Gastrointestinal bleeding associated with rivaroxaban administration in a treated patient infected with human immunodeficiency virus
DOI: https://doi.org/10.4414/smw.2014.13906
Botond
Lakatos, Marcel
Stoeckle, Luigia
Elzi, Manuel
Battegay, Catia
Marzolini
Summary
The use of rivaroxaban in fixed dosing regimens without need for routine coagulation monitoring may lead to the misconception that there is a minimal risk of drug-drug interactions. We describe the case of a patient infected with human immunodeficiency virus (HIV) on salvage therapy who developed gastrointestinal bleeding while receiving the standard dose of rivaroxaban for the prevention of venous thromboembolism after surgery. This case clearly sends a warning that protease inhibitors should not be co-administered with rivaroxaban. Furthermore, it highlights the importance of clinicians’ caution about potential drug-drug interactions.
Key words: drug-drug interaction; gastrointestinal bleeding; rivaroxaban; protease inhibitor
Introduction
Rivaroxaban, an oral direct factor Xa inhibitor, is currently licensed, among other indications, for the prevention of venous thromboembolism after orthopaedic surgery [1]. Owing to several pharmacokinetic improvements compared with the older vitamin K antagonists (rapid onset of action, high oral bioavailability that is not affected by food, predictable anticoagulant effect), rivaroxaban is used at a fixed dose without need for routine coagulation monitoring [2]. Despite its simplified administration, rivaroxaban has a potential for severe pharmacokinetic interactions [3], which may preclude its use with specific drugs, as illustrated in this HIV-infected patient who developed gastrointestinal bleeding associated with rivaroxaban administration while treated with a protease inhibitor-containing regimen.
Case report
Our patient was a 52-year-old Caucasian male who tested positive for human immunodeficiency virus (HIV) in 1984. His past HIV-related medical history included the occurrence of several opportunistic infections and oncological complications. His antiretroviral regimen underwent successive changes and several HIV drug resistances were acquired over the years as a result of suboptimal adherence. In the previous two years, the patient received salvage therapy including darunavir/ritonavir 600 mg/100 mg twice daily, lamivudine 150 mg twice daily, tenofovir 300 mg once daily, etravirine 200 mg twice daily and raltegravir 400 mg twice daily, which resulted in virological HIV supression (<20 copies/ml) and CD4+ T cell count consistently over 300 cells/mm3. Other comedications included lamotrigine, levetiracetam, gemfibrozil, pantoprazole and terbinafine.
In January 2012, the patient presented a Weber B fracture of the right malleolus due to a snowboard accident, which led to surgery. The postoperative period was uneventful and the patient returned home with a prescription for rivaroxaban 10 mg once daily for six weeks, initiated by the orthopaedic surgeon. Although the use of rivaroxaban was immediately questioned by the HIV specialist, owing to the risk of drug-drug interaction with darunavir/ritonavir, the surgeon insisted on maintaining rivaroxaban. Therefore, because of the risk of drug-drug interaction, the HIV physician planned to measure the rivaroxaban plasma concentration two weeks after the start of anticoagulant treatment. The rivaroxaban plasma level was 253 µg/l; of note, the median peak concentration (Cmax) value of rivaroxaban predicted from population pharmacokinetic analyses is 125 µg/l for 10 mg once daily [4]. The patient was informed of the elevated result and was advised, by a coagulation specialist, to halve the dosage of rivaroxaban. No additional rivaroxaban measurements could be performed as the patient travelled abroad. At the end of February 2012, while on a work trip in Mexico, the patient called his HIV physician in Switzerland to complain about acute bloody diarrhoea. He reported having watery diarrhoea with fresh blood accompanied by fever, asthenia, cramping abdominal pain and dizziness for the previous three days, which did not improve with ciprofloxacin 500 mg twice daily self-administered for two days. At this point, the patient was advised immediately to stop rivaroxaban and to go to the emergency unit. Laboratory work-up of the severely dehydrated patient did not show anaemia (haemoglobin: 139 g/l, haematocrit: 42.9%, platelets: 184x109/l). The rivaroxaban plasma level could not be documented since no specific measurement method was available in Mexico. The patient was treated with intravenous hydration and continued ciprofloxacin 500 mg twice daily for another five days with complete resolution of the bloody diarrhoea. Possible causes for the gastrointestinal bleeding were investigated in Mexico. Salmonellosis, Shigellosis and Campylobacter jejuni infections were excluded. Furthermore, the patient did not present any other gastrointestinal symptoms before the bleeding and had no history of colorectal disease. Thus, the exclusion of other possible causes, the chronology of events, the documented elevated plasma level of rivaroxaban and the resolution of the symptoms shortly after the interruption of the anticoagulant are highly suggestive of a drug-drug interaction between rivaroxaban and darunavir/ritonavir, which led to increased exposure to the anticoagulant and gastrointestinal bleeding. The other symptoms (fever, dizziness and diarrhoea) likely resulted from associated traveller’s diarrhoea. Since the treatment for the prevention of venous thromboembolism was almost completed, no other anticoagulant was further prescribed.
Discussion
Rivaroxaban is partly metabolised in the liver (≈60%) and partly eliminated unchanged (≈40%) in urine and to a minimal extent in faeces [5–6]. In the liver, metabolism is mainly performed by the cytochrome enzymes CYP3A4 (18%), CYP2J2 (14%) and hydrolytic enzymes (14%) [6]. In the kidney, elimination is mainly mediated by renal transporters such as P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) [7]. In-vitro studies indicate that protease inhibitors, in particular ritonavir, are strong inhibitors of CYP3A4, and possibly CYP2J2, P-gp and BCRP, thereby reducing the metabolism and renal elimination of rivaroxaban [8–10]. Of interest, a recent study in healthy volunteers showed that ritonavir 600 mg twice daily significantly increased rivaroxaban area under the curve (AUC) and Cmax by 153% and 55%, respectively [6]. These pharmacokinetic changes are consistent with the increase in rivaroxaban level measured in our patient. Interestingly, among the other drugs received by the patient, etravirine, a weak inducer of CYP3A4 and P-gp [11], did not attenuate the magnitude of the drug-drug interaction.
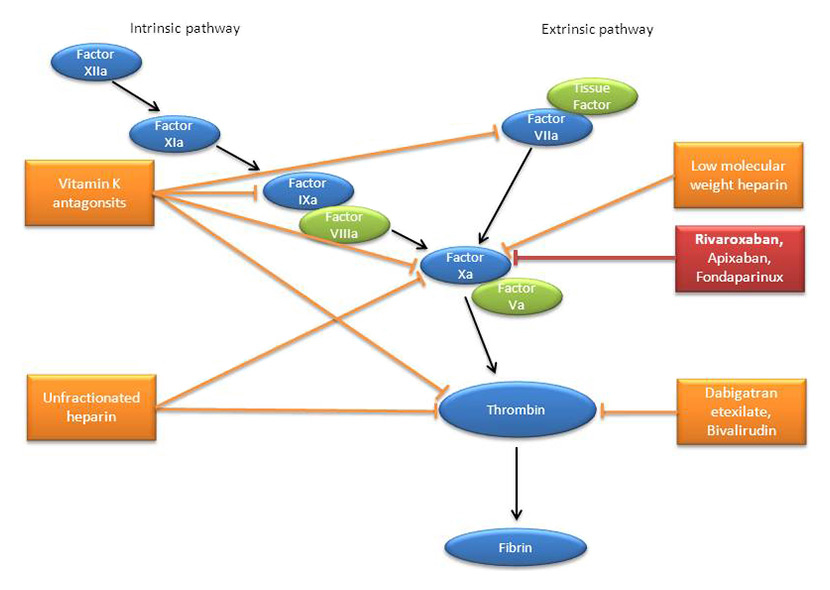
Figure 1
Schematic representation of the coagulation cascade with the sites of action of traditional and new anticoagulants. The cascade consists of the activation of subsequent coagulation factors (black arrows) in both the intrinsic and extrinsic pathways. The cascades converge upon the activation of factor Xa to a common path in which thrombin promotes the production of fibrin. The sites of inhibition of the various anticoagulants are shown by blunt arrows.
The gastrointestinal bleeding in our patient is compatible with the observed increase in rivaroxaban level. Clinical studies with rivaroxaban used for the prevention of venous thromboembolism after orthopaedic surgery have, indeed, shown that the risk of bleeding increases in a dose-dependent manner [12]. Given the dose-exposure correlation observed for rivaroxaban [4], the risk of bleeding would then be expected to increase with higher levels of the anticoagulant. Given the relatively short half-life of rivaroxaban, even in the presence of ritonavir [6], the anticoagulant effect is expected to resolve rapidly after the discontinuation of rivaroxaban, which is in line with our observation.
Protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) are prone to drug-drug interactions as a result of their inhibitory and inductive effects on cytochromes P450. Thus, the coadministration of these antiretroviral agents with anticoagulants should be cautious owing to the risk of altering the exposure to the anticoagulant drug. In this regard, it is important to remember that newer anticoagulants such as, for instance, rivaroxaban or apixaban are metabolised by cytochromes and, therefore, will be subject to drug-drug interactions. Furthermore, since their anticoagulant effect cannot be routinely monitored, their use is not recommended with a PI or NNRTI. Although vitamin K antagonists are metabolised by cytochromes, their effect can be easily monitored and their dosage adjusted according to the international normalised ratio (INR). Thus, vitamin K antagonists are being used with PI or NNRTI, although published cases indicate that the effect on the anticoagulant drug is not always predictable and that it is difficult to reach a stable INR [13–14]. Conversely, heparins are not subject to drug-drug interactions and, therefore, can be safely coadministered with antiretroviral agents. Additional information on drug-drug interactions between these two drug classes can be obtained from the Liverpool drug-drug interactions database [15]. The sites of action of traditional and new anticoagulants are shown in figure 1.
In conclusion, we present the first report on a drug-drug interaction between rivaroxaban and darunavir/ritonavir, which resulted in gastrointestinal bleeding. The use of rivaroxaban in fixed dosing regimens without need for routine coagulation monitoring may lead to the misconception that there is a minimal risk of drug-drug interactions. This case clearly sends a warning that protease inhibitors should not be coadministered with rivaroxaban. Furthermore, it highlights the importance of clinicians’ caution about potential drug-drug interactions.
Acknowledgement:We acknowledge Dr. Jan-Dirk Studt haematologist (University Hospital Zürich, Diagnostic Division of the Haematology Department) and Dr. Juan Steta medical doctor (Centro Medico, ABC Campus Santa Fe. Del Cuajimalpa, Mexico D.F.) for their contribution to the clinical management of the patient.
References
1 Eikelboom JW, Weizt JI. New oral anticoagulants for thromboprophylaxis in patients having hip or knee arthroplasty. BMJ. 2011;342:224–7.
2 Scaglione F. New oral anticoagulants: comparative pharmacology with vitamine K antigonists. Clin Pharmacokinet. 2013;52:69–82.
3 Graf L, Tsakiris DA. Anticoagulant treatment: the end of the old agents? Swiss Med Wkly. 2012;142:w13684.
4 Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–61.
5 Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37:1056–64.
6 Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–66.
7 Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338:372–80.
8 Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–4.
9 Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–81.
10 Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2004;310:334–41.
11 Schöller-Gyüre M, Kakuda TN, Raoof A, De Smedt G, Hoetelmans RM. Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin Pharmacokinet. 2009;48:561–74.
12 Fisher WD, Eriksson BI, Bauer KA, Borris L, Dahl OE, Gent M, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost. 2007;97:931–7.
13 Welzen MEB, van den Berk GEL, Hamers RL, Burger DM. Interaction between antiretroviral drugs and acenocoumarol. Antiviral Therapy. 2011;16:249–52.
14 Bonora S, Lanzafame M, D’Avolio A, Trentini L, Lattuada E, et al. Drug interactions between warfarin and efavirenz or lopinavir-ritonavir in clinical treatment. Clin Infect Dis. 2008; 46:146–7.
15 Liverpool drug-drug interactions database. http://www.hiv-druginteractions.org
