A large cohort of patients with hepatocellular carcinoma in a single European centre: aetiology and prognosis now and in a historical cohort
DOI: https://doi.org/10.4414/smw.2014.13900
Marion
Ganslmayer, Alexander
Hagel, Wolfgang
Dauth, Steffen
Zopf, Deike
Strobel, Volker
Mueller, Michael
Uder, Markus F.
Neurath, Juergen
Siebler
Summary
PRINCIPLES: The incidence of hepatocellular carcinoma is rising. However, this is occurring not only in developing nations, but in industrial countries as well. Surveillance programmes, classification systems and therapeutic options have improved, but there is a lack of data regarding their impact on the prognosis of this difficult-to-treat cancer.
MATERIALS AND METHODS: We evaluated 484 patients and reported on disease stage, therapeutic procedures and survival time. Data were compared with a historical cohort treated in the same centre 10 years before.
RESULTS: In this cohort, the main reason for liver disease was alcoholism, although hepatitis B remains the leading cause of hepatocellular carcinoma worldwide. Now, most patients have compensated liver function and hepatocellular carcinoma is diagnosed in the early tumour stages (it was diagnosed in the advanced disease stages in the previous cohort). Overall, median survival time was 62.4 weeks, 1-year survival was 58.6% and 3-year survival was 23.2%. Survival time correlated with the stage of liver disease, tumour stage and with therapeutic options.
CONCLUSION: Surveillance programmes lead to diagnosis in earlier tumour stages. Differentiated classification systems allow individualised therapeutic approaches. Earlier cancer stage and compensated liver function allow combination or sequential therapy, which was nearly impossible some years ago but is an option for most now. Primary liver cancer remains a difficult-to-treat malignancy, but the prognosis has improved remarkably, at least in the western world.
Abbreviations
AFP alpha-foetoprotein
BCLC Barcelona clinic liver cancer score
CI confidence interval
CLIP cancer of the liver Italian programme
DNA deoxyribonucleic acid
HBV hepatitis B virus
HCC hepatocellular carcinoma
HCV hepatitis C virus
HFTT high-frequency thermoablation
INR international normalised ratio
MELD model of endstage liver disease
MRI magnetic resonance imaging
MST median survival time
NASH nonalcoholic steatohepatitis
OLT liver transplant
RFA radiofrequency ablation
RNA ribonucleic acid
TACE transarterial chemoembolisation
WHO World Health Organisation
Introduction
Hepatocellular carcinoma (HCC) still remains one of the most commonly diagnosed cancer types [1]. According to World Health Organisation (WHO) data from 2008, it is the sixth most common cancer worldwide. Prognosis of liver cancer remains poor; in 2008, it was the third most common cause of cancer death, with 700,000 deaths worldwide. This number is not expected to decrease in the near future [1–3].
The incidence is much higher in developing countries than in the western world. This is explained by the rates of hepatitis B virus (HBV) infection and degraded food supplies contaminated with aspergillus. During the past decade, vaccination has reduced the number of HBV infections in Asia and the eastern world [1–3]. However, in Europe and the United States especially, hepatitis C Virus (HCV) infection and the increasing number of alcohol addicts has led to a higher rate of liver cirrhosis, leading to HCC [4, 5]. Therefore, HCC, a difficult-to-treat cancer, should remain a focus of research.
Since the liver is mostly able to compensate for impairment, symptoms usually occur in the far advanced tumour stages. However, curative therapeutic options exist for limited disease alone. These curative options are resection, radiofrequency ablation and liver transplantation [6–9]. Resection and local ablative strategies destroy vital tumour cell masses and are applicable only in limited HCC stages. Liver transplantation is possible only in accordance with the Milan criteria or within study protocols [10]. Other treatment options, such as transarterial chemoembolisation (TACE), are considered to be only life prolonging [9, 11]. Until 2010 there was no systemic treatment option: conventional chemotherapy produced no life-prolonging effect or was not applicable due to reduced liver function or poor overall condition. Therapeutic oestrogen receptor blockade with tamoxifen was shown to be ineffective [12]. Therefore, the prognosis of HCC patients was limited, and closely correlated with tumour stage at diagnosis and remaining liver function.
During the past decade, research has improved our knowledge of HCC, so that diagnosis and treatment could be reformed. Basic research decoded HCC development via specific cell mutations and genetic aberrations influencing treatability [4]. Effective screening procedures were not only introduced but also established. With the improvement in imaging techniques and better resolution of sonography and computed tomography, earlier diagnosis and (consequently) better treatability is to be expected [8, 13, 14]. Methods of diagnosis became simpler owing to the possibility of dynamic imaging and detection of the early arterial enhancement typical of HCCs. Most patients no longer need to be biopsied for diagnosis [6, 13].
Additionally, treatment options were enhanced. Radiofrequency ablation is applicable in much larger lesions [6]. With the development of drug-eluting beads, TACE can place chemotherapeutics more precisely in the tumour. TACE may offer a more effective therapeutic option than in the past. Sorafenib, the first effective systemic therapeutic option, acts at a subcellular level with combined antiproliferative and antiangiogenic effects. The substance was shown to be and introduced as the first systemic and life-prolonging agent for HCC [15]. These life-prolonging effects are only limited. Several substances are currently in phase III clinical trials. Progress in understanding the molecular mechanisms of HCC development is the key to the detection of new targets. For example, the c-MET inhibitor tivantinib has recently shown promising results in the subgroup of c-METhigh expressing HCC [4, 9, 16].
In the present study, we evaluated all consecutive HCC patients treated in a single European centre, namely the University Hospital Erlangen, between December 1999 and the end of January 2013. To date, the prognosis of HCC is known to be poor, with no realistic therapeutic hope for most of our patients. However, this assessment of the situation is based on the results of studies in other countries some years, or even decades, ago. Data describing the current situation of a freshly diagnosed HCC patient in the western world are lacking. Therefore, one aim of this study was to describe aetiology, diagnostic procedures, staging and outcome in a large group of HCC patients. In the same university hospital, a similar evaluation has been published for patients admitted between 1988 and 1999 [12]. Therefore, secondly, we present data on two cohorts which were diagnosed and treated in the same centre. We are thus able describe development of HCC epidemiology during the past decade in these representative patient groups.
Materials and methods
We included the data of 484 consecutive HCC patients admitted to the University Hospital of Erlangen between December 1999 and January 2013. The project was approved by the local ethics committee. The University Hospital Erlangen is the referral centre for Northern Bavaria, and at the time of data collection was capable of all therapeutic methods for HCC, including liver transplantation, liver surgery, interventional methods and the participation in multicentre trials. HCC was diagnosed via biopsy or in accordance with accepted European guidelines [6]. Contrast-enhanced ultrasound, multidetector computed tomography and magnetic resonance imaging were accepted imaging techniques. HCC was diagnosed in lesions >2 cm, if typical early arterial perfusion and late venous washout were shown with two different imaging methods, or when alpha-foetoprotein (AFP) was >400 U/l with positive results from one typical imaging method. With an update in 2005, a single dynamic imaging technique was sufficient for the diagnosis of HCC nodules >2 cm. Smaller lesions of 1–2 cm in diameter in pre-existing liver cirrhosis were diagnosed without biopsy by means of two approved imaging techniques. Owing to its lack of specificity, AFP was erased from diagnosis algorithms completely. In the case of uncertain imaging results, biopsies or close surveillance were performed in this cohort. The 2012 update of the EASL (European Association for the Study of the Liver) guidelines was put into practice after the conclusion of this study.
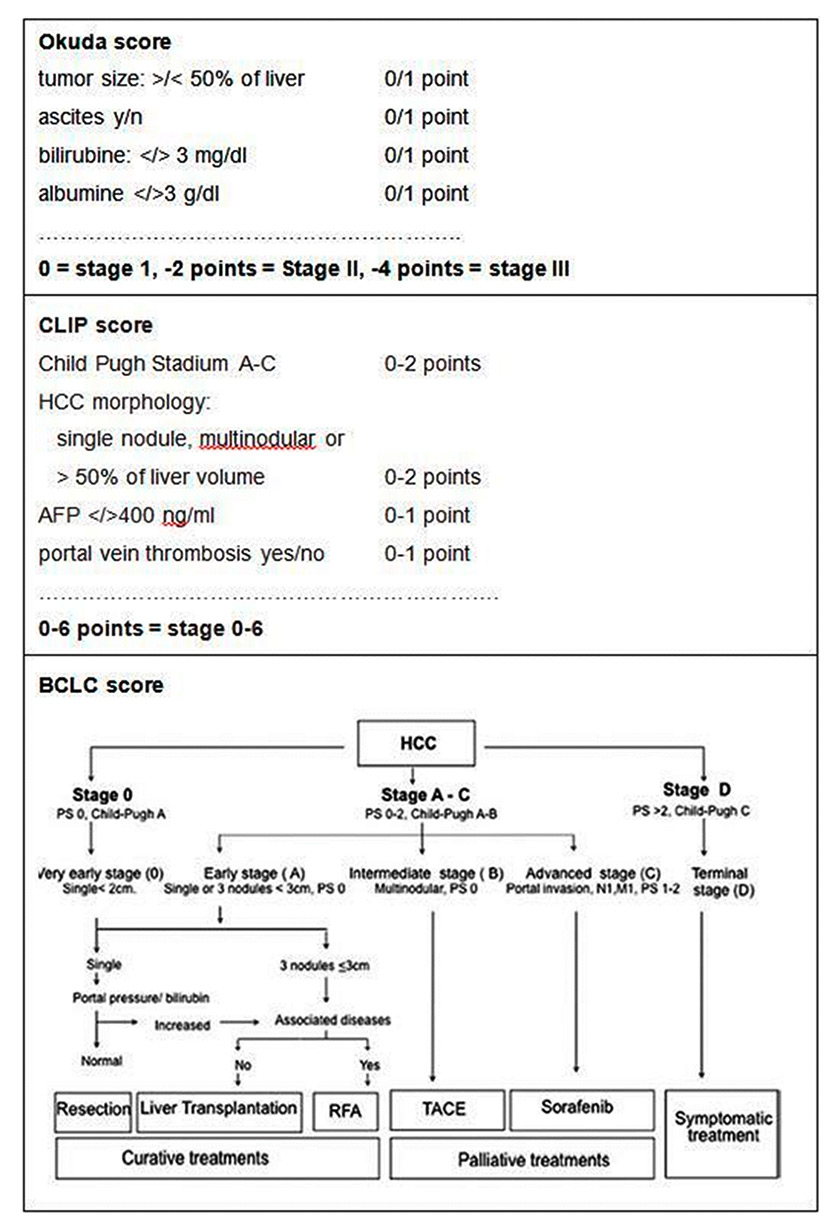
Figure 1
Principles of the Okuda, CLIP and BCLC scores as described in the literature [6, 12, 14].
AFP = alpha-foetoprotein; BCLC = Barcelona clinic liver cancer; CLIP = cancer of the liver Italian programme; RFA = radiofrequency ablation; TACE = transarterial chemoembolisation
If available, biopsy results and tumour grade were documented. We determined the stages and causes of underlying liver disease. Viral, autoimmune or metabolic liver disease were diagnosed with the usual blood tests (hepatitis B surface antigen antibody, hepatitis B core antigen antibody, hepatitis B deoxyribonucleic acid [DNA], hepatitis C antibody, hepatitis C ribonucleic acid [RNA], hepatitis D antibody, immunoglobulins, antimitochondrial antibody, antinuclear antibody, smooth muscle cell antibody, liver kidney microsomal antibody, coeruloplasmin, copper in urine, ferritin, ferrum/transferrin saturation and standard genetic tests for haemochromatosis and Wilson’s disease). Diagnosis of alcoholic liver disease versus nonalcoholic liver disease was based on histological parameters and the patients’ history of alcohol intake. Regular alcohol intake of >30 g/day in females and >70 g/day in males was defined as alcohol abuse and cause for existing steatohepatitis, fibrosis or liver cirrhosis.
Liver cirrhosis and function were staged according to the Child Pugh classification (ascites, bilirubin, albumin, encephalopathy, thromboplastin time) and the MELD score (model of endstage liver disease, a calculation including the international normalised ratio [INR], creatinine and bilirubin). HCC was classified according to the Okuda Score, the Barcelona clinic liver cancer score (BCLC) and the CLIP (cancer of the liver Italian programme) score, which have been described before [12, 14, fig. 1]. The Okuda score (tumour size, bilirubin, albumin and ascites) was the first classification system to include not only tumour-related parameters, but also liver function tests. Since the prognosis of HCC patients is closely related to the underlying liver disease, the Okuda score was superior to the TNM classification system. However, the three Okuda stages differentiate the prognosis only roughly and are no longer used in general. Here we used the Okuda score merely for comparison with the historical cohort.
The BCLC score combines the Okuda Score for tumour specific parameters, the Child Pugh score for liver function and the patient`s performance status for an estimate of overall condition. The combination of these parameters is used to define five stages (0 for very early HCC, A‒D for early to far advanced stages), which were correlated with the prognosis and the best therapeutic options [12].
The CLIP score includes the Child Pugh score and tumour specific parameters (morphology, AFP, vascularisation) and divides into seven stages (0 = early HCC to 6 = advanced HCC), which were correlated with the prognosis. CLIP does not suggest therapeutic procedures. Which score is the best prognostic tool for HCC patients is still under discussion [12, 14]. Both scores were used only for the study patients (1999–2013) and are not available for the historical cohort owing to the lack of some parameters, such as performance status.
The classification procedures were performed at the first diagnosis of HCC. In the University Hospital Erlangen, the optimal therapy for each HCC patient is discussed and decided at a weekly interdisciplinary round of specialists in liver surgery, interventional radiology and hepatology. We documented the therapeutic approach for each patient. In this series, liver transplant options in accordance with the Milan criteria and tumour resection were surgical approaches. Transarterial chemoembolisation with or without the use of drug-eluting beads and high-frequency thermoablation (HFTT) were the single or combined interventional options. Dependent on localisation of the tumour nodules, HFTT was guided via ultrasound or computed tomography. Patients unsuitable for interventional therapy and with sufficient performance status received treatment with sorafenib or were included in clinical trials. If no established therapy was available owing to the tumour stage or insufficient liver function, the patients received best supportive care. Some patients were treated with tamoxifen in the early years of the observation period. Those were pooled with the best supportive care patients for analysis.
Data were analysed statistically using WinStat for Exel (R Fitch Software, Bad Krozingen, Germany). Individual survival time was measured from the time of diagnosis until the end of the observation period, death or drop out. For survival time, a Kaplan-Meier analysis was performed. We analysed the differences in median survival time (MST) using the log-rank test or the t-test. For analyses with a large range of variables (CLIP 0‒6, MELD 6‒35), the Cox proportional hazard was calculated. Where applicable, we provided confidence intervals (CIs). A p-value of <0.05 was regarded as significant.
|
Table 1:Epidemiological data. |
|
|
Male
|
Caucasian
|
Eastern Europe
|
Italy
|
Alcohol abuse
|
NASH
|
HCV
|
HBV
|
Aetiology unknown
|
| Present cohort
1999‒2013 |
84% |
94% |
1.8% |
1.6% |
47% |
3.9% |
19% |
5.5% |
20% |
| Historical cohort
1988–1999 |
84% |
– |
– |
– |
59% |
– |
11% |
19% |
7% |
| HBV = hepatitis B virus; HCV = hepatitis C virus; NASH = nonalcoholic steatohepatitis |
|
Table 2: Child Pugh classification: distribution and survival time in the present and in the historical cohort. |
|
|
Child Pugh A
|
Child Pugh B
|
Child Pugh C
|
|
| Percentage (n) |
62.2% (n = 301) |
27.3% (n = 132) |
10.5% (n = 51) |
Present cohort
1999–2013
n = 484
|
| Median survival time (weeks)
[95% Confidence interval] |
83.01
[66.7–100.3] |
43.01,2
[29.7–67] |
12.91
[8.7–22.14] |
| Percentage |
35.0% (n = 98) |
41.0% (n = 115) |
17.0% (n = 48) |
Historical cohort
1988–1999
n = 261
|
| Median survival time (weeks) |
91 |
26 |
8.7 |
|
1 Survival Child Pugh A patients vs B and Child Pugh B vs C significant with p <0.0001
2 Survival Child Pugh A 1999–2012 vs historical cohort not signficant with p = 0.28; survival Child Pugh B 1999–2012 vs historical cohort significant with p = 0.0029; survival Child Pugh C 1999–2012 vs historical cohort not significant with p = 0.051 |
|
Table 3: Okuda Classification: distribution and survival time in the present and in the historical cohort. |
|
|
Okuda I
|
Okuda II
|
Okuda III
|
|
| Percentage (n) |
56.9% (n = 219) |
31.9% (n = 140) |
7.2% (n = 25) |
Present cohort
1999–2013
n = 484
|
| Median survival time (weeks)
[95% Confidence interal] |
101.11
[80.1–126.6] |
31.91
[22.9–41.14] |
13.71
[7.7–21.4] |
| Percentage (n) |
22% (n = 62) |
59% (n = 166) |
19% (n = 53) |
Historical cohort
1988–1999
n = 281
|
| Median survival time (weeks) |
1172
|
392
|
8.72
|
|
1 p <0.001 for Okuda I vs II and Okuda II vs III
2 Okuda I 1999–2012 vs historical cohort not signficant with p = 0.06; Okuda II 1999–2012 vs historical cohort not significant with p = 0.23; Okuda III 1999–2012 vs historical cohort not significant with p = 0.073 |
Results
Epidemiology and aetiology
Between December 1999 and January 2013, we registered 484 consecutive patients. The observation period ranged between 2 and 598 weeks. No patient was excluded. The dropout rate was <1%, 86% of the patients were male and 94.0% were Caucasian, the others being from Eastern Europe (Russia 1.4%, Rumania 0.4%, Croatia 0.4%), Turkey (1.5%), Italy (1.6%) and Greece (0.4%) (table 1). Overall, 90.5% of the patients suffered from cirrhosis, and the others showed signs of chronic liver disease at an earlier stage.
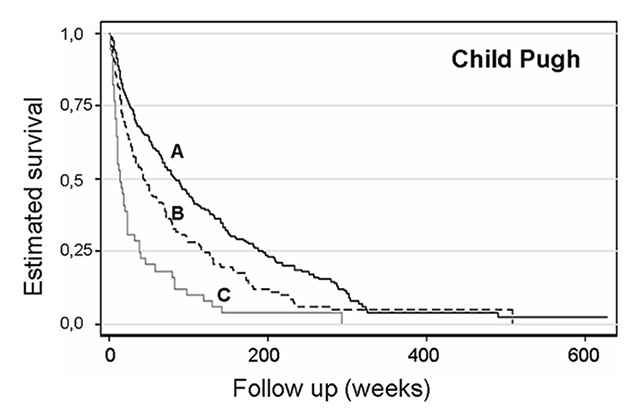
Figure 2
Kaplan-Meier curve for survival by Child Pugh score in patients with hepatocellular cancer (n = 301, 132 and 51 for Child Pugh A, B and C, respectively; p <0.001 for difference between the subgroups).
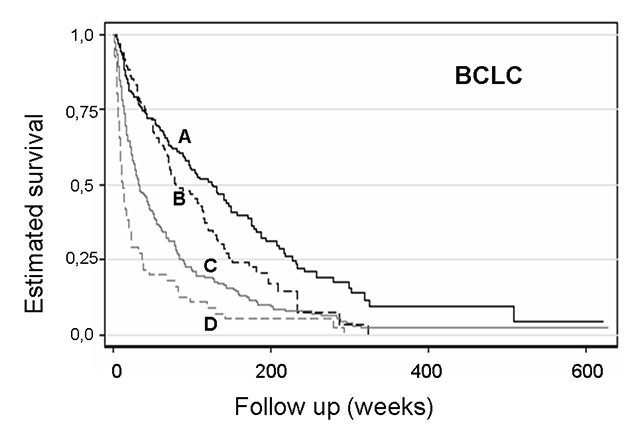
Figure 3
Kaplan-Meier curve for survival by BCLC score in patients with hepatocellular cancer (n = 6, 160, 94, 169 and 55 for BCLC 0, A, B, C and D, respectively; p <0.001 for B vs A, C vs B and D. C).
BCLC = Barcelona clinic liver cancer score
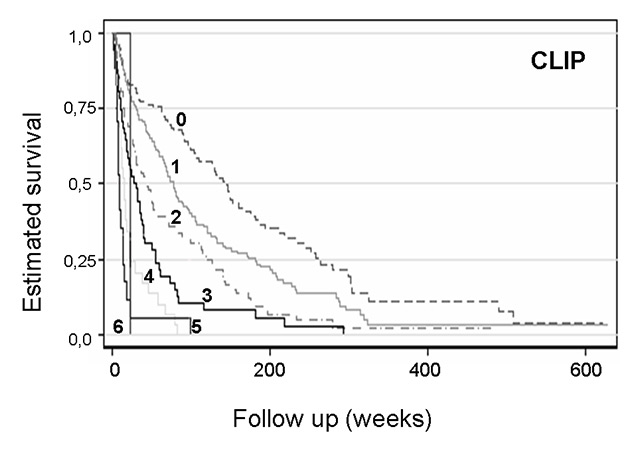
Figure 4
Kaplan-Meier curve for survival by CLIP score in patients with hepatocellular cancer (n = 131, 155, 98, 51, 29, 17 and 3 for CLIP 0‒6, respectively).
CLIP = cancer of the liver Italian programme
The aetiology varied widely: the largest subgroup showed alcohol-induced liver disease (47%); 19% suffered from chronic HCV infection and 5.5% were positive for HBV infection (1% were positive for both). Only 0.5% had HDV infection. Nonalcoholic steatohepatitis (NASH) was shown in 3.9% of the patients. Autoimmune hepatitis, primary biliary cirrhosis and haemochromatosis occurred less frequently, in 0.2%–2% of patients. Nearly 20% of the patients had a liver disease of an unknown origin (table 1).
In 63.5%, histology was used in the diagnosis of HCC, and 36.5% were diagnosed with the EASL criteria.
Liver function and hepatocellular carcinoma scores
The patients were mostly diagnosed at an early stage of liver disease (62.2% Child Pugh A, 27.3% Child Pugh B, 10.5% Child Pugh C; table 2:) and HCC stage (table 3: 56.9% Okuda I, 31.9% Okuda II, 7.2% Okuda III and table 4: 1.2% for BCLC 0 and 33.1%, 19.4%, 34.9% and 11.4%, respectively, for the stages A–D, and table 5: 27.1% for CLIP 0 and 32%, 20.2%, 10.6%, 6%, 3.5%, 0.6%, respectively for the stages 1–6.) All patients had <30 MELD points, 52.7% received ≤10 MELD points, and a further 41.7% ranged between 10 and 20 MELD points.
Overall MST was 62.4 weeks. Child Pugh A patients had a MST of 83 weeks, Child Pugh B patients 43 weeks and Child Pugh B patients 12.9 weeks (table 2, fig. 2). Patients with Okuda stages I, II and III had a MST of 101.1 week, 31.9 weeks and 13.7 weeks, (table 3). For BCLC, the MST was 301.9 weeks (stage 0), 127 weeks (BCLC A), 79.1 weeks (BCLC B), 31 weeks (BCLC C) and 12 weeks (BCLC D) (table 4, fig. 3). These scores reflected significant differences in survival time, with a decreasing prognosis for higher stages (p <0.001 for Child Pugh A vs B and B vs C; p <0.001 for Okuda I vs II vs III and p <0.001 for the BCLC stages).
The MELD score and the CLIP score (table 5, fig. 4) showed a worsening of prognosis with higher stages, as shown in the Cox proportional hazard analysis (p = 0.01). Owing to the low number of patients in some subgroups of MELD or CLIP, a survival analysis between the single groups did not appear reasonable (e.g. MST 16 weeks for MELD 15, n = 26; 43 weeks MELD 20, n = 20, complete data not shown).
Therapeutic procedures
Eight percent of the patients were listed for liver transplant, but only 4.5% received the transplant before death. 15.7% underwent tumour resection, 26.7% RFA and 6.5% received sorafenib or new therapeutic agents (within trials). The largest group of patients was treated with TACE (33.5%) and 27% received best supportive care (table 6).
The best survival time was achieved by the patients with a liver transplant (328 weeks). Liver resection, TACE or HFTT were less effective (survival time 96.7, 105.6 and 104.32 weeks, respectively, with no significant difference, table 6). As expected, sorafenib showed only a limited life-prolonging effect (38.6 weeks), which was nevertheless just significantly longer than the effect of best supportive care (14.1 weeks, p = 0.002).
Overall, 71.6% of the patients were treated with more than one therapeutic option (table 5, fig. 5). The combination therapy prolonged median survival significantly when compared with a monotherapeutic strategy (141 vs 33 weeks, p <0.001). Only 55% of the patients were treated as recommended in the BCLC system.
Comparison with historical cohort
The direct comparison with the historical cohort data previously published [12] showed a remarkable increase in the MST (62 vs 40 weeks). In the cohort of 1999–2013, more patients had limited liver disease and early HCC stages. Thus the number of patients with Child Pugh A at first diagnosis was significantly higher than in 1999–2012 (62.2% vs 35%, p <0.001) and the percentage of Child Pugh B and C patients decreased, but in a nonsignificant way (27.7 vs 41% and 10.5 vs 17% with p = 0.08 and p = 0.17, respectively). The fraction of patients with Okuda I at first diagnosis was higher in 1999–2013 (56.7% vs 22% p = 0.001) and the proportion of patients with Okuda II and III decreased significantly (36.1 vs 59%, p = 0.018 and 7.2% vs 19%, p = 0.048, respectively). Compared with the historical cohort, a significantly lower percentage had no therapeutic option and received the best supportive care (27.1% vs 48%, p = 0.015). The fraction of patients receiving HFTT or TACE increased significantly (27 vs 14% for HFTT and 33 vs 11% for TACE, p = 0.05 and p = 0.007, respectively). The number of patients undergoing liver resection decreased, but in a nonsignificant way (16% vs 26%, p = 0.15).
Survival time in the different substages of HCC or liver scoring systems (Child Pugh A, B and C and Okuda I‒III) did not show significant differences. Therapeutic procedures showed different survival times: MST after resection and best supportive care decreased (MST 96.7 vs 135 weeks and 48 vs 27.1 weeks, p = 0.03 and p = 0.04, respectively). MST after transplantation and TACE increased significantly (328 vs 257 weeks and 104 vs 65 weeks, p = 0.03 and p = 0.016, respectively). MST after HFTT did not change. The differences in the overall mean survival time are mostly based on the higher number of patients in low tumour and cirrhosis stages.
|
Table 4:BCLC score (present cohort 1999–2013). |
|
BCLC
|
|
A
|
B
|
C
|
D
|
| Percentage (n) |
1.2
(n = 6) |
33.1
(n = 160) |
19.4
(n = 94) |
34.9
(n = 169) |
11.4
(n = 55) |
| Median survival time (weeks)
[95% Confidence interval] |
301.9
[11.14–389] |
127
[91.7–149.3] |
79.1
[26–114.7] |
31
[24.4–45.6] |
12
[8–18.9] |
| BCLC = Barcelona clinic liver cancer score
p <0.001 for B vs A, C vs B and D vs C; percentage of BCLC 0 patients underpowered for statistical analysis |
|
Table 5:CLIP Score (present cohort 1999–2013). |
|
CLIP
|
|
1
|
2
|
3
|
4
|
5
|
6
|
| Percentage (n) |
27.1
(n = 131) |
32.0
(n = 155) |
20.2
(n = 98) |
10.6
(n = 51) |
6.0
(n = 29) |
3.5
(n = 17) |
0.6%
(n = 3) |
| Median survival time (weeks)
[95% Confidence interval] |
143
[104–176.2] |
76
[64–94.7] |
41
[28.4–52.7] |
30.4
[15.7–27.1] |
14.4
[8.7–20.14] |
8.3
[5.1–13.6] |
3.7
[3.3–4] |
| CLIP = cancer of the liver Italian programme |
|
Table 6:Therapeutic options. |
|
|
OLT
|
Resection
|
HFTT or PEI
|
TACE
|
Sorafenib + new drugs
|
BSC + tamoxifen
|
Multiple therapy
|
Single therapy
|
|
| Percentage
(n) |
4.5
(n = 22) |
15.7
(n = 76) |
26.7
(n = 81) |
33.5
(n = 162) |
6.5
(n = 31) |
27.1
(n = 131) |
71.6
(n = 347) |
28.3
(n = 137) |
Present
cohort
1999-2013
(n = 484) |
| Median survival time (weeks)
[95% CI] |
3282
[113–389] |
96.711
[52–140] |
105.61
[79–158] |
104.31
[82–130] |
38.62
[23–48.3] |
14.12
[11–22] |
1413
[114–170] |
32.63
[27.2–42] |
| Percentage (n) |
10
(n = 28) |
26
(n = 73) |
14
(n = 39) |
11
(n = 31) |
– |
48
(n = 134) |
1
(n = 28) |
99
(n = 278) |
Historical cohort
1988-99
(n = 281) |
| Median survival time (weeks) |
2574
|
1354
|
101 |
654
|
– |
30 |
– |
– |
| BSC = best supportive care; HFTT = high-frequency thermotherapy; OLT= liver transplant; PEI = percutaneous alcohol injection (only in historical cohort); TACE = transarterial chemoembolisation
New drugs = antiangiogenic substances, such as bevacizumab.
More than one therapy is possible.
1 No significant difference in median survival time with HFTT or resection vs TACE (p = 0.69 and p = 0.35 respectively).
2 Significant difference in median survival time with liver transplant, Sorafenib or BSC vs TACE (p = 0.04).
3 Significant difference in median survival time with multiple vs single therapy (p <0.001).
4 median survival time after resection and BSC present vs historical cohort significantly shorter (p = 0.03 and p = 0.04, respectively). Median survival time after OLT and TACE present vs historical cohort significantly longer (p = 0.016). Median survival time after HFTT present vs historic cohort similar (p = 0.96). |
Discussion
During the last few years, the rate of HCC increased in the United States and parts of Europe particularly [1]. The prognosis depends not only on tumour size, but also on the underlying liver disease and the remaining liver function. Exclusively limited HCC stages are treatable in a curative approach, if surgical or interventional destruction of the complete tumour tissue is possible. With the introduction of sorafenib, the first systemic treatment option, prognosis is prolonged by only a few weeks [9, 18]. During the past decade, clinical and basic research gave insights into liver cancer development and optimised the therapeutic approach [8, 16, 17]. At least in industrial countries, surveillance programmes for patients with liver cirrhosis and certain liver diseases were established. The WHO has been recommending HBV vaccination for an about 30 years and has reduced the number of cases of HBV-related liver cirrhosis and cancer worldwide [1, 4]. However, the impact on epidemiology, prognosis and treatment is described for only a limited number of HCC patients [7, 8, 14, 19, 20]. Here we evaluated a complete cohort of 484 HCC patients treated in a university hospital in northern Bavaria from 1999 to 2013. These patients were compared with a cohort (n = 281) treated from 1988 to 1999 in the same medical centre [12].
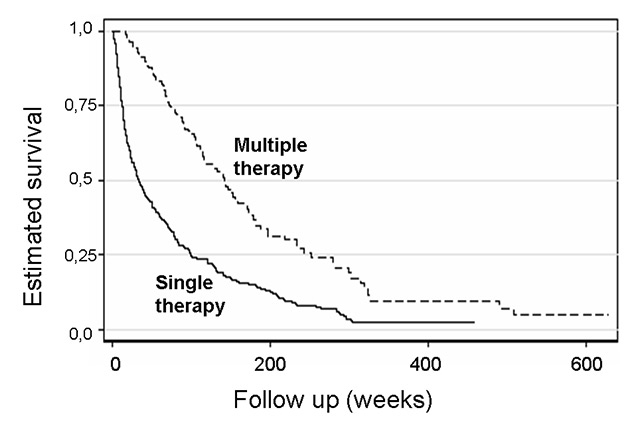
Figure 5
Kaplan-Meier curve for survival of patients with hepatocellular cancer receiving single or multiple therapies (n = 347 and 137 for multiple and single therapy, respectively, p <0.001).
Compared with the historical cohort, we see an increase in admitted HCC patients in the present group. Since the University Hospital Erlangen has been a referral centre for years, and the admission strategy has not been changed, this might be related to an increasing incidence of HCC in the region. Similar data have been reported from other German hospitals [14, 19] and also in the United States in Tulane University [21]. Unfortunately nationwide data sets are lacking in Germany, most European countries and the United States.
Alcoholic liver disease is the leading cause of liver disease in both the current and previous cohort, as reported by other European centres [12, 14, 19, 22]. Centres in the United States report that viral hepatitis is the leading cause for HCC-related liver disease, as was expected from the WHO data [1, 21]. In Europe especially, the increasing alcohol consumption by adolescents may be cause for concern [22]. We have to expect a growing number of young and difficult-to-treat patients with decompensating liver cirrhosis and HCC without the option of liver transplantation owing to alcoholism in this region [22]. Besides the number of patients with alcohol abuse, we see an increasing number of patients with HCV, whereas HBV-associated HCC is decreasing. This confirms the pattern described by other authors [2, 4, 23]. With better control of HCV infection, decreasing numbers of HCC patients might be expected (at least in the western world).
Changed diagnostic criteria influenced timing and methods of diagnosis: The typical vascular pattern of primary liver cancer allows a biopsy-free diagnosis. In this cohort, biopsy-free diagnostic procedures followed the EASL guidelines [6]. Dynamic magnetic resonance imaging (MRI) and computed tomography, as well as contrast enhanced sonography, were used for detection of the early arterial enhancement and venous washout, which is typical for primary liver cancer. In the updates of the EASL and American Association for the Study of Liver Diseases (AASLD) guidelines from 2012, the use of contrast-enhanced ultrasound for biopsy-free diagnosis was excluded because cholangiocellular carcinoma and precancerous dysplastic lesions are difficult to differentiate [13]. This decision and change of guidelines is still discussed widely by radiologists and sonographists [13]. Even before the change in guidelines, the liver team in Erlangen demanded definite typical patterns in the imaging procedures. If there were any doubts, an additional imaging technique or a biopsy were required. As a result of this diagnostic procedure, we expected a biopsy-based diagnosis in only a small number of patients. However, a biopsy was performed in 60% of our patients. Higher quality computed tomography and MRI would probably detect smaller lesions of less than 2 cm in diameter, which could not be classified with dynamic imaging. Compared with the historical control group, we can now diagnose liver cancer at an earlier tumour stage when liver function is mostly preserved. The Child Pugh score can still differentiate estimated survival time correctly. The MELD score showed early stages of liver disease as well. However, no MELD value was >30 points (more than 90% <20). Nowadays, (at least in Germany) a liver transplant is feasible with around 30 or more MELD points. This analysis showed a decrease in survival time with increasing MELD points, as expected. However, the MELD score is not able to estimate the prognosis of individual HCC patients correctly [24]. Obviously, HCC patients are at a disadvantage compared with patients with liver cirrhosis of a different origin in regard to organ assignment, so for HCC patients exception MELD criteria are applied. There are no published information on whether the exception MELD score for HCC patients equals or favours HCC patients. In the United States, MELD allocation for HCC patients has had to be adjusted several times. Evaluations of the situation in Europe are lacking. Our data indicate that the HCC staging scores (CLIP, BCLC, Okuda) and the Child Pugh score for liver function are superior to the MELD score for estimation of prognosis. Similar data have been reported before [10, 24–26].
Besides residual liver function, tumour expansion and vascular invasion influence survival time and applicable therapeutic strategies [8, 27]. The classical TNM classification does not describe primary liver cancer sufficiently and has not been recommended for a few decades. The first HCC score estimating tumour and liver function parameters was the Okuda score. However, the Okuda system is too vague for individual clinical evaluation and treatment decisions. Here we scored according to the Okuda method for sufficient comparability with the former cohort: The proportion of patients with limited disease (Okuda A) at the time of diagnosis increased significantly compared with the historical cohort. Beside the Okuda score, we determined the CLIP score and the BCLC score. Recently the CLIP score was highly recommended for estimation of prognosis in clinical and experimental settings [7, 14]. Here we describe a decrease in survival with higher CLIP stages as shown by Cox regression analysis. However, even with this fairly high number of patients, CLIP stages fail to provide an exact individual prognosis, with overlapping survival times between different stages (data not shown). The BCLC score was developed as a prognostic tool and guide for therapeutic decisions [7, 26]. With this the tumour stages were differentiated sufficiently. The BCLC score includes not only tumour-related parameters, but also the Child Pugh stage and the individual performance status of the patient. The largest subgroups in our cohort were those at the intermediate stages B and C, which are fit for interventional procedures. In the University Hospital Erlangen, each HCC patient is evaluated and discussed by an interdisciplinary board. However, treatment decisions followed the advice of the BCLC score in only 50% of the patients. The reason for this was mostly the proximity of portal veins or the liver capsule, the exact tumour position or difficult vascular patterns, which excluded HFTT or resection. This confirms the need of interdisciplinary boards and specialised HCC treatment centres with a range of different surgical, interventional and medical therapeutic options. The BCLC score may give guidance, but cannot replace a specialised centre for fixing individual treatment plans. Therefore, a final conclusion on the best score is not possible: for clinical prognostic evaluation and therapeutic advice, BCLC may be at advantage; for differentiation of risk groups as demanded in most clinical studies, the CLIP score may be superior.
Compared with the historical cohort, overall survival time was improved, but the survival time within the stages remained stable. Similar survival data have been reported from other industrial nations [7, 8, 20]. Surveillance programmes (as done in our centre) result in diagnosis at an earlier tumour stage and with sufficient residual liver function, as seen in our data-set. This allows more aggressive, individualised and successful therapy. Countries without close surveillance of high-risk patients still report HCC diagnoses at advanced tumour stages with limited therapeutic options and poor prognosis [28].
The techniques of interventional procedures have been enhanced during the last few years. The use of drug-eluting micro-beads for transarterial chemoembolisation in particular seems to enhance the efficacy, as indicated in our cohorts. This may influence the status of TACE among the other treatment options [11, 18]. Survival time after resection decreased, which is difficult to explain. The lower survival with best supportive care is explainable since systemic therapy with sorafenib and study drugs were not possible in the historical cohort.
Interestingly, we saw a change in therapeutic approach in the current data set: previously, limited liver function and insufficient therapeutic options allowed the application of only one treatment modality, whereas nowadays most patients are treated with sequential therapy. The possibility of combined treatment options increases survival time, especially in intermediate tumour stages. Among the new treatment options are sorafenib and various newly developed antitumour agents that reduce angiogenesis and proliferation of HCCs. The concept of a systemic treatment option was simply a milestone in HCC therapy [15, 18]. Our data regarding the efficacy of systemic treatment are comparable to the literature, with limited but significant effects. Therefore, development of more effective HCC therapeutics or combined treatment options is urgently needed [15, 16].
In conclusion, we describe an increasing incidence, but also a better prognosis in our cohort. Surveillance leads to the detection of HCC and liver disease at an early stage. The epidemiological analysis showed alcoholic liver disease to be a risk factor for HCC. Health education is urgently needed in the western world. Survival time increased owing to early diagnosis. HCC is still difficult to treat, but we are now able to offer more sufficient and life-prolonging therapeutic options to our patients. However, systemic treatment is still unsatisfactory. Certainly, there are further substances and combined treatment options on the way. So far, the improvement of interventional techniques (radiofrequency ablation and transarterial chemoembolisation) offers the most realistic options.
References
1 World Health Organization: World cancer statistics 2012.
2 Zidan A, Scherelein H, Schüle S, Settmacher U, Rauchfuss F. Epidemiological pattern of hepatitis B and hepatitis C as etiological agents for hepatocellular carcinoma in Iran and worldwide. Hepat Mon. 2012;12:6894–7000.
3 Ni YH, Chen DS. Hepatitis B vaccination in children: the Taiwan experience. Pathol Biol. 2010;58:296–300.
4 El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
5 The Federeal Ministry of Health, Germany. Drug abuse of teenagers and adolescents in the republic of Germany: alcohol, 2011.
6 European Association for the Study of the Liver. EASL practice guidelines for the treatment of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
7 Kim BK, Kim SU, Park JY, Kim Dy, Dang HA, Park MS, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve ptients with hepatocelullar carincoma. Liver Int. 2012;12:1120–7.
8 Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, et al. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2011;56:397–405.
9 Tinkle CL, Haas-Kogan D. Hepatocellucar carcicnoma: natural history, current management and emerging tools. Biologics: Targets and Therapy. 2012;6:207–19.
10 Weismüller TJ, Fikatas P, Schmidt J, Barreiros AP, Otto G, Beckebaum S, et al. Multicentric evaluation of model for end-stage liver disease-based allocation and survival after liver transplantation in Germany-limitations of the “sickest first”-concept. Transpl Int. 2011;24:91–9.
11 Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–50.
12 Herold C, Radespiel-Troeger, Ganslmayer M, Hahn EG, Schuppan D. Prognosis of a large cohort of HCC patients in a single European centre. Liver Int. 2012;22:23–8.
13 Dietrich CF, Cui XW, Boozari B, Hocke M, Ignee A. Contrast-enhanced ultrasound (CEUS) in the diagnostic algorithm of hepatocellular and cholangiocellular carcinoma, comments on the AASLD guidelines. Ultraschall Med. 2012;33:S57–66.
14 Den Winkel M, Nagel D, Sappl J, den Winkel P, Lamerz R, Zeck CJ, et al. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging sytems in a large western HCC cohort. Plos One. 2012;10:45066–80.
15 Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther. 2009;9:9739–45.
16 Trojan J, Zeuzem S. Tivantinib in hepatocellular carcinoma. Expert Opin Investig Drugs. 2013;22:141–7.
17 Fang P, Hu J, Cheng Z, Liu, Z. Wang, K, Jiao S. Efficacy and safety of Bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II Trials. Plos One. 2012;12:49717–25.
18 Cheng JW, Lv Y. New progress of non-surgical treatments for hepatocellular carcinoma. Med Oncol. 2013;30:381–8.
19 Erhardt A, Zhu E, Bondin D, Kubitz R, Knoefel WT, Mödder U, et al. Increasing number and improved survival of patients with hepatocellular carcinoma from 1988 to 2007: data of a German university clinic. Z Gastroenterol. 2011;49:720–7.
20 Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB. 2005;71:35–41.
21 Tangutur NK, Medvedev SF, Regenstein F, Balart LA. Hepatocellular carcinoma, a rapidly increasing public health problem: the Tulane experience 2003–2009. J LA State Med Soc. 2011;163:185–90.
22 Mancebo A, González-Diéguez ML, Cadahía V, Varela M, Pérez R, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol. 2013;11:95–101.
23 Fassio E. Hepatitis C and hepatocellular carcinoma. Ann Hepatol. 2010;9:119-2.
24 Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–43.
25 Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. OLT for HCC Consensus Group: Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:70175–9.
26 Shao YY, Lu LC, Hsu C, Shen YC, Hsu CH, Cheng AL. Prognosis of advanced hepatocellular carcinoma patients enrolled in cinical trials can be classified by current staging systems. Br J Cancer. 2012;107:1672–7.
27 Gomez-Rodriguez R, Romera-Gutierrez M, Artaza-Varaza R, Gonzales-Frutos C, Ciampi Dopazo JJ, et al. The value of the Bacelona Clinic Liver Cancer and alpha fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig. 2012;104:298–304.
28 Hucke F, Sieghart W, Schöniger-Hekele M, Peck-Radosavljevic M, Müller C. Clinical characteristics of patients with hepatocellular carcinoma in Austria – is there a need for a structured screening program. Wien Klin Wochenschr. 2011;123:542–51.




