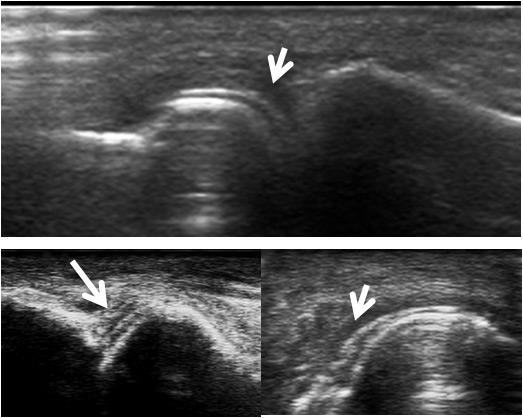
Figure 1
"Double contour" sign in gouty arthritis (white arrows): dorsal view of several metatarso-phalangeal joints performed outside an acute flare of gout.
DOI: https://doi.org/10.4414/smw.2013.13861
The diagnosis of rheumatoid arthritis (RA) is based essentially on clinical and biological parameters, although the new ACR/EULAR classification criteria include magnetic resonance imaging (MRI) and ultrasound (US) as additional tools to assess objectively joint involvement [1, 2].
The primary treatment goal in RA is to maximise long-term health-related quality of life by controlling the symptoms, preventing structural damage, normalising or preserving function, and enhancing social participation. Since the era of the new biologic treatments, these goals can be achieved in most patients and the benefit of tight disease control has been demonstrated. To optimise outcome, therapies should be adjusted according to disease activity. The ACR (American College of Rheumatism) response criteria were not established for clinical practice but rather for clinical trials. In contrast, the DAS (disease activity score), CDAI (clinical disease activity index) and SDAI (simplify disease activity index) are clinically useful for the continuous prospective monitoring of individual patients [3] . In many studies, however, these indexes, in particular the DAS, yielded a low reproducibility when applied in the setting of a large group of physicians [4]. Up to 30% of patients fulfilling remission criteria according to DAS, ACR and European League against rheumatism (EULAR) criteria still had progression in joint damage, suggesting that clinical criteria may not be reliable [5].
There are more and more data suggesting that US is useful in differential diagnosis, detection of early disease, disease activity monitoring, guidance of treatment decisions, and in the follow-up of remission of RA [6]. Over the last decade, US technology and machines have progressed such that good quality machines are now available for a reasonable price, allowing a wide distribution among rheumatologists in both clinical and private practice. In Switzerland, about 40% of rheumatologists have access to an US machine and have received basic training in musculoskeletal US. Moreover, US has been included in the formal training curriculum for all future Swiss rheumotologists.
In 2008, the Swiss Sonography in Arthritis and Rheumatism (SONAR) group was set up as an educational progamme focussing on the use of US in inflammatory arthritis in clinical practice. A semiquantitative grayscale (B) mode and doppler (PD) score was developed in accordance with several earlier published scores and based on the recommendations of the of the OMERACT [7]. The scoring system was taught to 108 rheumatologists.
This paper summarises a Swiss consensus on best clinical practice recommendations for the use of US in RA, based on the current literature and experience with the Swiss SONAR score since it was implemented in the Swiss RA registry database (SCQM).
The literature research, including the SONAR score data, was discussed by a panel of experts from leading Swiss rheumatology departments, researchers and clinicians, as well as members of the international Targeting US Initiative (TUI), and board certified rheumatologists in private practice with extensive training in US.
Synovitis can be demonstrated by US, appearing as hypoechogenic hypertrophy of synovial tissue in greyscale (B-mode). With the new US machines, synovial hypertrophy can be distinguished from fluid collection in most cases. For the detection of synovial hypertrophy, US is much more sensitive than clinical examination and can compare to MRI, especially when finger joints are evaluated [8]. The presence of vascularisation inside the synovial tissue in Doppler (PD) mode reflects active inflammation [9]. However the performance of PD is highly dependent on the quality of the machine, as well as subtle modification of the settings, the experience of the operator and the localisation of the examined joint (best in superficial joints) [10–11]. US can also differentiate between synovial inflammation and tenosynovitis, bursitis and other soft tissue lesions that can mimic clinical synovitis [7–8].
US can detect erosions at an earlier stage than traditional X-rays, especially in finger joints. Erosion scoring is, however, very time-consuming and can be difficult to perform in some joints [2].
Finally, cartilage lesions (loss of thickness, crystal deposits) can be assessed by this modality, especially in the small joints of the hands that are frequently involved in RA [12].
A pivotal element in the diagnosis of RA is the presence of synovitis in index joints. Joint involvement refers to any swollen or tender joint on examination, which may be confirmed by evidence of synovitis on imaging [13]. Several studies confirmed that US is a valid tool and is more sensitive than clinical examination in the detection of synovitis [8]. Hence, adding US to a clinical prediction rule (Leiden rule) in early RA raises the predictability of RA diagnosis [14].

Figure 1
"Double contour" sign in gouty arthritis (white arrows): dorsal view of several metatarso-phalangeal joints performed outside an acute flare of gout.
However, some precautions must be considered before using US systematically for the diagnosis of early RA. Only a very limited number of studies have, to our knowledge, evaluated the performance and the prognostic relevance of the new ACR/EULAR criteria with and without the use of US [15]. Moreover, significant US synovitis has not been clearly defined by the ACR/EULAR committee, and some studies have shown that US synovial hypertrophy can also be found in osteoarthritis and even in controls without any joint disease [16].
Some US signs can help to distinguish RA from other causes of arthritis. For example in early psoriatic arthritis (PsA), hypoechoic swelling surrounding the extensor digitorum tendon and peritendinous PD signal seem to predominate compared with intra-articular PD signal in early RA [17]. The described US pattern in PsA seems to confirm the presence of enthesitis [17–18]. US findings such as the presence of a hyperechoic signal within the cartilage layer (calcium pyrophosphate [CPPD] deposition disease) [19] or very superficial cartilage crystals (“double contour” sign in gout) [20] have been described (fig. 1). For CPPD crystal deposition, US imaging can give a positive likelihood ratio (LR) as high as 7.9–29, compared with a much lower positive LR for synovial fluid analysis (7.0) and plain radiography (0.36) [21–22]. Therefore, US has been proposed as an important method for the early diagnosis of CPPD crystal deposition [23]. There are, however, no controlled studies that have examined the capability of US to distinguish morphologically early RA from crystal-associated joint diseases. Osteophytes, one of the morphological hallmarks of osteoarthritis, can also reliably be assessed by US with a good agreement between MRI and US. Of note, US is more sensitive than conventional radiology and clinical examination [2, 4–25]. All of the above-mentioned findings support the idea that US may add important information to the differential diagnosis of articular diseases [26].
In clinical studies, the reliability of US repeatedly has been shown to be superior to the clinical evaluation for the differentiation between arthritis and arthralgia [8]. A normal US with nonspecific arthralgia could, therefore, help to differentiate arthritis from fibromyalgia or other nonspecific painful conditions. However, more studies are needed to evaluate the exact role of US in the diagnosis of inflammatory arthralgia and undifferentiated inflammatory arthritis.
US can be used to demonstrate the presence of synovitis in individual joints. RA is a polyarticular disease and, therefore, US has essentially been studied as a tool for diagnosis and follow-up by scoring many joints. How many and which joints should be included, which view of the joints to be examined (volar or palmar), the quantification of synovitis (yes/no, semiquantitative), which mode (B-mode only, separated from Doppler or combined) are still debated. At this stage, there is no commonly accepted standardised score, although the OMERACT has proposed minimal criteria for validation [26].
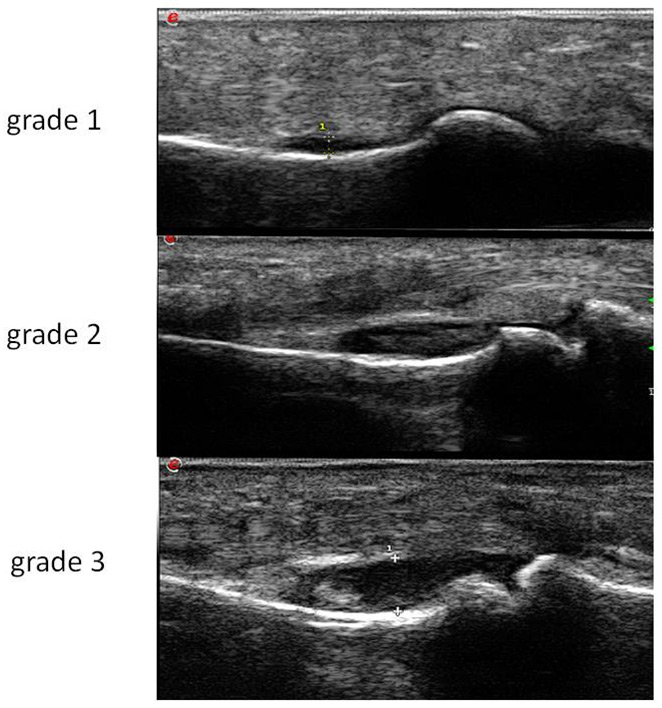
Figure 2
Synovitis grade 1-3 on B mode: proximal inter-phalangeal joint.
Several different scores for US have been studied and validated. The first ones comprised up to 68 joints. To render them more feasible for daily practice, investigators have progressively reduced the number of examined joints. The validated Spanish score examines 12 joints including the feet. It comprises B-mode and PD-mode with a semi-quantitative score according to the OMERACT recommendations [27]. In the hands and the feet, B-mode is performed by examining the dorsal side of the joints. The Berlin score examines seven joints, including hands and feet with a palmar view in the hands for B-mode [28].
RA and other forms of inflammatory polyarthritis like psoriatic arthritis can also involve tendons and bursae. Several attempts to develop scoring systems limited to tenosynovitis or including tenosynovitis in a synovitis score have been proposed [29]. Some have been validated like the German seven-joint score, which includes also tenosynovitis of the wrist and fingers [28], but their prognostic significance has not yet been fully evaluated.
The Swiss SONAR score analyses 22 joints in order to include approximately the same joints as the DAS, but excluding the thumbs and shoulders. These joints were excluded because they could be the site of common rheumatological problems not related to RA (osteoarthritis, rotator cuff lesions and other disorders). The score includes the palmar view of joints for B-mode, with a semiquantitative assessment in accordance with the Berlin score (fig. 2) and the dorsal view in PD-mode (fig. 3). The scanning time is between 10 and 15 minutes including PD evaluation. The cartilage thickness and presence of bony erosions and tenosynovitis can be assessed optionally.
Although the criteria of validation varied from one publication to the other, they usually included reproducibility (inter- and intra-operator with or without image acquisition) [30–32], correlation with the clinical scores (DAS, CDAI, etc.) [30–31] and responsiveness to treatments, usually assessed by measurement of standardised response mean (SRM) [30–31]. The Spanish and Berlin scores, were validated in selected patients by a limited number of well-trained and experienced US specialists with high-quality machines and after training as well as strict standardisation [27–28].
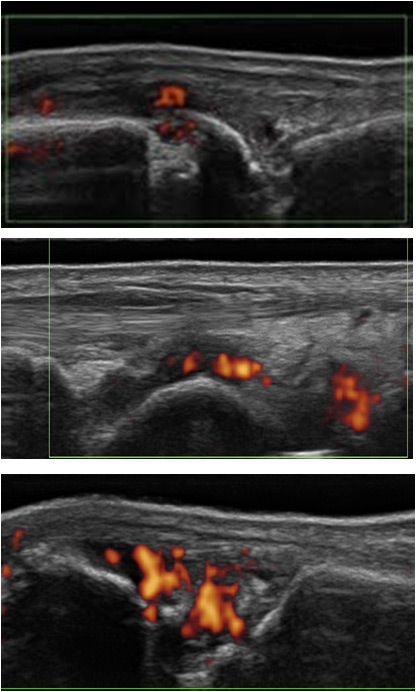
Figure 3
Synovitis grade 1-3, PD activity: dorsal view of a metacarpo-phalangeal joint. Presence of an erosion.
For the SONAR score, the inter-reader reproducibility was studied among a subset of 12 rheumatologists who had attended our teaching module 1 year before the validation procedure [33]. The reproducibility was fairly good (kappa: 0.53 for B-mode – 0.85 for Doppler) when the ultrasonographer had had some practical experience since the teaching (at least five patients with a SONAR score included in the SCQM). We used the data in the SCQM registry from 2009 onwards, where 980 US examinations (including 40 controls without rheumatic diseases) had been scored by different operators in real-life conditions in order to validate the correlation between the DAS and responsiveness to therapy by SRM measurements. The mean Pearson correlation coefficient, r (0.40 for B-mode – 0.41 for Doppler) and the mean SRM (0.80 for B-mode ‒ 0.86 for Doppler) were in the same ranges as those obtained in controlled studies [34].
Validation of the score under these conditions confirms the feasibility and possible integration of an US scoring system for RA monitoring into daily clinical practice. We must however emphasise that the SONAR, like most other scores, has been developed for the follow-up of RA patients and not for diagnosis or to differentiate RA from other types of inflammatory arthritis. No study evaluating the SONAR score for these topics has yet been done. It can. However. differentiate well controls without any musculoskeletal symptoms from RA patients, even when in remission [35].
US has been intensively studied in established RA. Different aspects have been evaluated: the correlation of US scores with other validated methods of measuring disease activity, such as the DAS; the ability of US to predict progression of structural damage such as erosions; the use of US as a single or a complementary outcome measure in a “treat-to-target” strategy; and, finally, the role of US imaging in defining remission.
Whichever score is studied, there is a significant correlation between US scoring and classical categories of DAS activities: remission (<2.6), low disease activity (≥2.6 to <3.2), moderately active disease (≥3.2 to <5), very active disease (≥5.1). On an individual level there are, however, many discordances. These discrepancies may have several explanations. The lack of reproducibility of the DAS, especially when performed by many different rheumatologists, is a well-known problem [4]. Another reason might be some heterogeneity in the quality of US examinations when performed by many different operators, as was the case in the SONAR score validation study. However, DAS measurements have been shown to be even more heterogeneous than US scorings [29]. Therefore, we think that US could be more reliable for the assessment of disease activity in routine practice than the classical clinical scores.
Individual discordances may also reflect real differences because clinical and US examinations provide complementary information on the arthritis status. Moreover, it seems that B- and Doppler-mode must be interpreted separately. The level of greyscale synovitis correlates better with disease duration, and probably reflects the level of previous inflammation and some fibrotic change, whereas the presence of PD signal seems less dependent on disease duration, but could be a better marker of ongoing inflammation [36].
Treating RA patients in accordance with clinical targets has been shown to improve outcome [37]. In randomised controlled studies, the ACR criteria response core dataset was the gold standard for assessing disease activity. In recent years, other scores such as the DAS, SDAI or CDAI have also been introduced and are increasingly applied in studies, as well as in practice.
A few studies have suggested that the addition of an US assessment to the management of patients with inflammatory arthritis improves the prediction of clinical outcomes [38]. Treatment in accordance with imaging measures could therefore provide better outcomes than treatment in accordance with clinical targets alone. This statement has not yet been fully confirmed and is under investigation in three prospective randomised multicentre studies. The use of US imaging to tailor the intensity of therapy should also be validated in registry cohorts such as the SCQM before its widespread use in routine practice is definitely recommended.
The detection of structural damage is of major prognostic importance and may determine future treatment decisions. In most randomised studies, erosion status before and after treatment has been assessed by standard X-rays with the application of validated scores, in particular the Sharp/van der Heijde or the Genant scores. In the early stages of the disease, computed tomography (CT) might be the gold standard for detection of the first erosions. Disadvantages of CT are radiation hazard, availability issues and costs. With MRI, very small erosions can be detected, and bone marrow oedema visualised with MRI even predicts the occurrence of erosions. On the other hand, several publications have confirmed that US performs almost as well as MRI and CT [39–40] for the detection of erosions. According to one randomised trial, PD US is an excellent predictor of subsequent joint damage in the affected joint [41]. However, although US is an excellent method to detect small and first erosions at accessible joints, such as the metacarpo-phalangeal, the proximal interphalangeal and the metatarsophalangeal joints, its value is more limited at sites such as the carpal or the midfoot joints. In follow-up it may be very difficult to compare erosive status assessed with US. Moreover, only a few controlled studies have compared US and regular X-rays for the prediction of long-term joint damage [42, 43].
Thus, the use of US for the detection of erosions in routine practice needs to be further evaluated. Indeed, there is, to our knowledge, no registry study in which the detection of erosions by US has been analysed. Although the SONAR score includes the assessment of bony erosions, comparison with plain X-rays has not been performed yet.
The main treatment goal in RA is to achieve a state of disease remission. However, no consensus exists on what constitutes clinical remission. A few remission criteria have been developed, in particular by the ACR and EULAR groups [43]. A DAS28 score of less than 2.6 is the most commonly used definition of remission because it can be easily applied in randomised clinical trials and in routine practice. The SDAI and the CDAI are less complex composite indices that have been derived from the DAS. They may more accurately reflect a state of disease remission than DAS28 remission scores [45]. These scores are not calculated in the SCQM registry and, therefore, are not used by most Swiss rheumatologists. In addition, many studies indicate that the DAS as well as the SDAI and CDAI remission definitions [2] reflect a measure of minimal RA disease activity rather than a true remission. Indeed, up to 30% of patients in remission show progression of erosions and joint damage on radiographs [46–47], and stopping the medication rapidly leads to flares in most of them [47].
In 2011, the ACR/EULAR committee proposed new, more stringent criteria for the definition of remission that could better predict the absence of structural damage progression [1]. The ACR/EULAR Boolean criteria include tender joint count of ≤1, swollen joint count of ≤1, serum C-reactive protein levels of ≤10 mg/l, and a patient global assessment of ≤10/100 mm on a visual analogue scale or a SDAI score of ≤3.3. The pertinence of this very strict remission definition has not yet been fully evaluated, in particular for the decision of stopping or tapering treatments, prediction of flares or absence of progression of erosions [1].
Six controlled trials have studied the use of US to define remission [38]. Concordant preliminary conclusions can be drawn [8], although four of the studies were performed by the same group, the number of joints scanned varied from one study to the other as did the definition of clinical and US remission . A significant number of patients fulfilling the clinical remission definitions still present US synovitis [2, 49–52]. This was recently confirmed in daily routine care by a study performed in patients included in the Swiss SCQM registry and using the SONAR score. More than one-third of all RA patients in clinical remission according to either the DAS28 definition (140 patients) or ACR/EULAR criteria (40 patients) showed persistence of US synovitis [35].
In patients considered to be in remission, two studies have evaluated whether US could better predict progression and flares after treatment discontinuation than the clinical scores [50–52]. The persistence of PD activity was the best predictor of subsequent radiological damage and flares in both studies [52–54].
In the absence of a consensus about a US scoring system, a unique definition of US remission cannot be proposed, each scoring system having its own definition and validation. However, it seems that the absence of synovitis according to PD activity should be considered as the main criterion of remission [54].
In our literature review, we have not done a systematic analysis that could allow us to classify our recommendations according to precise levels of evidence. In fact, there is, to our knowledge, as yet no peer review on these topics that has proposed international recommendations based on a class- and evidence-grading system. Therefore, the following practical recommendations for the use of US in clinical practice are based not only on literature data, but also on a critical review of our experience with the SONAR score by a panel of experts from leading Swiss rheumatology centres. They will be useful mostly for the Swiss rheumatologists who already are or intend to use US scoring for RA patients.
In the absence of a unique US scoring system recognised by the ACR or EULAR committees, we recommend that Swiss rheumatologists use the SONAR score. More than 100 rheumatologists have been trained in the application of this score and the score has been validated. Correlation of the SONAR score with disease activity scores, including remission and sensitivity to changes in disease activity, is similar to other published scores. Moreover, the SONAR score is unique because of its inclusion in a national registry database (SCQM). This allows assessment of not only the management of the clinical disease but also the application of US in RA. Results can be very easily documented online during the examination. The results are directly reported to the summary table where they can be compared with clinical assessments, in particular with the DAS28 score and the patient questionnaires. The combination of clinical composite scores with imaging data (X-Ray and US) is particularly useful for treatment monitoring in daily practice. The US score application in the SCQM database will be adapted according to future research data and the future development of a validated international score.
US is a useful tool in addition to clinical, biological and conventional radiographic data to establish the presence of arthritis. Some specific imaging patterns identified by US examination can help to clarify the differential diagnosis. The use of US examinations as a diagnostic procedure should not be limited to the SONAR score but must, for instance, include the assessment of additional joints or the presence of enthesites.
The new 2010 ACR/EULAR classification criteria can serve as a reasonable basis for the diagnosis of RA, especially at an early stage. It implies at least one clinically swollen joint. As the number of affected joints increases the probability of RA diagnosis, US can be used to identify the number of swollen joints. However, no clear definition of US synovitis was provided in the article including the RA classification criteria [2]. According to our experience, only grade 2 on B-mode without or with PD activity should be considered as significant for the presence of synovitis [4]. Of note, the SONAR score does not take into account the presence of synovitis in the feet and hips or shoulders. As the 2010 ACR/EULAR classification criteria of RA also includes these joints, the search for synovitis with US should not be restricted to the SONAR score.
When RA is recently diagnosed, we recommend a baseline SONAR score in addition to baseline standard X-rays, and the use of at least one of the following clinical activity indices (DAS28 or SDAI or CDAI) for all the patients, in particular those included in the SCQM registry. These baseline US data are useful not only for disease monitoring (see below) but also for assessing the prognostic value of US in comparison with other measurements of disease activity in future registry studies. For the SONAR score, a total B-mode score above 9 (out of 66) and more than one joint with grade 2 synovitis or any activity on PD strongly suggest the presence of active disease. When the DAS28 score is above 5.1 (very active disease), the mean total B-mode SONAR score is about 15 [34].
When both baseline US scoring and a clinical index such as DAS28 show the presence of either very active or moderately active disease, we recommend the use oof nly DAS28 or another validated clinical activity index to monitor therapy until remission or low disease activity is obtained.
In the case of a discrepancy between baseline US score and clinical indices, the clinician should re-evaluate the diagnosis. If RA is confirmed, we feel that the use of US should be preferred for treatment monitoring because it has been shown to be more reliable than clinical indices. During follow-up, the minimal clinically relevant change in total B-mode score should be above 3.5, and appearance or disappearance of grade 2 synovitis must be especially evaluated [34]. Change in Doppler activity should be preferred to B-mode changes prior to any treatment modification [3].
We recommend that US scoring is performed when treatment goals are achieved and remain sustained for at least 6 months, prior to considering treatment tapering. When signs of active synovitis on US (no Doppler activity or and less than 2 grade 2 synovitis on B-mode) [3] have disappeared, treatment tapering should be considered, since many studies have shown that this was possible with both traditional and biological disease-modifying antirheumatic drugs (DMARDs).
If signs of active synovitis are still present on US examination the clinician should be very cautious about stepping down therapy but instead evaluate reinforcement or change in therapy. The US examiner must pay special attention to the PD evaluation, in particular the absence of any residual PD activity [41].
As the SONAR erosion score has not been validated, we cannot recommend its use routinely. Nevertheless the possibility exists for the clinicians to include this information in the SCQM database for future studies. The same is true for the assessment of cartilage lesions in particular loss of thickness.
a) When tailoring therapy according to US (absence of correlation between US and clinical scores) intervals between two US examinations should be at least 3 months. However, changes in PD in response to therapy are more rapid than B-mode changes and can occur within a few days [7, 37].
b) When there is a good correlation with the clinical disease activity indices after reaching the treatment goals and in the absence of any symptomatic changes, we recommend repeating US evaluations once a year.
The best scoring system for US in the diagnosis and monitoring of RA has to be defined and validated in additional prospective studies, including randomised controlled trials and patient registries. Currently, studies are underway to assess the contribution of US to tight disease control and clinical outcome. Although the results of some prospective trials are still pending, a high level of expertise in US examination has been established in Switzerland, with many training sessions and more than 100 participating rheumatologists. Thus, there is a rationale for including US assessment in the daily clinical practice of rheumatologists in charge of RA patients.
1 Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86.
2 Balsa A, de Miguel E, Castillo C, Peiteado D, Martin-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology. 2010;49:683–90.
3 Sen D, Brasington R. Tight disease control in early RA. Rheumatic Dis Clin North America. 2012;38:327–43.
4 Hensor EM, Emery P, Bingham SJ, Conaghan PG, Consortium Y. Discrepancies in categorizing rheumatoid arthritis patients by DAS-28(ESR) and DAS-28(CRP): can they be reduced? Rheumatology. 2010;49:1521–9.
5 Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Wakefield R, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70:792–8.
6 Rowbotham EL, Wakefield RJ, Grainger AJ. The technique and application of ultrasound in the diagnosis and management of inflammatory arthritis. Semin Musculoskelet Radiol. 2012;16:360–6.
7 Alcalde M, D'Agostino MA, Bruyn GA, Moller I, Iagnocco A, Wakefield RJ, et al. A systematic literature review of US definitions, scoring systems and validity according to the OMERACT filter for tendon lesion in RA and other inflammatory joint diseases. Rheumatology. 2012;51:1246–60.
8 Ziswiler HR, Tamborrini G. [Musculoskeletal ultrasound II - <<why are bats better than physicians>>. Praxis. 2011;100:1297–302.
9 Carotti, F Salaffi, P Manganelli, D Salera, B Simonetti, W Grassi. Power Doppler sonography in the assessment of synovialtissue of the knee joint in rheumatoid arthritis: a preliminary experience. Ann Rheum Dis. 2002;61:877–82
10 Terslev L, Qvistgaard E, Kristoffersen H, Torp-Pedersen S, Bliddal H. Doppler ultrasonography in rheumatic diseases. Ugeskr Laeger. 2004;166:371–4.
11 Chavez-Lopez MA, Hernandez-Diaz C, Moya C, Pineda C, Ventura-Rios L, Moller I, et al. Inter- and intra-observer agreement of high-resolution ultrasonography and power Doppler in assessment of joint inflammation and bone erosions in patients with rheumatoid arthritis. Rheumatol Int. 2013;33(1):173–7.
12 Moller B, Bonel H, Rotzetter M, Villiger PM, Ziswiler HR. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis and rheumatism. 2009;61:435–41.
13 Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and rheumatism. 2010;62:2569–81.
14 Filer A, de Pablo P, Allen G, Nightingale P, Jordan A, Jobanputra P, et al. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann Rheum Dis. 2011;70:500–7.
15 Kawashiri SY, Suzuki T, Okada A, Yamasaki S, Tamai M, Nakamura H, et al. Musculoskeletal ultrasonography assists the diagnostic performance of the 2010 classification criteria for rheumatoid arthritis. Mod Rheumatol. 2013;23(1):36–43.
16 Millot F, Clavel G, Etchepare F, Gandjbakhch F, Grados F, Saraux A, et al. Musculoskeletal ultrasonography in healthy subjects and ultrasound criteria for early arthritis (the ESPOIR cohort). J Rheumatol. 2011;38:613–20.
17 Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70:1111–4.
18 Kaeley GS, D’Agostino MA, Grassi W, Ostergaard M, Olivieri I. GRAPPA 2011: Proceedings from the Ultrasound Imaging Module. J Rheumatol. 2012; 39:2211–3.
19 Filippou G, Bozios P, Gambera D, Lorenzini S, Bertoldi I, Adinolfi A, et al. Ultrasound detection of calcium pyrophosphate dihydrate crystal deposits in menisci: a pilot in vivo and ex vivo study. Ann Rheum Dis. 2012;71:1426–7.
20 Dalbeth N, Doyle A, McQueen FM. Imaging in gout: insights into the pathological features of disease. Curr Opin Rheumatol. 2012;24:132–8.
21 Howard RG, Pillinger MH, Gyftopoulos S, Thiele RG, Swearingen CJ, Samuels J. Reproducibility of musculoskeletal ultrasound for determining monosodium urate deposition: concordance between readers. Arthritis Care Res. 2011;63:1456–62.
22 Filippucci E, Riveros MG, Georgescu D, Salaffi F, Grassi W. Hyaline cartilage involvement in patients with gout and calcium pyrophosphate deposition disease. An ultrasound study. Osteoarthritis Cartilage. 2009;17:178–81.
23 Zhang W, Doherty M, Pascual E, Barskova V, Guerne PA, Jansen TL, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011;70:571–5.
24 Iagnocco A, Conaghan PG, Aegerter P, Moller I, Bruyn GA, Chary-Valckenaere I, et al. The reliability of musculoskeletal ultrasound in the detection of cartilage abnormalities at the metacarpo-phalangeal joints. Osteoarthritis Cartilage. 2012;20:1142–6.
25 Mathiessen A, Haugen IK, Slatkowsky-Christensen B, Boyesen P, Kvien TK, Hammer HB. Ultrasonographic assessment of osteophytes in 127 patients with hand osteoarthritis: exploring reliability and associations with MRI, radiographs and clinical joint findings. Ann Rheum Dis. 2013;72:51–6.
26 Ostergaard M. Can imaging be used for inflammatory arthritis screening? Semin Musculoskelet Radiol. 2012;16:401–9.
27 Naredo E, Moller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:2248–56.
28 Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum. 2009;61:1194–201.
29 Naredo E, D’Agostino MA, Wakefield RJ, Möller I, Balint PV, Filippucci E, et al . Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis. 2012 Sep 14. [Epub ahead of print]
30 Hammer HB, Bolton-King P, Bakkeheim V, Berg TH, Sundt E, Kongtorp AK, et al. Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:1995–8.
31 Mandl P, Balint P, Brault Y, Backhaus M, D'Agostino M, Grassi W, et al. Clinical and ultrasound-based composite disease activity indices in rheumatoid arthritis: Results from a randomized, multicentre study. Arthritis Care Res . 2012. Dec 4.: Epub ahead of print
32 de Miguel E, Cobo T, Munoz-Fernandez S, Naredo E, Uson J, Acebes JC, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68:169–74.
33 Brulhart L, Zufferey P, Tamborrini G, Moeller B, Gerber T, Krebs, et al. Reproducibility and feasibility of a 22 joints ultrasound score in rheumatoid arthritis: a study among rheumatologists with diverse expertise in musculoskeletal ultrasound L. Swiss Med Wkly. 2011;suppl 188:13.
34 Zufferey P SA, Tamborrini G, Brulhart L, Möller B Ziswiler HR. Sensitivity to change of the ultrasound synovitis SONAR score in RA patients: preliminary results of the Lausanne subset of the SCQM cohort. Swiss Med Wkly. 2012;suppl 195:5.
35 Zufferey P, Moeller B, Tamborrini T , Brulhart L, Scherer A, Ziswiler HR. Persistence of ultrasound synovitis in the patients fullfiling the das and/or the new acr/eular ra remission definitions: results of the sonar score applied to the patients of the scqm cohort. In: EULAR meeting. Berlin: Eular congress news 2012:13.
36 Koski JM, Saarakkala S, Helle M, Hakulinen U, Heikkinen JO, Hermunen H. Power Doppler ultrasonography and synovitis: correlating ultrasound imaging with histopathological findings and evaluating the performance of ultrasound equipments. Ann Rheum Dis. 2006;65:1590–5.
37 Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010,69:631–7.
38 Wakefield RJ, D’Agostino MA, Naredo E, Buch MH, Iagnocco A, Terslev L, et al. After treat-to-target: can a targeted ultrasound initiative improve RA outcomes? Ann Rheum Dis. 2012;71:799–803.
39 Dohn UM, Terslev L, Szkudlarek M, Hansen MS, Hetland ML, Hansen A, et al. Detection, scoring and volume assessment of bone erosions by ultrasonography in rheumatoid arthritis: comparison with CT. Ann Rheum Dis. 2013;72:530–4.
40 Amin MF, Ismail FM, El Shereef RR. The role of ultrasonography in early detection and monitoring of shoulder erosions, and disease activity in rheumatoid arthritis patients; comparison with MRI examination. Acad Radiol. 2012;19:693–700.
41 Baillet A, Gaujoux-Viala C, Mouterde G, Pham T, Tebib J, Saraux A, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology. 2011;50:1137–47.
42 Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
43 Scheel AK, Hermann KG, Ohrndorf S, Werner C, Schirmer C, Detert J, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006;65:595–600.
44 Shammas RM, Ranganath VK, Paulus HE. Remission in rheumatoid arthritis. Curr Rheumatol. 2010;12:355–62.
45 Balsa A, de Miguel E, Castillo C, Peiteado D, Martin-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology. 2010;49:683–90.
46 Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis. 2012;71:681–6.
47 Klarenbeek NB, Koevoets R, van der Heijde DM, Gerards AH, Ten Wolde S, Kerstens PJ, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1815–21.
48 Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, van Groenendael JH, Han KH, Kerstens PJ, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70:315–9.
49 Mandl P, Balint PV, Brault Y, Backhaus M, D’Agostino MA, Grassi W, et al. Metrologic properties of ultrasound versus clinical evaluation of synovitis in rheumatoid arthritis: results of a multicenter, randomized study. Arthritis Rheum. 2012;64:1272–82.
50 Scire CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology. 2009;48:1092–7.
51 Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73.
52 Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis. 2011;70:172–5.
53 Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;71:1316–21.
54 Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy ML, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64:67–76.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.