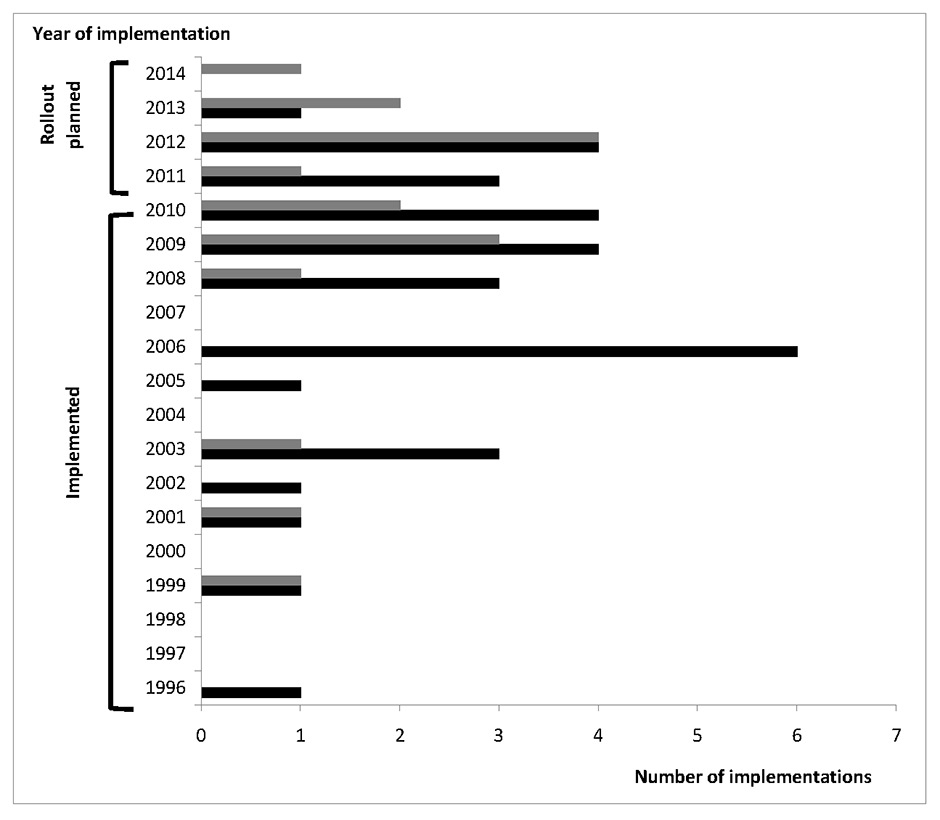
Figure 1
Number of implementations of CPOE and CDSS over the years.
Gray bars = implementation of clinical decision support (CDSS) systems; black bars = Implementation of computerised physician order entry (CPOE) systems.
DOI: https://doi.org/10.4414/smw.2013.13894
It has long been known that the prescription, delivery and use of drugs in healthcare is a process prone to errors that may jeopardise patient safety [1]. Early studies focused on inpatient settings and showed that errors occurred in all steps of drug treatment. Errors during the ordering or administration process account for about 90% of preventable adverse events [2–4]. Computerised provider order entry (CPOE), defined as the process of entering medication orders electronically instead of on paper charts [e.g., 5] has been considered and promoted as an important tool to improve the quality and safety of drug prescription and thus reduce errors [6]. In 2009, about 15% of hospitals in the USA had put CPOE into service and numbers have rapidly increased since then; penetration is lower in European countries [7].
CPOE systems usually address four key points, namely: (a) the standardised prescription structure that leads to clear and complete orders; (b) the integration of all types of associated decision support; (c) the communication of information and continuity of care; and (d) the introduction of (entirely) paperless processes. CPOE platforms have proven to be very effective in preventing errors due to unclear or incomplete orders, and to dramatically decrease transcription errors [8]. However, many mistakes are triggered by a lack of knowledge [9], and therefore, a CPOE platform should incorporate clinical decision support systems (CDSSs). Definitions of a CDSS vary; however, we consider a CDSS to be knowledge software designed to support clinical processes by linking patient data with medical- and/or drug-specific knowledge, and to provide advice for healthcare professionals while diagnosing, treating or monitoring patients [based on and modified from 10]. Such systems have been developed and put into service for various purposes, including disease diagnosis, prevention and management, and drug prescription [11]. The optimal way to design a CDSS and to integrate it into clinical workflow is still the subject of research. Moreover, in most cases, decision support is a combination of commercially and locally managed sources and systems. Thus, there is no broadly accepted standard to describe CDSS functionalities and content.
This work aimed to assess the level of CPOE and particularly CDSS implementation in Swiss healthcare institutions by analysing voluntary responses to a survey sent out to all hospitals and clinics in Switzerland.
The survey was a pdf-based, four-page document organised into three sections. Section 1 was devoted to the definition of institutional characteristics, such as the number of beds, the type of hospital and the care setting. Section 2 addressed general aspects of CPOE, such as deployment dates, and CPOE and CDSS coverage within the institution. Section 3 focussed on different types of CDSS and their integration into the workflow (see appendix http://www.smw.ch/fileadmin/smw/pdf/smw-13894-Appendix.pdf for the full questionnaire).
The different types of CDSS were classified into four dimensions:
1. Current drug-related information such as monographs (“drug-related CDSS”, e.g., dosage information).
2. Current drug with other active drugs for the patient (“comedication-related CDSS”, e.g., drug-drug interactions).
3. Context-related CDSS (“setting-related CDSS”, e.g., information on the hospital formulary).
4. Patient-specific CDSS (“patient-related CDSS”, e.g., drug-pregnancy information).
Each type of CDSS was defined by a set of functionalities and specifications. For instance, the CDSS type “drug-duplicate alerts” (with reference to the “comedication-related CDSS” dimension) was subdivided into the functionalities “information on identical substances with comparable bioavailability”, “information on substances from identical active drug class (ATC)”, “therapeutic duplications, and “exceptions requiring duplicate treatment, e.g., bridging”. For several types of CDSS, it was also possible to specify whether severity grades were available.
In total, 53 different CDSS functionalities were assessed, allowing for the definition of 20 types of CDSS that were allocated across the four dimensions (table 1).
Detailed information on functionalities (i.e., specifications) was gathered for each type of CDSS in order to allow a precise description of them.
Participants were also asked to specify how the CDSS was integrated into the workflow and the mode of user interaction. From the literature, we defined three categories related to how alerts were implemented [12, 13]:
1. Passive: all means of guiding prescribers with prefilled screens, such as default or pre-calculated values, mandatory fields, order sets, and frequent orders, etc.
2. Noninterruptive alerts: any type of interaction that can be perceived by the users during the process, but does not interrupt it and can be ignored without specific actions.
3. Interruptive alerts: any type of decision support that requires the user’s immediate attention, interrupts the usual or current process, and cannot be ignored without specific actions.
The survey was tested with two hospitals and iteratively refined before being sent out.
| Table 1:Classification of clinical decision support (CDSS) as employed in the questionnaire. | ||
| Dimension 1: Decision support related to the prescribed drug | ||
| Types | Functionalities | Specification |
| CDSS targeting drug dosage | (i) Information or (ii) calculation of | Information with regard to |
| Mininum doses | Age | |
| Usual | Weight | |
| Maximum doses | Renal function | |
| Single dose | comedication | |
| Daily dose | Route of administration | |
| Weekly maximal doses | Indication | |
| Cumulative doses | Genotype | |
| Timepoint of administration | ||
| Duration of treatment | ||
| Dosage at the beginning of therapy | ||
| Dosage at the end of therapy | ||
| CDSS supporting route of administration | Information on appropriate route of administration | |
| Information on inappropriate route of administration | ||
| Switch from i.v. to oral treatment | ||
| CDSS supporting administration of oral drugs | Information on whether a tablet can be split | |
| Information on whether a tablet can be crushed | ||
| Information on whether a capsule can be opened | ||
| Information on whether a drug can be given via tube | ||
| CDSS supporting administration of parenteral drugs | Information on how to prepare a drug for treatment including | |
| Solvent | ||
| Volume | ||
| Flow rate | ||
| Dimension 2: Decision support related to the comedication | ||
| Types | Functionalities | Specification |
| CDSS targeting drug-drug interactions | Information applies for two interacting drugs | Consideration of route of administration |
| Information applies for more than two drugs | Consideration of dosage | |
| Consideration of timepoint of administration | ||
| Consideration of measured lab values | ||
| CDSS targeting drug incompatibilities | Information on potentially incompatible drugs | Consideration of timepoint |
| Consideration of route of administration | ||
| CDSS targeting duplicate orders | Information on identical substances with comparable bio-availability | |
| Information on substances from the identical active drug class (ATC) | ||
| Therapeutic duplications | ||
| Exceptions requiring duplicate treatment | ||
| CDSS targeting corollary orders | CDSS targeting corollary orders | |
| Dimension 3: Decision support related to the setting | ||
| Types | Functionalities | Specification |
| CDSS supporting information on hospital formulary | CDSS supporting information on hospital formulary | |
| CDSS supporting information on cost-effectiveness | Information about drug prices | |
| Information about comparable cost-effectiveness | ||
| Proactive suggestion of the cheapest drug | ||
| CDSS supporting hospital admission | Switch of ambulatory medication | |
| CDSS supporting hospital discharge | Consideration of pre-hospital prescription | |
| Consideration of drug prices | ||
| Consideration of reimbursement in ambulatory care | ||
| Discharge information letter for patients | ||
| Dimension 4: Decision support related to the patient | ||
| Types | Functionalities | Specification |
| Diagnoses support | Diagnoses support | |
| CDSS targeting drug-disease interactions | Display of absolute contraindications | Consideration of encoded diagnoses |
| Display of relative contraindications | Consideration lab values | |
| Consideration patient notes | ||
| CDSS targeting drug allergy interactions | Information on allergy regarding | |
| A specific drug | ||
| Adjuvants | ||
| Cross-reactivity | ||
| Reverse allergy warning | ||
| CDSS targeting drug-lab interactions | Information on abnormal lab values | |
| Information on timely conduction of lab tests | ||
| CDSS supporting drug prescription in regard to genotype | Information for substances which might be affected by the patients’ genotype | Consideration of the patient’s genotype |
| CDSS supporting drug prescription during pregnancy | Information on usability of drug during pregnancy | Display of information only, if patient is female |
| Display of information only, if patient is female and at childbearing age | ||
| Display of information only, if patient is indeed pregnant (lab test, notes) | ||
| CDSS supporting drug prescription during breastfeeding | Information on usability of drug during breastfeeding | Display of information only, if patient is female |
| Display of information only, if patient is indeed breastfeeding (notes) | ||
| CDSS supporting drug prescription for specific age groups | Information on usability of drugs | Display of information taking actual patient age into account |
| Paediatrics | ||
| Elderly | ||
In February 2011, the survey was sent out as a pdf-questionnaire via e-mail to the medical directors or chief pharmacists of public hospitals (n = 120) and private clinics (n = 165) registered with the Swiss Hospitals Federation (H+). This federation includes most of the public and private inpatient organisations registered in Switzerland. The classification as public hospital or private clinic was derived from the Federal Statistical Office (OFS). Thereby, hospitals were defined according to the OFS as institutes for public health, which are financed by state and health insurance, compared to clinics which are private institutions. However, hospitals may have private treatments and clinics may have agreements with insurance companies to treat private patients [14].
In the absence of feedback after the first invitation via e-mail, non-responders were reminded a maximum of three times (one e-mail reminder after four weeks [March 2011], one telephone reminder [April 2011], and one reminder using a personally addressed letter [August 2011]).
The responses were manually transferred into an Excel sheet and the following parameters to describe institutions were included: (i.) the type of institution (hospital or clinic), (ii.) number of beds, and (iii.) region of Switzerland (i.e., French-speaking, German-speaking and Italian-speaking parts of Switzerland). If any of these details were missing, we looked up the institution’s homepage for more information. If we received several responses from one institution, we telephoned to ask which response should be considered for the analysis. Descriptive statistics were reported as frequencies, mean and standard deviation.
After four rounds of invitation and reminders, one-third of all hospitals and clinics responded to the survey (n = 92/285; 32.3%), with more hospitals (n = 52/120; 43.3%) than clinics (n = 40/162; 24.2%) answering. Forty-eight institutions (n = 48/92; 52.2%) responded to the first invitation, 23 (n = 23/92; 25.0%) to the first reminder, 5 (n = 5/92; 5.4%) to the second, and 16 (n = 16/92; 17.4%) to the third reminder. Among the responders, 37% were pharmacists, 14% physicians, 13% clinic managers, 8% medical directors, 4% directors of nursing and 3% quality managers. Nineteen (21%) responders did not state their occupation. Of all responders, 73 sent back a completed survey. A further 19 refused to participate, most often because the survey was not applicable or not of interest to the institution (n = 8), but also because it was not issued in German (n = 3), or due to time constraints (n = 2). Participation was highest in the Italian-speaking part (38%, vs 32% and 22% for the French- and German-speaking regions) (table 2). In five cases where we received two different questionnaire responses from the same organisation. However, we tested the degree of agreement in the two different questionnaires using the Cronbach alpha coefficient and obtained a mean value of 0.85 (0.98; 0.98; 0.97; 0.74; 0.59) for the five institutions, indicating a high degree of consistency [15].

Figure 1
Number of implementations of CPOE and CDSS over the years.
Gray bars = implementation of clinical decision support (CDSS) systems; black bars = Implementation of computerised physician order entry (CPOE) systems.
Of the 73 institutions participating in the survey, 42% had no CPOE in service (n = 31), 17.8% planned to introduce a CPOE (n = 13), and 39.7% did have a CPOE system in service in their institution (n = 29; two of those with partly implemented systems that were planned to be expanded).
Implementation of CDSSs was less frequent: 14 institutions were actually using a CDSS (19.1%) and another 18 institutions were planning to (24.6%). Of these, 10 institutions detailed which CDSS they planned to introduce. Of the 41 institutions indicating that they had no CDSS in general, 7 nevertheless specified that some specific types of CDSSs were available (probably paper-based versions).
Implementation rates for both CPOE and CDSSs were higher in hospitals than in clinics (51% vs 21% for CPOE and 24% vs 11% for CDSSs).
Sixty-five of 73 institutions specified their number of beds, and bed numbers for the 8 remaining institutions were found via their internet sites. According to these responses, a total of 22,076 beds were included in this survey. According to the responses to the survey, in which the numbers of beds with implemented CPOE or a CDSS were sometimes specified as a range, between 8,013 to 9,278 beds were equipped with CPOE (33.3%–44.1%) and between 4,904 to 5,264 with a CDSS (22.2%–23.8%).
Twenty-six of 29 institutions with a CPOE system in service indicated a year of implementation. The earliest documented introduction of CPOE was 1996, the latest was the year of the survey itself (2011). The median year of introduction of the CPOE systems was 2006 (25% quartile = 2003; 75% quartile = 2009). Nine of 14 institutions with CDSS specified the year of introduction, the earliest being 1999. The median year of introduction of CDSS systems was in 2009 (25% quartile = 2003; 75% quartile = 2009). The introduction of CPOE and CDSSs has been increasing, as highlighted by figure 1. Indeed, when looking at the number of systems introduced over 5-year periods, the number increased from 2 CPOE and 1 CDSS (1996–2000) to 6 CPOE and 2 CDSS (2001–2005) and 17 CPOE and 6 CDSS (2006–2010). Since 2010, eight institutions have already introduced a CPOE and eight a CDSS.
Of the 14 institutions with a CDSS in service, 12 institutions had introduced at least one module referring to the current drug (dimension 1), 12 institutions had modules related to the comedication (dimension 2), 13 had modules related to the setting (dimension 3) and 7 institutions had a CDSS relating to the patient (dimension 4). A total of 90 types of CDSS were implemented. The type of CDSS most often implemented was information on the formularies (n = 13) and information on drug-drug interactions (n = 11). Types of CDSS supporting prescriptions with regard to genotype were absent, and CDSS for drug-lab interactions, contraindications, specific age groups and corollary orders were rare (n = 1 institution each). Each institution had on average 6.2 ± 3.3 out of 20 different types of CDSS implemented. On the level of functionalities, each institution had a median of 8 of a possible 53 functionalities of CDSS implemented (25% quartile = 6, 75% quartile = 10.75). Hence, on average, only 15% of all possible CDSS functionalities were implemented. Of functionalities related to the prescription, to the comedication and the setting, one in four functionalities was implemented, but CDSS functionalities related to the patient were only rarely implemented (17 out of 148 functionalities, 11.5%) (table 3).
We obtained details about the implementation methodology for 85 of 90 types of CDSS, i.e., how the user had to interact with the software. Most CDSS types were implemented as passive or pre-structured information only (n = 56). Eight types were implemented as noninterruptive alerts only and eight types as interruptive alerts only.
| Table 2:Rate of acceptance for hospitals and clinics by regions of Switzerland. | |||
| Number of surveys sent out | Number of participating institutions [%] | ||
| French-speaking part | 54 | 19 | [35%] |
| German-speaking part | 218 | 49 | [22%] |
| Italian-speaking part | 13 | 5 | [38%] |
| Total | 285 | 73 | [26%] |
| Table 3:Rate of implementation stratified for different types of CDSS. In total, 92 implementations of different types of CDSS were reported (= 100%) in the 14 establishments with CDSS implementation counted. N = number of institutions. N1 = Number of establishments with at least one CDSS functionality being part of the respective dimension or a respective type of CDSS. N2 = Number of implementations of a distinct type of CDSS; N3 = Number of the respective implementations of a distinct functionality. If one functionality could have several specifications, these were summed up to one functionality. | |||||
| Dimension of CDSS | N1 | Type of CDSS | N2 | Functionality of CDSS (including distinct specification) | N3 |
| Prescription | 12 | CDSS targeting drug dosage | 8 | Information or calculation of miminum doses | 2 |
| Information or calculation of usual doses | 6 | ||||
| Information or calculation of maximum doses | 5 | ||||
| Information or calculation of single dose | 4 | ||||
| Information or calculation of daily dose | 3 | ||||
| Information or calculation of weekly maximal doses | 2 | ||||
| Information or calculation of cumulative doses | 1 | ||||
| Information or calculation of timepoint of administration | 3 | ||||
| Information or calculation of duration of treatment | 2 | ||||
| Information or calculation of dosage at the beginning of therapy | 3 | ||||
| Information or calculation of dosage at the end of therapy | 2 | ||||
| Consideration of specific characteristics | 7 | ||||
| CDSS supporting administration of oral drugs | 4 | Information on whether a tablet can be split | 3 | ||
| Information on whether a tablet can be crushed | 2 | ||||
| Information on whether a capsule can be opened | 2 | ||||
| Information on whether a drug can be given via tube | 3 | ||||
| CDSS supporting administration of parenteral drugs | 5 | Information on how to prepare a drug for treatment including solvent | 5 | ||
| Information on how to prepare a drug for treatment including volumen | 5 | ||||
| Information on how to prepare a drug for treatment including flow rate | 4 | ||||
| CDSS supporting route of administration | 8 | Information on appropriate route of administration | 8 | ||
| Information on inappropriate route of administration | |||||
| Switch from iv to oral treatment | |||||
| Comedication | 12 | CDSS targeting drug-drug interactions | 11 | Information applies for two interacting drugs | 9 |
| Information considers more than two drugs | 3 | ||||
| CDSS targeting duplicate orders | 7 | Information on identical substances with comparable bio-availability | 3 | ||
| Information on substances from the identical active drug class (ATC) | 2 | ||||
| Therapeutic duplications | 4 | ||||
| Exceptions requiring duplicate treatment | |||||
| CDSS targeting drug incompatibilities | 5 | Information on potentially incompatible drugs | 6 | ||
| CDSS targeting corollary orders | 1 | Information on corollary orders | 1 | ||
| Setting | 13 | CDSS supporting information on hospital formulary | 13 | Information on hospital formulary | 13 |
| CDSS supporting information on cost-effectiveness | 5 | Information about drug prices | 5 | ||
| Information about comparable cost-effectiveness | |||||
| Pro-active suggestion of the cheapest drug | 1 | ||||
| CDSS supporting hospital admission | 4 | Switch of ambulatory medication | 4 | ||
| CDSS supporting hospital discharge | 6 | Consideration of prehospital prescription | 5 | ||
| Consideration of drug prices | |||||
| Consideration of reimbursement in ambulatory care | |||||
| Discharge information letter for patients | 3 | ||||
| Patient | 7 | Diagnoses support | 4 | Information on diagnoses support | 4 |
| CDSS targeting drug-disease interactions | 1 | Display of absolute contraindications | |||
| Display of relative contraindications | 2 | ||||
| CDSS targeting drug allergy interactions | 2 | Information on allergy regarding a specific drug | 2 | ||
| Information on allergy regarding adjuvants | 1 | ||||
| Information on allergy regarding cross-reactivity | 1 | ||||
| Information on allergy regarding reverse allergy warning | |||||
| CDSS targeting drug-lab interactions | 1 | Information on abnormal lab values | 1 | ||
| Information on timely conduction of lab tests | 1 | ||||
| CDSS supporting drug prescription in regard to genotype | Information for substances which might be affected by the patients’ genotype | ||||
| CDSS supporting drug prescription during pregnancy | 2 | Information on usability of drug during pregnancy | 2 | ||
| CDSS supporting drug prescription during breastfeeding | 2 | Information on usability of drug during breastfeeding | 2 | ||
| CDSS supporting drug prescription for specific age groups | 1 | Information on usability of drugs paediatrics | 1 | ||
| Information on usability of drugs elderly | |||||
| Total | 90 | 148 | |||
Via a country-wide survey, we assessed the self-reported grade of CPOE and CDSS implementation in Swiss hospitals and clinics. Results showed that 39.7% (n = 29) of responders were using a CPOE. Of those, 13 institutions had additional CDSS functionalities with another 3 institutions were planning to introduce a CDSS. However, of the 13 institutions planning to introduce CPOE, 12 also planned to introduce a CDSS (and 7 of them already used some CDSS functionalities, potentially available either on paper or via the intranet). Since CPOE systems have been shown to be very successful in preventing transcription errors, especially when linked to electronic medication administration records [16]. However, they have been less effective in reducing decision-based errors [17], indicating that CPOE systems in this subset might be “second-generation-type” systems already are linked to CDSS systems at the time of introduction. However, this study also shows that the types of CDSS currently in service is rather basic, primarily providing information on monographs and driving prescribers with passive order-sets and protocols. Active decision-support, such as the extensive usage of patient-specific information or workflow-driven clinical pathways, that could be considered as third and fourth generation systems, were almost nonexistent, even though the specificity and hence positive predictive value of such alerts is considered higher [18]. In order to maximise the added-value of CDSSs, significant efforts need to be made. Switzerland’s small, fractured market is surely one problem, as each of the 26 Cantons has legal autonomy over its own health regulation framework; this could discourage certain large companies from addressing this market. Moreover, it is clear that the required customisation of commercially available CDSSs requires significantly more manpower at each different institution [19].
This study has several limitations. After three rounds of reminders, the response rate was still only 32.3%. Nevertheless, this figure can be considered as appropriate when taking into account the length of the questionnaire. Previously published studies on cross-sectional surveys of healthcare practices reported response rates within a comparable range (28.8% for a survey on clinical pharmacist services [20] and 42% for a survey on self-reported acceptance and use of CDSSs [21]). Nevertheless, the response rate could have been increased if the survey had been translated into the respective regional languages. Furthermore, association with national organisations could have been beneficial, either to promote the survey or to provide (financial) incentives for institutions to fill it out. The facultative aspect of the survey with about 70% of nonresponders has probably generated a selection bias, with an over-selection of responders using CPOE or a CDSS. Thus, the overall estimate of CPOE or CDSS in service in Switzerland would be most probably too high and may not reflect the actual implementation rate. However, this study was intended to evaluate the types of CDSS in CPOE rather than the overall implementation of CPOE. Moreover, the occupation and professions of the responders (e.g., quality manager, pharmacist, medical director) themselves might have influenced the survey’s interpretation given their individual views on the functionalities and implementation of the system.
The classification of CDSSs was piloted but not assessed in a large set of hospitals. Thus, some dimensions of the evaluation might not have been defined clearly enough. For example, we had to withdraw the question about being a teaching hospital because almost all hospitals indicated that they were a teaching hospital – whether or not they were linked to a university. Whilst the survey allowed responders to indicate that a CDSS was both commercially and locally developed (i.e., a mixed system), it was not possible to declare a “mixed” system for CPOE. However, some hospitals did report having both in-house and commercially developed CPOEs. This option should be included in future questionnaires. In a few cases, the questionnaire revealed contradicting results, e.g., reporting that no CDSS is available, but describing a CDSS in service. In such case, we assumed the “no CDSS” answer to be wrong and that a CDSS was available.
Moreover, we did not assess any additional effects related to the introduction of CPOE or a CDSS, e.g., satisfaction, cost-effectiveness, potential benefit on prescription quality, or adverse outcomes. These were not within in the scope of this survey, but they are fields of research that require attention [22, 23]. Lastly, our survey reflects the situation in Switzerland and as such it is not readily transferable to other countries, since the introduction of CPOE and CDSS systems varies considerably between countries and is subject to nationally guided campaigns, incentives or constraints.
According to this survey, the introduction of CPOE in Swiss healthcare facilities is increasing. The types of CDSS currently in service usually include only basic decision support related to drug, the co-medication or the setting, and only scarcely taking into account patient characteristics.
Acknowledgment:The authors are grateful to all those who answered our survey and would like to thank them for their cooperation. In order to preserve anonymity they cannot be named. This work could not have been carried out without their help.
1 Lesar TS, Lomaestro BM. Pohl H. Medication-prescribing errors in a teaching hospital. A 9-year experience. Arch Intern Med. 1997;157:1569–76.
2 Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34.
3 Smith DH, Perrin N, Feldstein A, Yang X, Kuang D, et al. The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Arch Intern Med. 2006;166:1098–104.
4 Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16.
5 Ash JS, Gorman PN, Seshadri V, Hersh WR. Computerized Physician Order Entry in U.S. Hospitals: results of a 2002 Survey. J Am Med Inform Assoc. 2004;11:95–9.
6 Hubert P, Treluyer JM. Medication errors among hospitalized children. Arch Pediatr. 2005;12:915–7.
7 Stürzlinger H, Hiebinger C, Pertl D, Traurig P. Computerized Physician Order Entry – effectiveness and efficiency of electronic medication ordering with decision support systems. GMS Health Technol Assess. 2009; 5:Doc07.
8 Mekhjian HS, Kumar RR, Kuehn L, Bentley TD, Teater P, Thomas A, et al. Immediate benefits realized following implementation of physician order entry at an academic medical center. J Am Med Inform Assoc. 2002;9:529–39.
9 Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164:785–92.
10 Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, et al. Clinical Decision Support Systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8:527–34.
11 Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. J Am Med Assoc. 2005;293:1223–38.
12 Chaffee BW, Zimmerman CR. Developing and implementing clinical decision support for use in a computerized prescriber-order-entry system. Am J Health Syst Pharm. 2010;67:391–400.
13 Miller RA, Waitman LR, Chen S, Rosenbloom ST. The anatomy of decision support during inpatient care provider order entry (CPOE): empirical observations from a decade of CPOE experience at Vanderbilt. J Biomed Inform. 2005;38:469–85.
14 OFS.org [Internet]. Statistiques des établissements de santé (soins intra-muros), Statut juridico-économique des établissements (publics-subventionnés/privés), Version 3.0, Neuchâtel (février 2000) [cited 2013 April 15]. Available from: http://www.bfs.admin.ch/bfs/portal/fr/index/themen/14/03/01/key/01.html
15 Cronbach JL Warrington WG. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334.
16 Poon EG, Keohane CA, Yoon CS, Ditmore M, Bane A, Levtzion-Korach O, et al. Effect of bar-code technology on the safety of medication administration. N Engl J Med. 2010;362:1698–707.
17 Ballentine AJ, Kinnaird D, Wilson JP. Prescription errors occur despite computerized prescriber order entry. Am J Health Syst Pharm. 2003;60:708–9.
18 Eppenga WL, Derijks HJ, Conemans JM, Hermens WA, Wensing M, De Smet PA. Comparison of a basic and an advanced pharmacotherapy-related clinical decision support system in a hospital care setting in the Netherlands. J Am Med Inform Assoc. 2012;19:66–71.
19 Ash JS, McCormack JL, Sittig DF, Wright A, McMullen C, Bates DW. Standard practices for computerized clinical decision support in community hospitals: a national survey. J Am Med Inform Assoc. 2012;19:980–7.
20 Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing-2010. Am J Health Syst Pharm. 2011;68:669–88.
21 Spina JR, Glassman PA, Simon B, Lanto A, Lee M, Cunningham F, et al. Potential safety gaps in order entry and automated drug alerts: a nationwide survey of VA physician self-reported practices with computerized order entry. Med Care. 2011;49:904–10.
22 Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157:29–43.
23 Schnipper JL, Linder JA, Palchuk MB, Yu DT, McColgan KE, Volk LA, et al. Effects of documentation-based decision support on chronic disease management. Am J Manag Care. 2010;16(12 Suppl HIT):SP72–81.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported. For part of her work, HMS received a scholarship from the Dr. August and Dr. Anni Lesmüller Stiftung.
Authors’ contribution: DCG and HMS contributed equally to the work. DCG and HMS designed the survey, conducted the data collection and analysis, and wrote the first draft of the manuscript. PB and CL reviewed the survey, supported the data collection, monitored the data analysis, and reviewed the manuscript.