substitution at the 4-position of the phenethylamine confers hallucinogenic activity (2C-series or ring substituted phenethylamines). Example drugs of the large 2-C series are 2,5-dimethoxy-4-bromophenethylamine (2C-B) and 2,5-dimethoxy-4-iodophenethylamine (2C-I) (fig. 3). The hallucinogenic properties of these drugs are further enhanced by a methyl-group at the α-carbon (D-Series or ring substituted amphetamines or hallucinogenic amphetamines). Example drugs included in this group are 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-bromoamphetamine (DOB), and 2,5-dimethoxy-4-iodoamphetamine (DOI) (fig. 3). Nausea and tachycardia but also long-lasting hallucinogenic effects, agitation, and ergotism (vasospasms) have been reported in intoxications with these hallucinogenic amphetamines. The hallucinogenic activity of the 2-C and D series drugs is mediated by an interaction with the serotonergic 5-HT2A receptor [54].

Figure 3
Structures of representative novel psychoactive substances (hallucinogenic phenethylamines, furans and difurans, and hallucinogenic tryptamines). Chemically, the shown substances include phenethylamines of the 2-C series (2C-B, 2C-I, and 25I-NBOMe) and of the 2-D series (DOM and DOI), a bezofuran (6-APB), a benzodifuran (2C-B-fly), and tryptamines (DMT, AMT, and 4-OH-MET). Pharmacologically, all presented substances are hallucinogens and potent serotonin 5-HT2A receptor agonists except for 6-APB which is an empathogen and indirect serotonin agonist similar to MDMA.
Various N-methoxybenzyl-substituted phenethylamines have emerged on the EU drug market since 2012 [2]. These drugs such as 4-Iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (25I-NBOMe, fig. 3) exert an even higher potency at the 5-HT2A receptor and possibly also at other receptors compared with the already very potent classic hallucinogens [55]. Severe and fatal intoxications including agitation, hallucinations, seizures, and hyperthermia have been reported with 25I-NBOMe [56, 57] consistent with serotonergic but also sympathomimetic toxicity. Importantly these novel hallucinogens are extremely potent and psychoactive at microgram doses. This is likely to result in overdosing.
Benzofurans and benzodifurans
Benzofurans and benzodifurans are also groups of ring substituted amphetamines. Benzofurans containing one furan ring are 6-(2-aminopropyl)benzofuran (6-APB, fig. 3) and 5-(2-aminopropyl)benzofuran (5-APB) and several others. These drugs are structurally related to MDMA and similarly inhibit monoamine transporters [16] and release serotonin and noradrenaline (unpublished data by the author). Users report the effects of 5-APB and 6-APB to be comparable to MDMA but more intense [58]. Adverse effects include nausea, sympathomimetic stimulation, and agitation [58]. Benzodifurans are a group also known as the “fly” drugs (bromo-dragon fly, 2C-B-fly [fig. 3], etc.) and are hallucinogen. These drugs may produce paranoia, agitation, tachycardia, and hyperthermia and have been implicated in several fatalities [58].
Piperazines
Piperazines are commonly found in ecstasy pills as substitutes for MDMA [59]. The phenylpiperazines meta‑chlorophenylpiperazine (m-CPP, fig. 1) and trifluoromethylphenylpiperazine (TFMPP) are indirect and direct serotonergic agonists without dopaminergic activity. Consistently these drugs are not reinforcing [60]. m-CPP and TFMPP have less desirable psychotropic effects and more adverse effects, including dysphoria, anxiety, and nausea, compared with MDMA [61–63]. Benzylpiperazine (BZP, fig. 1) is an indirect dopamine and noradrenaline agonist without serotonergic properties [9] and exerts stimulant effects in humans [64]. The clinical toxicity of BZP mainly includes hallucinations, agitation, seizures, and hyperthermia [65]. Drug users report more unpleasant effects and hallucinations with BZP than with MDMA [61]. BZP is sometimes combined with m-CPP or TFMPP and sold as ecstasy [59] mimicking the mixed dopaminergic-serotonergic profile of MDMA. However, the BZP-TFMPP combination is not well tolerated at higher doses and produces agitation, anxiety, hallucinations, and vomiting [66].
Aminoindanes
Aminoindanes such as 5,6-methylenedioxy-2-aminoindane (MDAI, fig. 1) and 5-iodoaminoindane (5-IAI) are allegedly less-neurotoxic alternatives to MDMA. MDAI and 5-IAI release serotonin and noradrenaline comparable with MDMA [9]. Consistently the subjective effects of MDAI are also reported to be very similar to those of MDMA [67]. Complications include serotonin syndrome and hyperthermia [67], also similar to MDMA.
Pipradrols/piperidines
Intoxications with “ivory wave”, which contains the pipradrol derivative desoxypipradrol (2-diphenyl-methylpiperidine [2-DPMP, fig. 1]) or diphenylprolinol (diphenyl-2-pyrrolidinemethanol [D2PM]) have been reported starting in 2010 [68, 69]. D2PM and 2-DPMP are selective and very potent catecholamine transporter inhibitors without transporter-mediated substrate-releasing properties, similar to methylphenidate [9]. This pharmacological profile is also very similar to MDPV, α-PVP, and other pyrovalerone cathinones [7, 36] and likely associated with high abuse liability and an increased risk of psychiatric complications. The clinical toxicity of 2-DPMP and D2PM is long-lasting (24-72 h) and involves sympathomimetic stimulation with hypertension, agitation, hallucinations, and insomnia [45, 68, 69].
Tryptamines
Tryptamines contain an indole structure joined to an ethylamine group in simple tryptamines (for a classification see [15]). Natural tryptamines include serotonin and hallucinogens such as psilocybin in magic mushrooms and dimethyltryptamine (DMT, fig. 3) in Ayahuasca. Ergolines are more complex tryptamines and include the prototypic hallucinogen LSD. Commonly misused novel synthetic tryptamines (designer hallucinogens) are alpha-methyltryptamine (AMT) [13] and 4-hydroxy-N-methyl-N-ethyltryptamine (4-HO-MET) [45] (fig. 3). Tryptamine-induced hallucinations are mostly mediated by direct interaction with serotonergic 5-HT2A receptors [54]. However, many tryptamines also release serotonin [70] and in some cases dopamine and noradrenaline [71]. As a result serotonin syndrome and sympathomimetic toxicity may occur and tryptamines have stimulant as well as hallucinogenic properties [13]. Typically visual hallucinations predominate. Consistent with their action as serotonergic agonists tryptamines are not addictive [71].
Prevalence of novel psychoactive substance use
In a German survey, 9% of 15-18 year-old school students reported having smoked spice products at least once in their lives and 2% also within the last 30 days. Other “legal highs” (research chemicals, bath salts) had been used by 3% at least once and in 1% within the last 30 days [72]. In contrast, in the UK, life-time prevalence rates of mephedrone use of 20.3% have been reported from some areas in 2010 [73].
In Switzerland, the prevalence of novel psychoactive substances use is yet unknown. In 2009–2010, 37% of people using classic drugs such as amphetamines or MDMA tested positive for novel psychoactive substances in hair [74]. For example, m-CPP was found in 10.5%, mephedrone in 3%, 4-fluoroamphetamine in 4% [74]. However, many substances were possibly not yet used at the time of analysis or were not yet detectable. Considering our ongoing surveillance data collections from emergency department visits, presentations of intoxications with novel psychoactive substances are infrequent compared with those with alcohol, cannabis or cocaine [4, 75].
|
Table 1:Monoamine transporter inhibition. |
|
NET
|
DAT
|
SERT
|
DAT/SERT
|
|
Substance
|
Chemical class
|
Pharmacological class
|
IC50 [µM]
|
IC50 [µM]
|
IC50 [µM]
|
Ratio
|
| PMA |
Amphetamine |
Empathogen |
0.8 |
71 |
2.4 |
0.03 |
| m-CPP |
Piperazine |
Empathogen |
1.7 |
31 |
1.2 |
0.04 |
|
MDMA
|
Amphetamine |
Empathogen |
0.45 |
17 |
1.4 |
0.08 |
| Methedrone |
Cathinone |
Empathogen |
2.2 |
35 |
4.7 |
0.14 |
| MDAI |
Aminoindane |
Empathogen |
0.65 |
31 |
8.3 |
0.21 |
| Mephedrone |
Cathinone |
Empathogen-stimulant |
0.25 |
3.3 |
4.6 |
1.4 |
| Naphyrone |
Cathinone |
Empathogen-stimulant |
0.25 |
0.47 |
0.96 |
2.0 |
|
Cocaine
|
Cocaine |
Stimulant |
0.45 |
0.8 |
2.4 |
3.1 |
| Methylone |
Cathinone |
Empathogen-stimulant |
0.54 |
4.8 |
16 |
3.3 |
| BZP |
Piperazine |
Stimulant |
0.41 |
17 |
57 |
3.4 |
| Pentylone |
Cathinone |
Stimulant |
0.99 |
1.3 |
8.4 |
6.2 |
|
Methamphetamine
|
Amphetamine |
Stimulant |
0.06 |
1.1 |
24 |
22 |
| Buphedrone |
Cathinone |
Stimulant |
0.65 |
4.2 |
104 |
25 |
| Methcathinone |
Cathinone |
Stimulant |
0.09 |
1.1 |
33 |
30 |
|
Amphetamine
|
Amphetamine |
Stimulant |
0.09 |
1.3 |
52 |
40 |
| MDPV |
Pyrovalerone-cathinone |
Stimulant |
0.04 |
0.03 |
9.3 |
300 |
| 2-DPMP |
Pipradrol |
Stimulant |
0.14 |
0.07 |
93 |
1328 |
| PVP |
Pyrovalerone |
Stimulant |
0.02 |
0.05 |
301 |
6020 |
|
Methylphenidate
|
Pipradrol |
Stimulant |
0.13 |
0.12 |
807 |
6725 |
| NET = noradrenaline transporter; DAT = dopamine transporter; SERT = serotonin transporter. Values are concentrations at which the transporter is inhibited by 50%. A low value indicates greater potency.
DAT/SERT ratio = 1/DAT IC50: 1/SERT IC50. A low DAT/SERT ratio (<0.1) indicates tenfold greater relative serotonergic vs dopaminergic activity similar to MDMA. A high DAT/SERT ratio (>10) indicates greater relative dopaminergic vs serotonergic activity similar to methamphetamine.
Data are from Simmler et al. 2013 and 2014 and unpublished by the author (PVP) |
|
Table 2: Acute clinical toxicity associated with novel psychoactive substances. |
|
Substances
|
Classification
|
Leading acute toxicity
|
| PMA |
(Para-substituted) amphetamine, empathogen |
Serotonergic toxidrome, hyperthermia, nausea, seizures, fatalities |
| MDMA |
Amphetamine, empathogen |
Serotonergic and sympathomimetic toxidrome, hyperthermia, hyponatraemia, renal and liver failure |
| MDAI |
Aminoindane, empathogen |
Serotonergic and sympathomimetic toxidrome, hyperthermia |
| Mephedrone, methylone |
(Cocaine-MDMA-mixed) cathinone, empathogen/stimulant |
Sympathomimetic toxidrome, agitation, vomiting, psychosis, chest pain, seizures, insomnia |
| 3-FMC, flephedrone, methcathinone |
(Methamphetamine-like) cathinone, stimulant |
Sympathomimetic toxidrome, psychosis, agitation, chest pain, insomnia |
| MDPV, α-PVP |
(Pyrovalerone-)cathinone, stimulant, high potency drug |
Psychosis, agitation, combative behaviour, sympathomimetic toxidrome, chest pain, prolonged insomnia |
| m-CPP, TFMPP |
(Phenyl)piperazine, entactogen |
Serotonergic toxicity, nausea, vomiting, anxiety, headache, dizziness, dysphoria, confusion, hallucinations, tachycardia |
| BZP |
(Benzyl)piperazine, stimulant |
Mostly sympathomimetic toxicity, agitation, anxiety |
| 2-DPMP, methylphenidate |
Pipradrol, stimulant |
Sympathomimetic toxidrome, insomnia, agitation, hallucination, anxiety, insomia |
| 6-APB |
Benzofuran, empathogen |
Serotonergic and sympathomimetic toxidrome, nausea, agitation, anxiety, dizziness, hyperthermia |
| “fly” drugs |
Benzodifurans, hallucinogens |
Psychosis, agitation, hyperthermia, sympathomimetic toxicity, vasospasm, limb pain/ischaemia, seizures, fatalities |
| 2C-B |
(Ring-substituted) phenethylamine, hallucinogen |
Psychosis, agitation,vomiting, vasospasms |
| 25-I-NBOMe |
"NBOM"-phenethylamine, hallucinogen, very high potency drug |
Serotonergic and sympathomimetic toxidrome, psychosis, agitation, seizures, hyperthermia |
| AMT |
Tryptamine, hallucinogen |
Serotonergic and sympathomimetic toxidrome, psychosis, agitation, hyperthermia, nausea |
| Synthetic cannabinoids |
Cannabinoid, hallucinogen |
Psychosis, agitation, anxiety, sympathomimetic toxidrome, chest pain, myocardial infarction, renal injury, seizure, vomiting |
| Sympathomimetic toxidrome typically includes hypertension, tachycardia, mydriasis, agitation, and sweating
Serotonergic toxidrome typically includes tremor, clonus, hyperreflexia, sweating, hyperthermia, mydriasis, agitation, and confusion
Psychosis includes hallucinations and paranoia
AMT = alpha-methyltryptamine; α-PVP = alpha-pyrrolidinopentiopenone; 6-APB = 6-(2-aminopropyl)benzofuran; BZP = benzylpiparazine; 2C-B = 2,5-dimethoxy-4-bromophenethylamine; m-CPP = meta-chlorophenylpiperazine; 2-DPMP = 2-diphenylmethlpiperidine; 3-FMC = 3-fluoromethcathinone; MDAI = 5,6-methylenedioxy-2-aminoindane; MDMA = 3,4-methlylenedioxymethamphetamine; MDPV = 3,4-methlyenedioxypyrovalerone; 25I-NBOMe = 4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine; PMA = paramethoxyamphetamine; TFMPP = trifluoromethylphenylpiperazine. |
Treatment of intoxications
Table 2 lists typically reported acute medical problems associated with novel substances from each class. The clinical toxicity of novel psychoactive substances is generally similar to that of other amphetamines including MDMA. The majority of patients (85-95%) presenting at emergency departments with acute medical problems associated with the use of novel psychoactive substances are minor or moderate poisonings [12, 45, 76]. Common clinical features are hypertension, tachycardia, chest pain, agitation, and hallucinations [12, 76, 77]. Severe and fatal poisonings manifest as serotonin syndrome [67], hyperthermia [38, 78], seizures, and brain oedema due to hyponatremia [79]. These are also the severe complications of MDMA use [31, 80]. Treatment is mainly supportive. Heart rate, blood pressure, and body temperature should be monitored. Depending on clinical features laboratory tests including electrolytes, creatine kinase, liver enzymes, and cardiac enzymes may be indicated. Acute treatment of a sympathomimetic toxidrome primarily includes benzodiazepines and fluid replacement to control agitation, cardiovascular stimulation and hyperthermia. The use of haloperidol without benzodiazepines is generally not recommended because seizure and dysrhythmia thresholds are lowered and negative drug-induced psychological effects including anxiety may increase [81]. Hypertension should be treated primarily with nitrates. β-blockers should be avoided because of unopposed α-adrenergic stimulation resulting in further increases in blood pressure [82]. PhentoIamine may be useful but α-blockade increases stimulant-induced tachycardia [83]. Combined α-β-blockade with carvedilol reduced MDMA-induced elevations in blood pressure, heart rate and also body temperature [84], but it is not a routine treatment for stimulant-induced toxicity [85]. Physical cooling and relaxation may be needed in cases of severe hyperthermia, but antipyretics are of no use.
Drug screening
Most stimulant-type novel psychoactive substances are not detected by standard immunoassay urine drug screens. Thus, intoxication would typically present as sympathomimetic or serotonergic toxidromes with a negative screening result for amphetamines. Ideally, urine and blood should be sampled and analysed by liquid chromatography-mass spectrometry by a specialised laboratory. Typically the identity of the novel psychoactive substances will not be readily available for the management of the acute intoxication. Nevertheless, the novel psychoactive substances should be identified to better document the substances and their associated toxicity.
Conclusion
Stimulants inhibit the transport of dopamine and noradrenaline (pipradrols, pyrovalerone cathinones) or induce the release of these monoamines (amphetamines and methamphetamine-like cathinones), entactogens predominantly enhance serotonin release (phenylpiperazines, aminoindanes, para-substituted amphetamines, and MDMA-like cathinones) similar to MDMA, and hallucinogens (tryptamines, hallucinogenic phenethylamines) are direct agonists at serotonergic 5-HT2A receptors. Synthetic cannabinoids act as agonists at the cannabinoid CB1 receptor similar to THC. In particular, the dopamine/serotonin transporter inhibition ratio in vitro can be helpful to predict the expected psychotropic but also the toxic effects of novel substances.
Acknowledgement:The author thanks Anna Rickli for designing Figure 1 and 3 and Anna Rickli, Yasmin Schmid, and Linda D. Simmler for comments on the manuscript. Anna Rickli, Linda Simmler, and Marius Hoener contributed to the original research reviewed in this paper.
References
1 Smith SW, Garlich FM. Availability and supply of novel psychoactive substances. In: Dargan PI, Wood DM, Eds. Novel psychoactive sustances: classification, pharmacology and toxicology. Amsterdam, Elsevier Academic Press, 2013.
2 European Drug Report 2014. European Monitoring Center for Drugs and Drug Addiction (EMCDDA). 2014; www.emcdda.europa.eu.
3 Hillebrand J, Olszewski D, Sedefov R. Legal highs on the Internet. Subst Use Misuse. 2010;45:330–40.
4 Wood DM, Heyerdahl F, Yates CB, Dines AM, Giraudon I, Hovda KE, et al. The European Drug Emergencies Network (Euro-DEN). Clin Toxicol (Phila). 2014;52:239–41.
5 Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone). Clin Toxicol (Phila). 2011;49:691–3.
6 Derungs A, Schwaninger AE, Mansella G, Bingisser R, Kraemer T, Liechti ME. Symptoms, toxicities, and analytical results for a patient after smoking herbs containing the novel synthetic cannabinoid MAM-2201. Forensic Toxicol. 2012;31:164–71.
7 Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–70.
8 Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–60.
9 Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem Pharmacol. 2014;88.
10 Wood DM, Hill SL, Thomas SH, Dargan PI. Using poisons information service data to assess the acute harms associated with novel psychoactive substances. Drug Test Anal. 2014. in press
11 Monte AA, Bronstein AC, Cao DJ, Heard KJ, Hoppe JA, Hoyte CO, et al. An outbreak of exposure to a novel synthetic cannabinoid. N Engl J Med. 2014;370:389–90.
12 Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49:499–505.
13 Kamour A, James D, Spears R, Cooper G, Lupton DJ, Eddleston M, et al. Patterns of presentation and clinical toxicity after reported use of alpha methyltryptamine in the United Kingdom. A report from the UK National Poisons Information Service. Clin Toxicol (Phila). 2014;52:192–7.
14 Dargan PI, Wood DM. Novel psychoactive substances: classification, pharmacology and toxicology. Amsterdam, Elsevier Academic Press, 2013.
15 Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49:705–19.
16 Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol. 2013;700:147–51.
17 Fattore L, Fratta W. Beyond THC: The new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60.
18 Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. “Spice” and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–7.
19 Cohen J, Morrison S, Greenberg J, Saidinejad M. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129:e1064–7.
20 Castellanos D, Thornton G. Synthetic cannabinoid use: recognition and management. J Psychiatr Pract. 2012;18:86–93.
21 Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2013;8:523–6.
22 Moosmann B, Kneisel S, Girreser U, Brecht V, Westphal F, Auwarter V. Separation and structural characterization of the synthetic cannabinoids JWH-412 and 1–[(5–fluoropentyl)-1H-indol-3yl]-(4–methylnaphthalen-1–yl)methanone using GC-MS, NMR analysis and a flash chromatography system. Forensic Sci Int. 2012;220:e17–22.
23 Iversen L. Speed, Ecstasy, Ritalin: the science of amphetamines. Oxford University Press, Oxford, 2008.
24 Morgan CJ, Noronha LA, Muetzelfeldt M, Fielding A, Curran HV. Harms and benefits associated with psychoactive drugs: findings of an international survey of active drug users. J Psychopharmacol. 2013;27:497–506.
25 Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. 2013 in press.
26 Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, et al. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone and in combination. Int J Neuropsychopharmacol. 2014;17:371–81.
27 Hysek CM, Simmler LD, Nicola V, Vischer N, Donzelli M, Krähenbühl S, et al. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012;7:e36476.
28 Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl). 2012;222:293–302.
29 Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, et al. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4–methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–59.
30 Liechti ME. “Ecstasy” (MDMA): pharmacology, toxicology, and treatment of acute intoxication. Dtsch Med Wochenschr. 2003;128:1361–6.
31 Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use: case-series of emergency department visits. Swiss Med Wkly. 2005;135:652–7.
32 Simmler LD, Hysek CM, Liechti ME. Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. J Clin Endocrinol Metab. 2011;96:2844–50.
33 Simmler LD, Wandeler R, Liechti ME. Bupropion, methylphenidate, and 3,4–methylenedioxypyrovalerone antagonize methamphetamine-induced efflux of dopamine according to their potencies as dopamine uptake inhibitors: implications for the treatment of methamphetamine dependence. BMC Res Notes. 2013;6:220.
34 Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–54.
35 Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41.
36 Meltzer PC, Butler D, Deschamps JR, Madras BK. 1–(4–Methylphenyl)-2–pyrrolidin-1–yl-pentan-1–one (pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–32.
37 Eiden C, Mathieu O, Cathala P, Debruyne D, Baccino E, Petit P, et al. Toxicity and death following recreational use of 2–pyrrolidino valerophenone. Clin Toxicol (Phila). 2013;51:899–903.
38 Vevelstad M, Oiestad EL, Middelkoop G, Hasvold I, Lilleng P, Delaveris GJ, et al. The PMMA epidemic in Norway: comparison of fatal and non-fatal intoxications. Forensic Sci Int. 2012;219:151–7.
39 Lurie Y, Gopher A, Lavon O, Almog S, Sulimani L, Bentur Y. Severe paramethoxymethamphetamine (PMMA) and paramethoxyamphetamine (PMA) outbreak in Israel. Clin Toxicol (Phila). 2012;50:39–43.
40 Daws LC, Irvine RJ, Callaghan PD, Toop NP, White JM, Bochner F. Differential behavioural and neurochemical effects of para-methoxyamphetamine and 3,4–methylenedioxymethamphetamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:955–77.
41 Carmo H, Remiao F, Carvalho F, Fernandes E, de Boer D, dos Reys LA, et al. 4–Methylthioamphetamine-induced hyperthermia in mice: influence of serotonergic and catecholaminergic pathways. Toxicol Appl Pharmacol. 2003;190:262–71.
42 De Letter EA, Coopman VA, Cordonnier JA, Piette MH. One fatal and seven non-fatal cases of 4–methylthioamphetamine (4–MTA) intoxication: clinico-pathological findings. Int J Legal Med. 2001;114:352–6.
43 Wikstrom M, Thelander G, Nystrom I, Kronstrand R. Two fatal intoxications with the new designer drug methedrone (4–methoxymethcathinone). J Anal Toxicol. 2010;34:594–8.
44 Winstock AR, Wolff K, Ramsey J. 4–MTA: a new synthetic drug on the dance scene. Drug Alcohol Depend. 2002;67:111–5.
45 Helander A, Backberg M, Hulten P, Al-Saffar Y, Beck O. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci Int. 2014;243C:23–9.
46 Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–2.
47 Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4–methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38:552–62.
48 Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–15.
49 Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–61.
50 Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22.
51 Aarde SA, Wright MJ, Buczynski MW, Angrish D, Parsons LH, Houseknecht KL, et al. Behavioral and termoregulatory effects of novel cathinone derivative drugs 4–MMC and MDPV. Neuropsychopharmacology. 2011;36:S441.
52 Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4–methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–40.
53 Green AR, King MV, Shortall SE, Fone KC. The preclinical pharmacology of mephedrone; not just MDMA by another name. Br J Pharmacol. 2014;171:2251–68.
54 Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81.
55 Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5–HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–64.
56 Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, et al. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin Toxicol (Phila). 2013;51:487–92.
57 Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35:20–5.
58 Greene SL. Benzofurans and Benzodifurans. In: Dargan PI, Wood DM, eds. Novel Psychoactive Substances:classification, pharmacology and toxicology. Elsevier, Amsterdam, 2013.
59 Wood DM, Button J, Lidder S, Ramsey J, Holt DW, Dargan PI. Dissociative and sympathomimetic toxicity associated with recreational use of 1–(3–trifluoromethylphenyl) piperazine (TFMPP) and 1–benzylpiperzine (BZP). J Med Toxicol. 2008;4:254–7.
60 Fantegrossi WE, Winger G, Woods JH, Woolverton WL, Coop A. Reinforcing and discriminative stimulus effects of 1–benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend. 2005;77:161–8.
61 Brunt TM, Koeter MW, Niesink RJ, van den Brink W. Linking the pharmacological content of ecstasy tablets to the subjective experiences of drug users. Psychopharmacology (Berl). 2012;220:751–62.
62 Bossong MG, Brunt TM, Van Dijk JP, Rigter SM, Hoek J, Goldschmidt HM, et al. mCPP: an undesired addition to the ecstasy market. J Psychopharmacol. 2010;24:1395–401.
63 Jan RK, Lin JC, Lee H, Sheridan JL, Kydd RR, Kirk IJ, et al. Determining the subjective effects of TFMPP in human males. Psychopharmacology (Berl). 2010;211:347–53.
64 Lin JC, Bangs N, Lee H, Kydd RR, Russell BR. Determining the subjective and physiological effects of BZP on human females. Psychopharmacology (Berl). 2009;207:439–46.
65 Gee P, Gilbert M, Richardson S, Moore G, Paterson S, Graham P. Toxicity from the recreational use of 1–benzylpiperazine. Clin Toxicol (Phila). 2008;46:802–7.
66 Thompson I, Williams G, Caldwell B, Aldington S, Dickson S, Lucas N, et al. Randomised double-blind, placebo-controlled trial of the effects of the “party pills” BZP/TFMPP alone and in combination with alcohol. J Psychopharmacol. 2010;24:1299–308.
67 Corkery JM, Elliott S, Schifano F, Corazza O, Ghodse AH. MDAI (5,6–methylenedioxy-2–aminoindane; 6,7–dihydro-5H-cyclopenta[f][1,3]benzodioxol-6–amine; “sparkle”; “mindy”) toxicity: a brief overview and update. Hum Psychopharmacol. 2013;28:345–55.
68 Murray DB, Potts S, Haxton C, Jackson G, Sandilands EA, Ramsey J, et al. “Ivory wave” toxicity in recreational drug users; integration of clinical and poisons information services to manage legal high poisoning. Clin Toxicol (Phila). 2012;50:108–13.
69 Wood DM, Puchnarewicz M, Johnston A, Dargan PI. A case series of individuals with analytically confirmed acute diphenyl-2–pyrrolidinemethanol (D2PM) toxicity. Eur J Clin Pharmacol. 2012;68:349–53.
70 Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB. Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology (Berl). 2014.
71 Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–9.
72 Werse B, Müller O, Schell C, Morgenstern C. Jahresbericht Monitoring-System Drogentreds: Drogentrends in Frankfurt am Main 2010. Goethe-Universität, Centre for Drug Research, Frankfurt am Main, 2011. URL: http://www.frankfurt.de/sixcms/media.php/738/MoSyD-Jahresbericht%202010%20%20Gesamtdokument.pdf. Accessed Aug 13th, 2014.
73 Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103:875–9.
74 Rust KY, Baumgartner MR, Dally AM, Kraemer T. Prevalence of new psychoactive substances: a retrospective study in hair. Drug Test Anal. 2012;4:402–8.
75 Bodmer M, Enzler F, Liakoni E, Bruggisser M, Liechti ME. Acute cocaine-related health problems in patients presenting to an urban emergency department in Switzerland: a case series. BMC Res Notes. 2014;7:173.
76 Warrick BJ, Hill M, Hekman K, Christensen R, Goetz R, Casavant MJ, et al. A 9–state analysis of designer stimulant, “bath salt”, hospital visits reported to poison control centers. Ann Emerg Med. 2013;62:244–51.
77 Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila). 2010;48:924–7.
78 Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4–methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–5.
79 Balmelli C, Kupferschmidt H, Rentsch K, Schneemann M. Fatal brain edema after ingestion of ecstasy and benzylpiperazine. Dtsch Med Wochenschr. 2001;126:809–11.
80 Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al. The harmful health effects of recreational ecstasy: a systematic review of observational evidence. Health Technol Assess. 2009;13:iii-iv, ix-xii, 1–315.
81 Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol. 2000;10:289–95.
82 Hysek CM, Vollenweider FX, Liechti ME. Effects of a b-blocker on the cardiovascular response to MDMA (ecstasy). Emerg Med J. 2010;27:586–9.
83 Hysek CM, Fink AE, Simmler LD, Donzelli M, Grouzmann E, Liechti ME. a-Adrenergic receptors contribute to the acute effects of MDMA in humans. J Clin Psychopharmacol. 2013;33:658–66.
84 Hysek CM, Schmid Y, Rickli A, Simmler LD, Grouzmann E, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Swiss Medical Forum. 2012;12:110S.
85 Hysek CM, Schmid Y, Rickli A, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans: lost in translation. Br J Pharmacol. 2013;170:1273–5.


