Figure 1
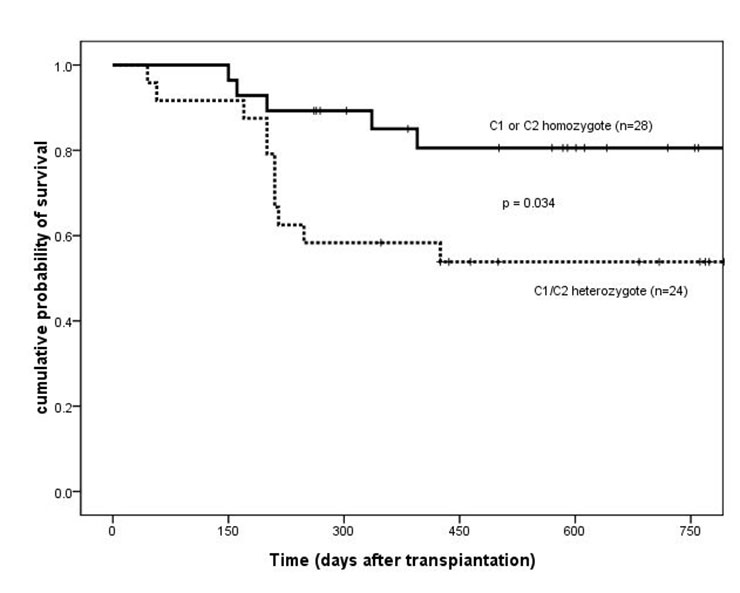
Overall survival of patients based on HLA-Cw ligand groups (C1 or C2 homozygote vs C1/C2 heterozygote). HLA = human leukocyte antigen.
DOI: https://doi.org/10.4414/smw.2013.13717
Allogeneic haematopoietic stem cell transplantation (HSCT) is a possible treatment for many patients with haematological malignancies. The alloreactivity of NK cells and certain subsets of T lymphocytes is regulated by the interaction between killer immunoglobulin-like receptors (KIRs) of donor cells and human leukocyte antigen (HLA)-class I molecules on the target recipient cells. HLA-Cw is the main ligand for most inhibitory KIRs and can be classified into two subgroups, C1 and C2, which bind to KIR2DL2/3 and KIR2DL1, respectively [1]. Other human inhibitory KIRs with known ligands include KIR3DL1, which binds to the HLA-Bw4 epitope [2] and KIR3DL2, which binds to HLA-A3 or HLA-A11 [3]. Previous studies showed that HLA-KIR interactions have a significant impact on the outcomes of haploidentical [4–10], unrelated donor [11–13] and matched related donor (MRD) [14–17] HSCT, including relapse, graft versus host disease (GVHD) and transplant-related mortality (TRM). Other studies revealed that alloreactivity in the HSCT had a confounding effect for which there are many explanations and hypotheses whilst the underlying mechanism remains to be elucidated.
The aim of the study was to assess the impact of interaction between recipient HLA-Cw and donor KIR on patient outcome. Recipient HLA ligands and donor KIRs were genotyped for 52 allogeneic HSCT patients.
From January 2006 to December 2007, 52 patients with myeloid malignancies underwent HLA-matched, sibling donor HSCT at the Institute of Haematology and Blood Diseases Hospital, CAMS and PUMC. The median duration of follow-up was 500.5 (range, 45–965) days. The median age of the patients was 38 (range, 14–55) years and that of donors was 36 (range, 9–52) years. Of the recipients, 18 were females and 34 were males; of the donors, 22 were females and 30 were males. There were 20 acute myeloid leukaemia (AML) cases, 31 chronic myeloid leukaemia (CML) cases, and one myelodysplastic syndrome (MDS) case. Patients were assigned to either a standard or a high pre-transplant risk group. The high-risk group included patients with AML other than first complete remission (CR1), CML other than first chronic phase (CML not CP1), and refractory anaemia with excess blasts (RAEB). Standard-risk patients were those with AML CR1 and CML CP1. There were 10 cases in the high-risk group and 42 cases in the standard-risk group.
This study was approved by the Research Ethics Committee of the Institution.
All patients and donors were HLA typed by nucleic acid-based molecular methods. HLA class I (HLA-A*, -B* and -Cw*) and II (DRB1*) typing were performed by polymerase chain reaction- sequence-specific priming (PCR-SSP) (Pel Freez) according to the manufacturer’s instructions. These methods provided intermediate-resolution allele assignment, as well as high-resolution allele assignment in some cases. KIR genotyping was also performed by the PCR–SSP (Pel Freez) method according to the manufacturer’s instructions. The typing determined the presence or absence of KIR genes and provided information about particular KIR alleles or variants. In 48 cases, donor DNA samples were available; thus, KIR genotyping was performed retrospectively to determine the inhibitory KIR (KIR2DL1, KIR2DL2, KIR2DL3, KIR3DL1, and KIR3DL2) and activating KIR (KIR2DS1, KIR2DS2) genotypes. Patients were grouped based on the expression of HLA ligand: (1) HLA-A3 or -A11, (2) HLA-Bw4, and (3) HLA-Cw groups (homozygous C1 or C2 and heterozygous C1/C2). The patients were further sub-grouped based on the combination of HLA and KIR genotypes: patients with three HLA ligands (C1, C2, Bw4) for donor inhibitory KIRs were assigned to the matched group and the remaining to the mismatched group [18].
The majority of patients received a busulfan – (3.2 mg/kg/day for three days, intravenously) and cyclophosphamide – (60 mg/kg/day for two days) based preparatory/conditioning regimen. For acute GVHD prophylaxis, methotrexate plus cyclosporine (25 cases) or tacrolimus (27 cases) based regimens were used. The median dose of CD34+cells in the graft was 2×106/kg of patient body weight. No manipulation of the graft, such as ex vivo T-cell depletion, was performed in any of the cases.
The endpoints included overall survival (OS), calculated from the date of stem cell infusion until the date of death from any cause, or the last follow-up; disease-free survival (DFS), calculated from the date of stem cell infusion until the date of relapse or death from any cause, or if the patient was alive in CR at the last follow-up; transplant-related mortality (TRM), defined as all causes of death without evidence of initial disease.
Acute GVHD was diagnosed and graded according to previously reported criteria [19]. All patients surviving more than seven days after transplant were considered at risk for developing acute GVHD. Chronic GVHD defined as GVHD occurring in patients after day 100 post-transplantation, classified as previously described [20]. Only patients still alive at day 100 and for whom chronic GVHD information had been collected were included in the chronic GVHD analysis.
Cytomegalovirus (CMV) infection was diagnosed when CMV screening became positive by quantitative plasma PCR with a detection threshold of 103/ml.
For categorical variables, the chi-square statistic or Fisher’s exact test was used to establish differences in their distributions; a 2-sided pvalue of 0.05 was considered significant. The incidences of post-transplant variables (platelet engraftment >20,000/μl, GVHD, TRM, relapse, CMV infection) were estimated as cumulative incidence. Kaplan-Meier method was used for analysis of OS and DFS and log-rank method was used to establish their differences between subgroups. SPSS version 10.0 statistical software was used to perform the analysis.
All 52 patients achieved platelet engraftment; 37 of 52 patients were alive, three patients relapsed, and 12 patients died of TRM. The overall incidence of acute GVHD was 65% (34 patients) and that of chronic GVHD was 56% (28 patients). Acute GVHD of grades II–IV occurred in 13% (7 patients) of patient, and extensive chronic GVHD occurred in 14% (7 patients). CMV infection was noted in 42% (22 patients) of patients.

Figure 1
Overall survival of patients based on HLA-Cw ligand groups (C1 or C2 homozygote vs C1/C2 heterozygote). HLA = human leukocyte antigen.
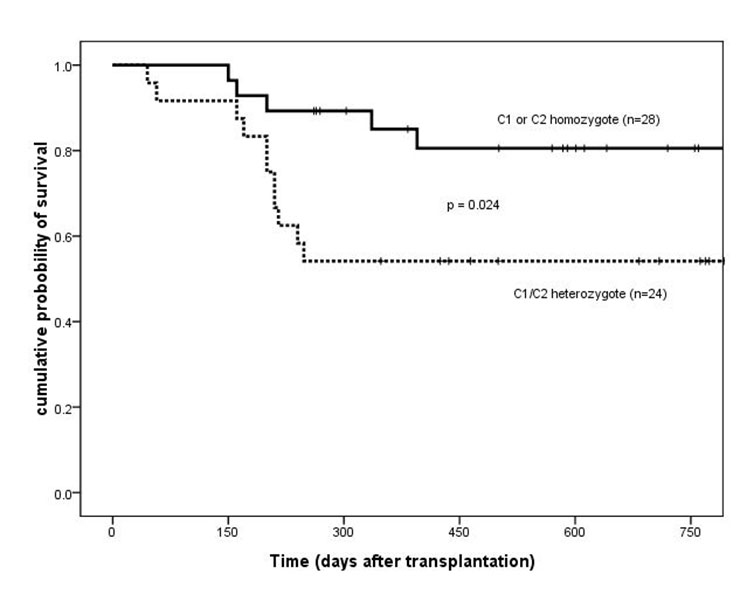
Figure 2
Disease-free survival of patients based on HLA-Cw ligand groups (C1 or C2 homozygote vs C1/C2 heterozygote). HLA = human leukocyte antigen.
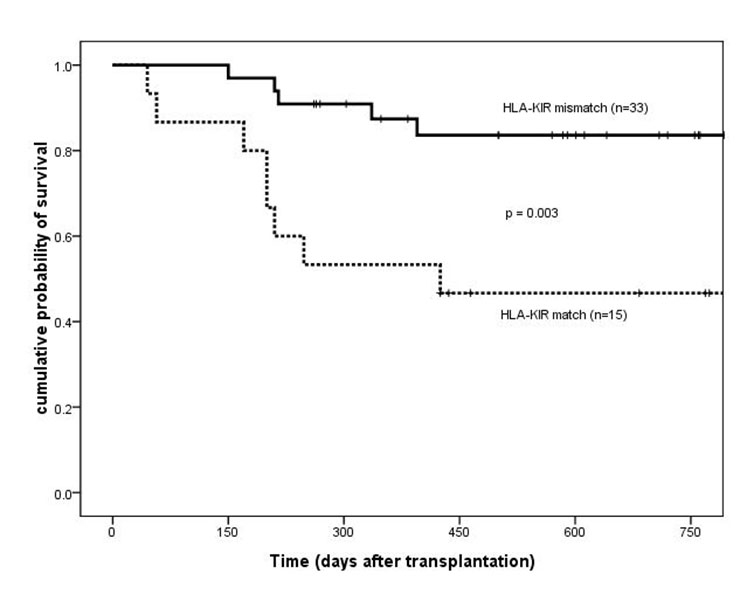
Figure 3
Overall survival of patients based on HLA-KIR match/mismatch group. HLA =human leukocyte antigen; KIR = killer immunoglobulin-like receptor.
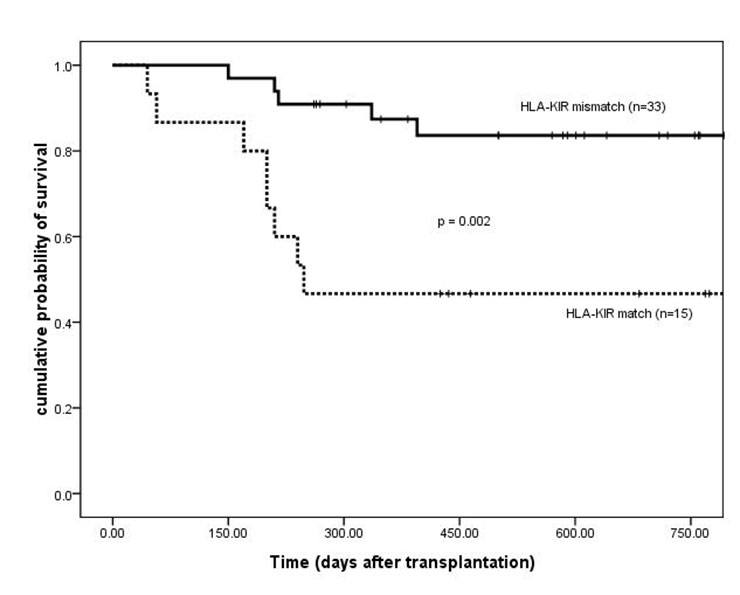
Figure 4
Disease-free survival of patients based on HLA-KIR match/mismatch group. HLA = human leukocyte antigen; KIR = killer immunoglobulin-like receptor.
The impact of recipient HLA ligand subtypes on clinical outcome was evaluated. 28 (54%) patients were homozygous for either C1 (25 patients) or C2 (3 patients), and 24 (46%) were C1/C2 heterozygotes. The cumulative incidence of platelet engraftment was similar between C1/C2 heterozygotes and C1 or C2 homozygotes, and grade II‒IV acute GVHD was the same (5/24 vs 2/28, p = 0.146). In comparison to homozygotes, C1/C2 heterozygotes had a significantly higher incidence of chronic GVHD (85% vs 31%, 19/22 vs 9/28, p = 0.000), including cases with extensive chronic GVHD (6/7 vs 1/7, p = 0.005).
Two years after transplantation, OS and DFS were significantly higher for C1 or C2 homozygotes than for C1/C2 heterozygotes (OS, 81% ± 8% vs 54% ± 10%, p = 0.034; DFS, 81% ± 8% vs 54% ± 10%, p = 0.024) (fig. 1 and 2). No difference in the baseline characteristics was found between these two groups (table 1). Three relapsed cases were C1/C2 heterozygous. The cumulative incidence of relapse two years after transplantation was significantly higher for C1/C2 heterozygous cases (15%) than for homozygous cases (0%; p = 0.048). No significant difference was found as to TRM between the heterozygous (8 patients, 35%) and the homozygous cases (4 patients, 18%; p = 0.149).
Platelet engraftment and the incidence of acute GVHD were similar between HLA-KIR matched and mismatched patients. For HLA-KIR-mismatched patients, there was a significantly lower incidence of chronic GVHD (43% vs 85%, 14/33 vs 11/13, respectively; p = 0.007). OS and DFS two years after transplantation were higher in HLA-KIR mismatched patients than in matched cases (OS, 84% ± 7% vs 47% ± 13%, p = 0.003; DFS, 84% ± 7% vs 47% ± 13%, p = 0.002) (fig. 3 and 4). The baseline patient characteristics were similar (table 2). The incidence of relapse and TRM at two years after transplantation were lower in HLA-KIR mismatched patients than in HLA-KIR matched cases (relapse, 0% vs 17%, p = 0.023; TRM, 15% vs 43%, p = 0.018).
The types of specific KIR genes were examined in the donors. All donors expressed 2DL1, 2DL2/L3, and 3DL2, and 51 donors (98%) expressed 3DL1. The expression rate of activating KIR differed (18–98%). The impact of the expression of donor activating KIR genes on the clinical outcomes was further evaluated, but there was no significant difference as to platelet engraftment, incidence of acute and chronic GVHD, OS, DFS, and TRM.
The impact of recipient HLA ligand, HLA-KIR matching, and donor activating KIR on the incidence of active CMV infection was evaluated. The presence of KIR2DS2 was significantly correlated with CMV infection: a higher incidence of CMV infection was observed in patients with donor KIR2DS2 (70% vs 34%, 7/10 vs 13/38, respectively; p= 0.041). There was no other significant correlations with CMV infection.
Multivariate analysis showed that C1/C2 heterozygosity and HLA-KIR match were independent risk factors for OS and DFS (table 3 and 4).
| Table 1:Patient characteristics based on HLA-Cw group. | |||
| C1 or C2 homozygote (n = 28) | C1/C2 heterozygote (n = 24) | p value | |
| Age at transplant (years) | 37.5 (14–51) | 38 (15–55) | 0.85 |
| Gender (female/male) | 12/16 | 6/18 | 0.18 |
| Standard risk / high risk | 22/6 | 20/4 | 0.74 |
| CD34+ cell dose (×106/kg) | 2.954 (1.14–6.90) | 2.10 (0.9–6.48) | 0.18 |
| Total nucleated cell dose (×108/kg) | 5.0 (1.47–8.80) | 5.11 (1.16–7.72) | 0.63 |
| ABO match/mismatch | 18/10 | 10/14 | 0.10 |
| HLA =human leukocyte antigen. | |||
| Table 2: Patient characteristics based on HLA-KIR match/mismatch group. | |||
| HLA-KIR match (n = 15) | HLA-KIR mismatch (n = 33) | p value | |
| Age at transplant (years) | 38 (27–54)a | 38 (14–55) | 0.89 |
| Gender (female/male) | 4/11 | 13/20 | 0.39 |
| Standard risk / high risk | 12/3 | 26/7 | 1.0 |
| CD34+ cell dose (×106/kg) | 2.48 (1.25–4.62) | 2.35 (0.90–6.90) | 0.62 |
| Total nucleated cell dose (×108/kg) | 5.0 (1.16–6.30) | 5.0 (1.47–8.80) | 0.31 |
| ABO match / mismatch | 7/8 | 18/15 | 0.61 |
| HLA = human leukocyte antigen; KIR = killer immunoglobulin-like receptor. | |||
| Table 3: Independent risk factors for DFS in patients. | |||
| Parameter | OR | 95% CI | p value |
| C1/C2 heterozygotes and homozygotes | 3.829 | 1.228–11.941 | 0.021 |
| HLA-KIR match / mismatch | 0.219 | 0.071–0.672 | 0.008 |
| Low / high risk | 2.071 | 0.504–8.514 | 0.313 |
| Type of disease (AML/CML) | 0.875 | 0.515–1.485 | 0.620 |
| CMV infection | 1.774 | 0.652–4.831 | 0.262 |
| HLA = human leukocyte antigen; KIR = killer immunoglobulin-like receptor. | |||
| Table 4: Independent risk factors for OS in patients. | |||
| Parameter | OR | 95% CI | p value |
| C1/C2 heterozygotes and homozygotes | 3.791 | 1.207–11.911 | 0.022 |
| HLA-KIR match / mismatch | 0.229 | 0.075–0.703 | 0.010 |
| Low / high risk | 2.168 | 0.521–9.019 | 0.287 |
| Type of disease (AML/CML) | 0.874 | 0.515–1.482 | 0.617 |
| CMV infection | 1.867 | 0.686–5.081 | 0.222 |
| HLA = human leukocyte antigen; KIR = killer immunoglobulin-like receptor. | |||
The alloreactivity mediated by the interaction between donor KIR and recipient HLA ligand showed some influence on the outcome of HSCT. Controversy still exists in terms of this observation and the underlying mechanism. Some data suggest that alloreactive cells exert a marked GVL effect and improve the outcome [4–6, 15], whilst others suggest that alloreactivity is associated with higher TRM and has a deleterious effect [7, 11].There are hypothetical explanations, including conditioning regimen, T-cell depletion, disease type and disease status. It should be noted that KIR receptors are also expressed by some subsets of T cells and they might contribute to alloreactivity in HSCT [7].
The results of the present study indicated worse outcomes in C1/C2 heterozygotes than in C1 or C2 homozygotes, which is in agreement with the report of Sobecks et al. [16, 21]. C1/C2 heterozygosity was correlated to a higher incidence of chronic GVHD (cGVHD), particularly extensive cGVHD, and was associated with lower OS and DFS and higher disease relapse. This difference in outcome may be due to the weaker alloreactivity among C1/C2 heterozygotes than in C1 or C2 homozygotes. As C1/C2 heterozygotes have more opportunity to engage inhibitory KIRs than C1 or C2 homozygotes, they have greater inhibitory effects on KIR-positive NK and T cell populations involved in the alloreactivity. Theoretically, greater inhibition leads to weaker alloreactivity. Therefore, C1/C2 heterozygotes activate alloreactive cells to a lesser extent.
Meanwhile, the present study showed that HLA-KIR mismatched patients had a better outcome, which is in line with some earlier reports [15, 16, 22]. HLA-KIR mismatch was associated with reduced incidences of cGVHD and TRM, a lower relapse rate and prolonged OS and DFS. This impact is in concordance with the influence of C1 or C2 homozygosity. Stronger alloreactivity exists in HLA-KIR mismatched cases. These studies support the hypothesis that alloreactivity may decrease cGVHD and relapse and thus lead to a better prognosis. The incidences of aGVHD between HLA-KIR matched and mismatched patients were the same, the reasons are unknown and may be connected with the different pathogenesis of aGVHD and cGVHD or the relatively small cohort of patients.
We analysed donor KIR genotype. It is important to note that nearly 100% of the donors possessed inhibitory KIRs corresponding to the HLA ligands. It is known that alloreactivity is induced by the absence of an HLA ligand that corresponds to the specific inhibitory KIR. In other words, the absence of an HLA ligand indicates alloreactivity [15, 22, 23], and less HLA ligand leads to stronger alloreactivity. This is consistent with the present findings that C1 or C2 homozygosity leads to a lesser inhibitory effect, which translates to stronger alloreactivity and better outcome. Limited information is available on specific ligands and functions of activating KIRs [18, 24–28]. We hypothesize that activating KIRs may be associated with clinical outcomes.
Some studies have shown that the incidence of active CMV infection correlated with activating KIR [29–31], but the results remain contradictory. Hadaya et al. [32] showed that the presence of activating KIR genes in the recipients was associated with a lower rate of CMV infection after kidney transplantation. We have no information on the recipient’s KIR. The present study demonstrates that activating KIR2DS2 increased the incidence of CMV infection. The relationship between CMV infection and activating KIRs remains unclear and warrants further investigation.
There are some limitations in the present study. Firstly, we performed HLA-DQ typing of seven patients and donors, they were matched. We have no information as to HLA-DP and other HLA-DQ typing. The influence of HLA-DQ and HLA-DP on the results is unknown. Secondly, a relatively small cohort of patients from a single centre was included, and the study size was limited. Many centres and larger scale studies are required to confirm our findings.
Analysis of the role of HLA ligand and HLA-KIR interactions has important clinical implications for allogeneic HSCT. The finding that C1/C2 heterozygosity and HLA-KIR match are poor prognostic factors suggests that additional intensified therapy, such as stronger conditioning regimens or stronger GVHD prophylactic regimens, may be appropriate for such patients. Future approaches examining the outcome in relation to specific KIR phenotype may also be useful in optimising the selection of haematopoietic stem cell donors for haematopoietic stem cell transplantation.
Acknowledgement: We express appreciation here for the support given by all colleagues in the study centre.
Funding / potential competing interests: This work was supported by the Natural Science Foundation of Shandong Province (ZR2010HQ014), and the Tianjin Municipal Science and Technology Commission Grant (10JCYBJC13100), China.
1 Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer immunoglobulin-like receptors. Mol Immunol. 2002;38(14):1007–21.
2 Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181(3):1133–44.
3 Farag S, Fehniger T, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukaemia effect. Blood. 2002;100(6):1935–47.
4 Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100.
5 Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukaemia: challenging its predictive value. Blood. 2007;110(1):433–40.
6 Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172(1):644–50.
7 Bishara A, De Santis D, Witt CC, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63(3):204–11.
8 Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after non myeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16(4):533–42.
9 Moretta A, Pende D, Locatelli F, Moretta L. Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidentical haemopoietic stem cell transplantation to cure high-risk leukaemias. Clin Exp Immunol. 2009;157(3):325–31.
10 Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukaemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for paediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–29.
11 Schaffer M, Malmberg KJ, Ringdén O, Ljunggren HG, Remberger M. Increased infection- related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78(7):1081–5.
12 Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR- ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. 2007;109(11):5058–61.
Gagne K, Busson M, Bignon JD, Loiseau P, Dormoy A, et al. Donor KIR3DL1/3DS1 gene and recipient Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(11):1366–75.
14 Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA identical sibling hematopoietic stem cell transplantation for myeloid leukaemia. Blood. 2004;103(4):1521–6.
15 Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem cell transplantation for acute myelogenous leukaemia (AML) predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–84.
16 Sobecks RM, Ball EJ, Maciejewski JP, Rybicki LA, Brown S, Kalaycio M, et al. Survival of AML patients receiving HLA-matched sibling donor allogeneic bone marrow transplantation correlates with HLA-Cw ligand groups for killer immunoglobulin-like receptors. Bone Marrow Transplant. 2007;39(7):417–24.
17 Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukaemia. 2005;19(8):1446–51.
18 Kim HJ, Choi Y, Min WS, Kim TG, Cho BS, Kim SY, et al. The activating killer cell immunoglobulin-like receptors as important determinants of acute graft-versus host disease in hematopoietic stem cell transplantation for acute myelogenous leukaemia. Transplantation. 2007;84(9):1082–91.
19 Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304.
20 Storb R, Prentice RL, Sullivan KM, Shulman HM, Deeg HJ, Doney KC, et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anaemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med. 1983;98(4):461–6.
21 Clausen J, Kircher B, Auberger J, Schumacher P, Ulmer H, Hetzenauer G, et al. The role of missing killer cell immunoglobulin-like receptor ligands in T cell replete peripheral blood stem cell transplantation from HLA-identical siblings. Biol Blood Marrow Transplant. 2010;16(2):273–80.
22 Beelen DW, Ottinger HD, Ferencik S, Elmaagacli AH, Peceny R, Trenschel R, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogenic hematopoietic stem cell transplantation in patients with myeloid leukaemias. Blood. 2005;105(6):2594–600.
23 Leung W, Iyengar R, Triplett B, Turner V, Behm FG, Holladay MS, et al. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol. 2005;174(10):6540–5.
24 Bignon JD, Gagne K. KIR matching in hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17(5):553–9.
25 Schellekens J, Rozemuller EH, Petersen EJ, van den Tweel JG, Verdonck LF, Tilanus MG. Activating KIRs exert a crucial role on relapse and overall survival after HLA-identical sibling transplantation. Mol Immunol. 2008;45(8):2255–61.
26 Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukaemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for paediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–29.
27 Sivori S, Carlomagno S, Falco M, Romeo E, Moretta L, Moretta A. Natural killer cell expression the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood. 2011;117(16):4284–92.
28 Scquizzato E, Zambello R, Teramo A, Baesso I, Varotto S, Albergoni MP, et al. KIR/HLA-I mismatching and risk of relapse in paediatric patients undergoing non-haploidentical allogeneic haematopoietic stem cell transplantation. Pediatr Transplant. 2011;15(2):198–204.
29 Chen C, Busson M, Rocha V, Appert ML, Lepage V, Dulphy N, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38(6):437–44.
30 Cook M, Briggs D, Craddock C, Mahendra P, Milligan D, Fegan C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107(3):1230–2.
31 Gallez-Hawkins GM, Franck AE, Li X, Thao L, Oki A, Gendzekhadze K, et al. Expression of activating KIR2DS2 and KIR2DS4 genes after hematopoietic cell transplantation: relevance to cytomegalovirus infection. Biol Blood Marrow Transplant. 2011;17(11):1662–72.
32 Hadaya K, de Rham C, Bandelier C, Bandelier C, Ferrari-Lacraz S, Jendly S, et al. Natural killer cell receptor repertoire and their ligands, and the risk of CMV infection after kidney transplantation. Am J Transplant. 2008;8(12):2674–83.