Figure 1
Sample size considerations.
DOI: https://doi.org/10.4414/smw.2013.13728
Methodology of a prospective, population-based cohort study
Hypertension currently affects about 1 billion individuals worldwide [1]. Because of its close correlation with age and obesity, the number of adults with hypertension is expected to increase by about 60% over the next 20 years [1]. In a Western population the lifetime risk of developing overt hypertension in middle-aged to elderly individuals is approximately 90% [2]. Elevated blood pressure and hypertension are major risk factors for the development of myocardial infarction, stroke, cardiovascular death, congestive heart failure and end-stage renal disease [3–6]. Cardiovascular risk is directly associated with blood pressure across a wide spectrum of blood pressure levels without evidence of a threshold down to at least 115/75 mm Hg [3, 4, 7].
Both environmental and genetic factors have been implicated in the occurrence of elevated blood pressure. Elevated blood pressure, dyslipidaemia and impaired glucose metabolism are closely associated with obesity and frequently occur together in an individual[8–10]. Many studies have shown that these metabolic abnormalities are closely associated with an increased risk of blood pressure progression and incident hypertension [11–15]. A common underlying mechanism such as inflammation, insulin resistance or genetic factors may therefore be implicated in several of these pathways.
With regard to genetic effects, the heritability estimates of mean 24-hour systolic and diastolic blood pressure were 63% and 68% in a population-based sample from the United Kingdom [16]. Similar results have been reported from the Framingham Heart Study, where investigators found heritability estimates of 57% and 56% for long-term systolic and diastolic blood pressure respectively [17]. These values are much higher compared to single-examination blood pressure values, which are estimated at some 40% [17]. Reliance on single blood pressure values may be one reason why identification of genetic polymorphisms associated with blood pressure and the development of hypertension has been slow and of limited success. Although we and others have identified a substantial number of polymorphisms in various candidate genes that might be related to blood pressure progression or incident hypertension, few of these gene variants have actually been validated in independent studies [18–21]. A recent meta-analysis of several large genome-wide association studies described 29 loci that were significantly associated with blood pressure and/or hypertension [22]. However, these variants explained only about 0.9% of the overall phenotypic variance of systolic and diastolic blood pressure, suggesting that many other genetic associations remain to be detected [22].
The main objective of this project is to assemble a large population-based cohort study among young and healthy adults with the aim of gaining further insights into the genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors and their progression over time. Other objectives include assessment of subclinical cardiac arrhythmias in the general population and evaluation of the role of sleep and sleep quality on the progression of cardiovascular risk factors.
The genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP) study is a population-based cohort study in the Principality of Liechtenstein. All inhabitants of the Principality of Liechtenstein aged 25–41 years are invited to participate in the study. The GAPP study is being performed in the Principality of Liechtenstein for the following reasons: (1) Possibility of targeting the entire population for enrolment, as opposed to a random subset in many other cohort studies; (2) efficient use of existing infrastructure including office space and sample collection and (3) possibility of close collaboration with several local authorities. Inclusion and exclusion criteria are listed in table 1. The study protocol was approved by the local ethics committee. Informed written consent is obtained from every individual participant.

Figure 1
Sample size considerations.
Specifically, a list of all current inhabitants of the Principality of Liechtenstein is provided by the National Office of Public Health. All inhabitants included on the list receive a study letter. Subsequently, potential participants are approached by phone and all consenting participants without exclusion criteria are invited in writing to the non-institutionalised study centre in Schaan, FL, which is located close to the study laboratory. Trained study nurses complete all measurements in a standardised manner. Multiple efforts are undertaken to minimise the non-response rate: (1.) Several attempts to contact participants including outside regular working hours, (2.) enrolment visits at week-ends, (3.) close cooperation with official Liechtenstein authorities to maintain an up-to-date address list, (4.) public relations activities to raise the population’s familiarity with the study and (5.) involve professional associations in Liechtenstein in formal support for the study. Details of all examinations are provided below. Follow-up examinations are scheduled every 3–5 years and should begin during 2013.
Baseline examination includes standardised assessment of several personal, medical, lifestyle and nutritional factors by questionnaire. Medication, former diseases and familial history of diseases is assessed. Physical activity is determined with the validated IPAQ questionnaire [23] while for the assessment of nutritional factors the official questionnaire of the Federal Office of Public Health is used (Swiss Health Survey, 2007). Smoking behaviour is determined with the Fagerström questionnaire [24].
Weight is measured in underwear with a calibrated balance (Seca 877, Hamburg). Height is determined without shoes backwards in front of a wall with a calibrated measuring tape (Seca 202, Hamburg). Waist circumference is obtained at the tightest part between costal arch and iliac crest, while hip circumference is measured round the widest portion of the buttocks.
Conventional office blood pressure is obtained in a quiet environment after at least 5 minutes’ rest with the participant sitting. The same validated oscillometric device (Microlife BP3AG1) is used in all participants. Three consecutive blood pressure measurements are obtained on the non-dominant arm. To minimise the white coat effect, the first reading is discarded and the average of the last two measurements is used for analyses relating to conventional blood pressure. Hypertension is defined as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg and/or use of antihypertensive treatment.
For ambulatory blood pressure monitoring we use a fully automatic, non-invasive device (Schiller BR-102 plus) which has been validated by international protocols [25]. The devices undergo periodic recalibration to maintain longitudinal consistency of blood pressure measurements. The monitoring device is fitted to the non-dominant arm by a trained study nurse and blood pressure is recorded during a 24-hour period. Blood pressure is measured every 15 minutes between 7.30 am and 10 pm, and every 30 minutes between 10 pm and 7.30 am. All participants are instructed to engage in their usual activities during the measurement period, but to keep their arm still during recordings. Daytime and nighttime blood pressure is defined according to a diary to be kept by all participants during the measurement period. Ambulatory blood pressure recordings are repeated if <80% of possible recordings are available, but remain otherwise unedited for analysis.
A fasting venous blood sample is obtained from every participant by a trained study nurse using a minimally traumatic venipuncture. Morning spot urine samples are collected from every participant. Urinary sodium, creatinine and albumin levels are measured in all individuals. Specimens are analysed in an accredited medical laboratory (ISO 17025) located next to the study centre. This allows optimal pre-analytical sample handling including immediate analysis. Urine samples for all participants are stored at–80 °C for future analyses. A large panel of blood biomarkers are assayed, including established cardiovascular risk marker including total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, plasma glucose and C-reactive protein. A complete list of all biomarkers with their corresponding analytical characteristics is provided in table 2. Several aliquots including whole blood for genetic analyses are entered into a biobank for long term storage at –80 °C.
Standard 12-lead ECGs are obtained in all participants. All ECGs are interpreted by a trained physician. A 24-hour Holter EKG is performed using the Medilog AR12plus device (Schiller AG, Baar, Switzerland). All Holter recordings are imported into the software Medilog®DARWIN (Schiller AG, Baar, Switzerland) for post-processing. All 24-hour Holter ECGs are extensively scrutinised by a trained physician to remove artefacts and adjust templates. The system used is programmed to stop at any rhythmic event or ectopic beat, corresponding to a true count of all events on the full disclosure.
Bioelectrical impedance analysis is used to assess body composition of all participants using standardised methodology and a validated device (BIA ego fit, 2010, Germany).
In June 2011, the study steering committee decided to add lung function testing and nighttime pulse oximetry to the study protocol. These exams are therefore not available from the first 604 participants. Lung function is tested using simple spirometry without bronchodilation or challenge tests, using a commercially available device (EasyOne, ndd Medizinaltechnik, Zürich, Switzerland). The test is performed by trained study nurses using internationally accepted standardisation guidelines [26].
We use the validated ApneaLink device for sleep pulse oximetry and nasal flow measurement [27, 28]. Pulse oximetry is a standard screening approach in outpatients with excessive daytime sleepiness. Pulse oximetry has been shown to have a high specificity but low sensitivity for the diagnosis of obstructive sleep apnoea. Due to its relatively low sensitivity for diagnosis of obstructive sleep apnoea, pulse oximeters were combined with nasal flow measurement. Nasal flow can be monitored using a nasal cannula connected to a pressure transducer. A drop in pressure can reliably detect apnoeas and hypopnoeas. It has been shown that measurement of nasal-cannula flow compared to polysomnography has a high sensitivity and specificity in detecting patients with obstructive sleep apnoea [27, 28].
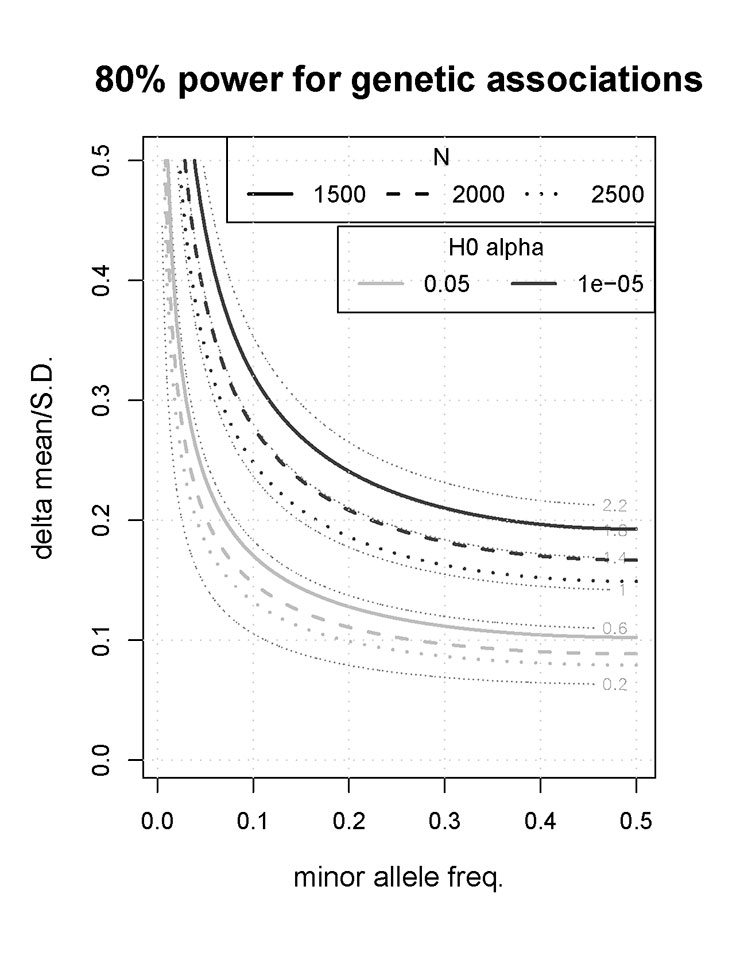
The principal sample size considerations were based on a genome-wide study approach involving at least 300,000 to 500,000 polymorphisms for each participant. We assumed that polymorphisms with a p value of <10–5 will be included in the replication set, a threshold similar to that used in prior studies [29]. Assuming a power of 80% and an alpha level of 10–5, a sample size of about 2,000 participants allows detection of genetic polymorphisms that explain about 1.4% of the variance (fig. 1.) For example, we shall have 80% power to characterise genetic variants that are associated with a blood pressure difference of about 2 mm Hg, assuming a standard deviation of 10 mm Hg for ambulatory blood pressure differences (i.e. delta mean/standard deviation = 0.2) [16] and a minor allele frequency of at least 0.1. Associations in this order of magnitude have recently been discovered in genome-wide association studies for various cardiovascular risk factor traits [30–33]. Finally, the figure also highlights that if for whatever reason the study recruits slightly fewer than 2,000 participants, the resultant loss of power is minimal.
Even more power is available for other types of genetic study where less stringent p values are required (e.g., candidate-gene studies or independent confirmation of promising findings from other studies), as shown in the figure. In this context, assuming a minor allele frequency >0.1, an alpha of 0.05 and a power of 80%, a study of 2,000 participants allows detection of genetic polymorphisms which explain some 0.4% of the variance. For example, we have 80% power to characterise genetic variants that are associated with a blood pressure difference of 1–1.5 mm Hg, assuming a standard deviation of 10 mm Hg for blood pressure differences (i.e., delta mean/standard deviation = 0.10–0.15) [16] and a minor allele frequency of at least 0.1.
Similar statistical criteria (i.e. alpha = 0.05) are required for phenotype-phenotype association studies. Because association studies using continuous traits are more efficient than studies using categorical traits (e.g. genotypes), this project has substantial power to find previously unknown phenotype-phenotype associations. Finally, given that spirometry and pulse oximetry was not performed in the first 604 participants, this study may have a somewhat reduced power to assess these phenotypes.
| Table 1:GAPP inclusion and exclusion criteria. |
| Inclusion criteria |
| Age between 25 and 41 years. |
| Inhabitant of the Principality of Liechtenstein. |
| Exclusion criteria |
| Any known cardiovascular disease, including coronary artery disease, stroke, transient ischaemic attack, peripheral artery disease, renal disease, atrial fibrillation or congestive heart failure. |
| Body mass index >35 kg/m2. |
| Current intake of antidiabetic drugs or insulin. |
| Current pregnancy or lactation (enrolment postponed until breast feeding is completed). |
| Current daily intake of nonsteroidal anti-inflammatory drugs including aspirin, regular intake of steroids (>1 day per week), regular intake of liquorice (>1 day per week), or regular intake of sympathomimetic drugs (>1 day per week). |
| Documented obstructive sleep apnoea syndrome. |
| Any other major illness. |
| Intention to leave Liechtenstein/Switzerland on a permanent basis. |
| Table 2: Baseline blood and urinary biomarkers in GAPP. | ||||||
| Analyte | Method | Unit | CV 1 (%) | Mean 1 | CV 2 (%) | Mean 2 |
| Amylase | Roche Cobas | U/l | 0.92 | 81.41 | 0.90 | 193.92 |
| ALAT | Roche Cobas | U/l | 2.04 | 46.23 | 0.89 | 135.05 |
| ASAT | Roche Cobas | U/l | 1.60 | 44.14 | 1.21 | 113.47 |
| Alkaline phosphatase | Roche Cobas | U/l | 4.10 | 92.3 | 2.86 | 199.62 |
| Total bilirubin | Roche Cobas | µmol/l | 3.44 | 14.59 | 2.35 | 60.20 |
| Calcium | Roche Cobas | mmol/l | 1.81 | 2.06 | 1.47 | 3.30 |
| Total cholesterol | Roche Cobas | mmol/l | 3.26 | 2.36 | 1.52 | 4.58 |
| HDL-cholesterol | Roche Cobas | mmol/l | 2.38 | 0.72 | 2.05 | 1.49 |
| LDL-cholesterol | Roche Cobas | mmol/l | 1.63 | 1.45 | 1.27 | 2.53 |
| Complete blood count | Sysmex XE 5000 | n.a. | n.a. | n.a. | n.a. | |
| Copeptin | Brahms Luminometry | pmol/l | 5.4 | 11.6 | 5.3 | 108.9 |
| Creatine kinase | Roche Cobas | U/l | 1.51 | 167.29 | 1.06 | 316.02 |
| Creatinine | Roche Cobas | µmol/l | 2.44 | 96.02 | 1.88 | 320.65 |
| C-reactive protein | Roche Cobas | mg/l | 2.04 | 5.85 | 2.46 | 36.16 |
| Cystatin C | Roche Cobas | mg/l | 2.73 | 1.12 | 1.22 | 4.39 |
| Ferritin | Roche Cobas | ng/ml | 1.88 | 25.43 | 2.73 | 168.48 |
| Iron | Roche Cobas | µmol/l | 2.17 | 20.31 | 1.13 | 44.07 |
| Transferrin | Roche Cobas | g/l | 1.75 | 2.08 | 2.15 | 3.65 |
| GGT | Roche Cobas | U/l | 2.29 | 46.86 | 1.00 | 204.75 |
| Fasting glucose | Roche Cobas | mmol/l | 1.12 | 5.63 | 0.99 | 12.8 |
| Haemoglobin | Sysmex XE 5000 | g/l | 1.0 | 59 | 0.6 | 124 |
| HbA1c | Biorad D10 | % | 1.91 | 5.6 | 1.6 | 10.71 |
| LDH | Roche Cobas | U/l | 1.35 | 172.98 | 1.25 | 321.05 |
| NT-proBNP | Roche Cobas | pg/ml | 4.32 | 157.94 | 3.0 | 4,792.45 |
| Phosphate | Roche Cobas | mmol/l | 1.91 | 1.24 | 1.09 | 2.08 |
| Potassium | Roche Cobas | mmol/l | 1.01 | 3.64 | 0.78 | 6.40 |
| Total protein | Roche Cobas | g/l | 1.30 | 49.06 | 1.10 | 74.57 |
| Sodium | Roche Cobas | mmol/l | 1.02 | 113.06 | 0.85 | 137.52 |
| Triglycerides | Roche Cobas | mmol/l | 1.50 | 1.26 | 1.12 | 2.35 |
| hsTroponin T | Roche Cobas | µg/l | 2.37 | 0.03 | 2.88 | 2.13 |
| TSH | Roche Cobas | mIE/l | 6.42 | 0.20 | 4.99 | 1.70 |
| Urea | Roche Cobas | µmol/l | 1.97 | 302.05 | 1.38 | 598 |
| Uric acid | Roche Cobas | mmol/l | 1.45 | 6.72 | 1.17 | 19.46 |
| Urine dipstick analysis | Roche Miditron | n.a. | n.a. | n.a. | n.a. | |
| Urinary albumin | Roche Cobas | mg/l | 6.04 | 35.58 | 1.71 | 113.76 |
| Urinary creatinine | Roche Cobas | µmol/l | 1.55 | 8,653.87 | 1.50 | 4,458.7 |
| Urinary protein | Roche Cobas | mg/l | 4.1 | 204.68 | 1.3 | 1,071.25 |
| ALAT = alanine aminotransferase; ASAT = aspartate aminotransferase; CV = coefficient of variation; GGT = gamma-glutamyl transpeptidase; HbA1c = haemoglobin A1c; hs = high-sensitive; LDH = lactic acid dehydrogenase; NT-proBNP = N-terminal pro B-type natriuretic peptide; TSH = thyroid-stimulating hormone. The laboratory methods employed are displayed with their corresponding imprecision at up to 3 different levels of mean analyte concentrations. The CVs and mean concentrations are calculated from at least 20 daily measurements of commercially available control materials, as performed in the laboratory analysing the study samples. | ||||||
Between June 2010 and July 2012 trained study nurses called 2,843 potential participants, of whom 514 could not be reached and 876 declined to participate. BMI ≥35 kg/m2 was found in 24 individuals, 44 had other exclusion criteria and 31 cancelled the study appointment for unknown reasons. Furthermore, the inclusion of 29 women was delayed due to pregnancy and 158 individuals were interested in participating but delayed the appointment for scheduling reasons, leaving 1,167 individuals who were included in GAPP. An additional 166 eligible participants spontaneously made an appointment at the study centre, such that by July 2012 1,333 had been enrolled. We estimate that enrolment will be completed by mid-2013.
Baseline characteristics of the participants included so far are shown in table 3. Mean age is 36.7 years and 47.5% of all participants are male. Around 20% of all participants are current smokers, and 57% have never smoked. Mean levels of all traditional risk factors are within normal ranges, confirming the inclusion of an overall healthy cohort of young adults. Nevertheless, among male participants the prevalence of hypertension and prediabetes is 25% and 32% respectively. The corresponding numbers among female participants are 6% and 23% respectively. Only 26 individuals receive blood pressure lowering treatment. As expected, levels of N-terminal B-type natriuretic peptides are low, and only 4% of the participants have a NT-proBNP value ≥125 ng/l, which is considered elevated according to the manufacturer’s package insert. Troponin levels are more often measurable in men than in women (24.7% versus 10.1%, respectively), although most participants have troponin values below the detection threshold independent of sex.
| Table 3: Gender-specific baseline characteristics. | |||
| n = 1,333 | Men (n = 632) | Women (n = 701) | p |
| Age (y) | 36.8 ± 4.85 | 36.6 ± 4.89 | 0.41 |
| Height (cm) | 178.7 ± 7.0 | 165.6 ± 6.1 | <0.0001 |
| Weight (kg) | 83.3 ± 11.3 | 64.4 ± 10.7 | <0.0001 |
| BMI (kg/m2) | 26.1 ± 3.1 | 23.5 ± 3.9 | <0.0001 |
| Smoking (%) | 0.07 | ||
| Current | 141 (22.3) | 133 (19.0) | |
| Past | 149 (23.6) | 144 (20.5) | |
| Never | 341 (54.0) | 421 (60.1) | |
| Fat mass (%) | 20.6 ± 5.1 | 28.8 ± 5.0 | <0.0001 |
| Muscle mass (%) | 37.6 ± 3.3 | 33.2 ± 3.7 | <0.0001 |
| Body water (%) | 57.1 ± 4.3 | 52.0 ± 5.1 | <0.0001 |
| Systolic BP (mm Hg) | 128.3 ± 11.9 | 113.6 ± 10.2 | <0.0001 |
| Diastolic BP (mm Hg) | 82.7 ± 9.1 | 74.5 ± 8.4 | <0.0001 |
| Systolic 24h BP (mm Hg) | 130.6 ± 9.7 | 117.4 ± 8.3 | <0.0001 |
| Diastolic 24h BP (mm Hg) | 80.2 ± 7.7 | 74.2 ± 6.6 | <0.0001 |
| Hypertension (%)* | 156 (24.7) | 40 (5.7) | <0.0001 |
| Prediabetes (%)† | 203 (32.1) | 162 (23.1) | 0.0002 |
| Triglycerides (mmol/l) | 1.10 (0.77; 1.60) | 0.73 (0.57; 1.00) | <0.0001 |
| LDL cholesterol (mmol/l) | 3.34 ± 0.86 | 2.75 ± 0.74 | <0.0001 |
| HDL cholesterol (mmol/l) | 1.34 ± 0.32 | 1.74 ± 0.40 | <0.0001 |
| HbA1c (%) | 5.5 ± 0.37 | 5.44 ± 0.37 | 0.003 |
| Fasting glucose (mmol/l) | 4.9 (4.7; 5.3) | 4.7 (4.4; 4.9) | <0.0001 |
| hsCRP (mg/l) | 0.9 (0.5; 1.7) | 0.9 (0.5; 2.0) | 0.57 |
| Troponin ≥3 ng/l (%) | 156 (24.7) | 71 (10.1) | <0.0001 |
| Copeptin (pmol/l) | 3.6 (2.5; 5.5) | 2.2 (1.6 ; 3.3) | <0.0001 |
| NT- proBNP (ng/l) | 20.0 (11.0; 34.0) | 50.0 (34.0; 80.0) | <0.0001 |
| Haemoglobin (g/l) | 150.1 ± 8.2 | 130.4 ± 9.2 | <0.0001 |
| Data are presented as mean ± SD, median (interquartile range) or number (percentage). BMI = body mass index; BP = blood pressure; LDL = low density lipoprotein, HDL = high density lipoprotein; HbA1c = haemoglobin A1c; hsCRP = high sensitivity C-reactive protein; NT-proBNP = N-terminal B-type natriuretic peptide. * Hypertension is defined as a systolic office BP of ≥140 mm Hg, a diastolic office BP of ≥90 mm Hg and/or current use of blood pressure lowering drugs [44]. † Prediabetes was defined as HbA1c between 5.7% and 6.4% [45]. | |||
While many established cohort studies have performed large scale comprehensive genetic analyses in the evaluation of elevated blood pressure and other cardiovascular risk factors [34–37], there are several unique features that distinguish the current project from those studies. First, almost all previous large scale cohort studies used conventional office blood pressure instead of ambulatory blood pressure measurement. Blood pressure measurement may therefore not have been reliable enough to uncover potentially important genetic associations. Second, rapid progress in sequencing technologies will enable application of this technology in the not too distant future. Third, the availability of DNA, blood and urine specimens in one large, well-characterised cohort study provides an ideal platform to assess in detail predictors and causes of cardiovascular risk factors, as well as their interrelationships. This large platform also allows great flexibility for future, as yet unanticipated, analyses or ancillary projects, and is an ideal starting point for collaboration with large international consortia looking at the genetic basis of cardiovascular risk factors. Fourth, unlike previous studies which enrolled mainly middle-aged to elderly individuals, a sizable proportion of them with prevalent hypertension, diabetes mellitus or cardiovascular disease, the current project focuses on young and healthy individuals. Studies on cardiovascular risk factor progression among young adults are scarce [38, 39], and most of them do not have ambulatory blood pressure measurement as well as a wide range of biological specimens available for analysis. The importance of studying the pathobiology of cardiovascular risk factor progression among young individuals is underscored by prior studies showing that arteriosclerotic disease is a long-term process initiated early in life [40, 41]. Finally, the current study offers a unique opportunity to assess the significance of subclinical cardiac arrhythmias and the role of sleep and sleep quality in the progression of cardiovascular risk factors in the general young population.
Cumulative long term exposure to a proatherogenic environment over a lifetime, in combination with prevalent disease, may mask a potentially important effect of genetic polymorphisms that remain stable over time. The great advantage of studying cardiovascular risk factors among young, healthy individuals has been highlighted in at least two prior publications. In a cohort study of 1,160 male former medical students over more than 50 years of follow-up, parental history of hypertension was a highly significant predictor for developing hypertension, even after taking into account potential confounders (hazard ratio [95% confidence interval] 2.4 [1.8–3.2] for individuals where both parents had received a diagnosis of hypertension), suggesting that genetic factors play an important role in the pathogenesis of incident hypertension [42]. Importantly, a parental history of hypertension greatly increased the risk of early onset hypertension in offspring by a factor of 6.2 throughout adult life and by a factor of 20.0 at the age of 35 years, suggesting that investigation of genetic and other risk factors is more efficient and precise in younger populations. Similar findings were reported from the Swedish Twin Registry [43]. In this study, the relative risk (95% confidence interval) of dying from coronary heart disease among monozygotic twins was 8.1 (2.7–24.5) and 2.1 (1.4–3.2), depending on whether their respective twins had died of coronary heart disease before age 55 years or between 76 and 85 years of age respectively. These highly differential risk estimates indicate that genetic susceptibility to death from coronary heart disease becomes less pronounced with higher age.
The present project offers an excellent opportunity to assess in detail genetic and phenotypic predictors of cardiovascular risk factors and their progression over time in young and healthy adults from the general population using a variety of state-of-the-art assessment tools. Findings from this study will improve our understanding in the regulation and pathobiology of blood pressure and other cardiovascular risk factors, improve cardiovascular risk stratification in individual patients and potentially define potential new drug targets. By combining the advantage of a lower confounding potential in a young homogeneous sample population with precise measurement of blood pressure and other cardiovascular risk factor phenotypes as well as availability of various samples for future analyses, the present project maximises the likelihood of discovering previously unknown genetic and phenotypic risk markers and replicating previously established findings in a unique study sample.
Acknowledgment:We would like to thank the local study team in Schaan, FL, including Manuela Schoeb, Regina Boss, Ariane Brehm, Liliane Nipp, Ursula Scattolin, Susanne Wäger, Priska Senn and Arnulf Holzknecht. We would also like to thank Gian Völlmin for administrative support and Jörg Leuppi and David Miedinger for supporting the set-up of lung function testing and nighttime pulse oximetry. Finally, we thank the different professional societies in Liechtenstein (Liechtensteinische Ärztekammer, Liechtensteinische Patientenorganisation, Verband Liechtensteinischer Samaritervereine) and the Liechtenstein Office of Public Health, especially Peter Gstöhl and Helen Naeff, for their support.
1 Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J.Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
2 Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al.Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–10.
3 Conen D, Ridker PM, Buring JE, Glynn RJ.Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: prospective cohort study. BMJ. 2007;335:432.
4 Lewington S, Clarke R, Qizilbash N, Peto R, Collins R.Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
5 Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK.The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62.
6 Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C.Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–8.
7 Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al.Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7.
8 Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
9 Alberti KG, Zimmet PZ.Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998;15:539–53.
10 Haslam DW, James WP.Obesity. Lancet. 2005;366:1197–209.
11 Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD.A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007;20:370–7.
12 Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al.Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–65.
13 Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB.Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72.
14 Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ.Blood pressure and risk of developing type 2 diabetes mellitus: The Women’s Health Study. Eur Heart J. 2007;28:2937–43.
15 Sesso HD, Buring JE, Chown MJ, Ridker PM, Gaziano JM.A prospective study of plasma lipid levels and hypertension in women. Arch Intern Med. 2005;165:2420–7.
16 Tobin MD, Raleigh SM, Newhouse S, Braund P, Bodycote C, Ogleby J, et al.Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation. 2005;112:3423–9.
17 Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, et al.Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension. 2000;36:477–83.
18 Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY.Natriuretic peptide precursor gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50:1114–9.
19 Conen D, Cheng S, Steiner LL, Buring JE, Ridker PM, Zee RY.Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: The Women’s Genome Health Study. J Hypertens. 2009;27:476–83.
20 Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY.Association of renin-angiotensin and endothelial nitric oxide synthase gene polymorphisms with blood pressure progression and incident hypertension: prospective cohort study. J Hypertens. 2008;26:1780–6.
21 Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al.Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53.
22 The International Consortium for Blood Pressure Genome-Wide Association Studies.Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9.
23 Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al.International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
24 Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO.The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27.
25 Denchev SV, Simova, II, Matveev MG.Evaluation of the SCHILLER BR-102 plus noninvasive ambulatory blood pressure monitor according to the International Protocol introduced by the Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Blood pressure monitoring. 2007;12:329–33.
26 Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al.Standardisation of spirometry. Eur Respir J: official journal of the European Society for Clinical Respiratory Physiology. 2005;26:319–38.
27 Erman MK, Stewart D, Einhorn D, Gordon N, Casal E.Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med: official publication of the American Academy of Sleep Medicine. 2007;3:387–92.
28 Weinreich G, Armitstead J, Topfer V, Wang YM, Wang Y, Teschler H.Validation of ApneaLink as screening device for Cheyne-Stokes respiration. Sleep. 2009;32:553–7.
29 Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al.Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–65.
30 Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al.Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet. 2008;82:1185–92.
31 Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al.Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–97.
32 Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, et al.Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–6.
33 Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al.Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9.
34 Cupples LA, Arruda HT, Benjamin EJ, D’Agostino RB, Sr., Demissie S, DeStefano AL, et al.The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet. 2007;8(Suppl 1):S1.
35 Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, et al.Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;54:249–55.
36 Vollenweider P, Hayoz D, Preisig M, Pecoud A, Waterworth D, Mooser V, et al.L’etat de sante des Lausannois: premiers resultats de l’etude CoLaus. Rev Med Suisse. 2006;2:2528–30, 2532–3.
37 Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, et al.LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008;371:483–91.
38 Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., et al.CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16.
39 The Bogalusa Heart Study 20th anniversary symposium. Am J Med Sci. 1995;310(Suppl):S1–S138.
40 McNamara JJ, Molot MA, Stremple JF, Cutting RT.Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216:1185–7.
41 Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al.High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–10.
42 Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ.Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins Precursors Study. Arch Intern Med. 2008;168:643–8.
43 Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U.Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–6.
44 Mancia G, De Backer G, Dominiczakc A, Cifkovad R, Fagarde R, Germanof G, et al.2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension. ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751–62.
45 American Diabetes A.Standards of medical care in diabetes – 2012. Diabetes care. 2012;35(Suppl 1):S11–63.
Funding / potential competing interests: The GAPP study is supported by the Liechtenstein Government, the Swiss National Science Foundation (PP00P3_133681, to David Conen), the Swiss Heart Foundation, the Swiss Society of Hypertension, the University of Basel, University Hospital Basel, the Hanela Foundation, Schiller AG and Novartis.