Figure 1
Newborn hearing screening with measurement of automated OAE.
DOI: https://doi.org/10.4414/smw.2013.13905
Universal newborn hearing screening (UNHS) was recommended in 1998 in the European Consensus Statement on Neonatal Hearing Screening in Newborns [1]. In Switzerland, UNHS was introduced in 1999 under the auspices of the “Swiss Working Group: Hearing Screening in Newborns”. This working group included representatives of the Swiss societies for otorhinolaryngology (ENT), paediatrics and neonatology, and developed the necessary foundations for UNHS in Switzerland. The working group recommends that screening should be performed by estimating otoacoustic emissions (OAEs) during hospitalisation on the maternity ward. (Women in Switzerland typically spend several days on the maternity ward even after an uncomplicated birth.)

Figure 1
Newborn hearing screening with measurement of automated OAE.
OAEs are completely safe for the baby and screening is generally performed between the 2nd and 4th day after birth [2]. The test takes a few minutes and is able to assess the cochlear function of a newborn. OAEs utilise the natural phenomenon of sound echoes from the outer hair cells of the cochlea following a sound stimulus, and can be recorded in 99% of normally hearing ears. The response can be absent for technical reasons (e.g. poorly fitting probe, excessive background noise, ear wax, middle-ear effusion) and is generally absent in ears with a hearing loss of 30 dB or greater [3]. The test may be performed by a semiskilled person without specialist knowledge, (e.g. a nurse or midwife after instruction; fig. 1).
The Swiss Working Group has decided that screening is passed if the OAEs are detectable in at least one ear. If both ears fail, further investigation, usually with a second OAE measurement on the same or next day, is recommended. If during maternal admission no OAEs are detectable on either side, the child is referred for further investigation within the first few months of life.
The first aim of our present study was to evaluate how widespread UNHS was in Switzerland in 2012. We also wanted to investigate the implications of a failed screen by retrospectively evaluating records of the University Hospital Zurich in order to determine how many newborns were appropriately followed-up and how many actually had a hearing impairment.
All birth clinics officially registered in Switzerland were contacted in February 2012 by postal questionnaire (table 1). These clinics were of two types: firstly, hospital maternity wards, which are generally midwife-run and doctor-supervised; secondly, purely midwife-run “birth-centres” (“Geburtshaus” in Swiss German; “maison de naissance” in French). It should, of course, also be said that some women in Switzerland give birth at home, with or without medical supervision. These births could not be included in our analysis.
Institutions that did not respond within 6 weeks were contacted by telephone. The number of births at each institution was recorded from the birth register.
If an institution replied that it did routinely perform hearing screening, it was assumed at 100% of children born in this institution had screening. This is clearly a substantial assumption, but we did not have direct access to patient records. We then correlated the number of births in each institution with whether or not they routinely performed screening to give as an overall estimate of how many newborns in Switzerland undergo screening.
To investigate the follow-up of newborns failing the screening process, a retrospective consecutive cohort analysis of all neonates born in the years 2005‒2010 at the University Hospital of Zurich was carried out. Data were extracted from the electronic hospital database. Preterm babies of less than 34 weeks gestation were excluded as all premature infants receive thorough follow-up separately from the screening programme because of the increased risk of hearing impairment.
Data analysed included timing and type of follow-up testing (e.g. OAEs, brainstem audiometry, free field play audiometry, side specific audiometry etc.), as well as the ultimate diagnosis and treatment.
| Table 1: Questionnaire (translated into English from the original German, French or Italian). |
| Do you perform the newborn hearing screening? Yes, all No, only when risks No |
| How do you perform the measurement? One ear, if this passes the screening Both ears OAE Other methods (BERA, ALGO) |
| Who performs the test? Nurse, midwife Audiometrist Physician |
| Do the parents have to pay for the test? Yes No |
| Who performs the follow-up of newborns with failed hearing screening? Again in the maternity clinic / birth-centre Paediatrician ENT |
A total of 110 maternity wards and 14 birth-centres are officially registered in Switzerland. All were successfully contacted and returned the questionnaire. A total of 102/110 (92.7%) maternity clinics and 1/14 (7%) birth-centres routinely perform UNHS. When the data are appropriately weighted according to the number of births, 97.9% of all newborns are screened (table 2). Of note, all institutions with >160 births per year performed screening.
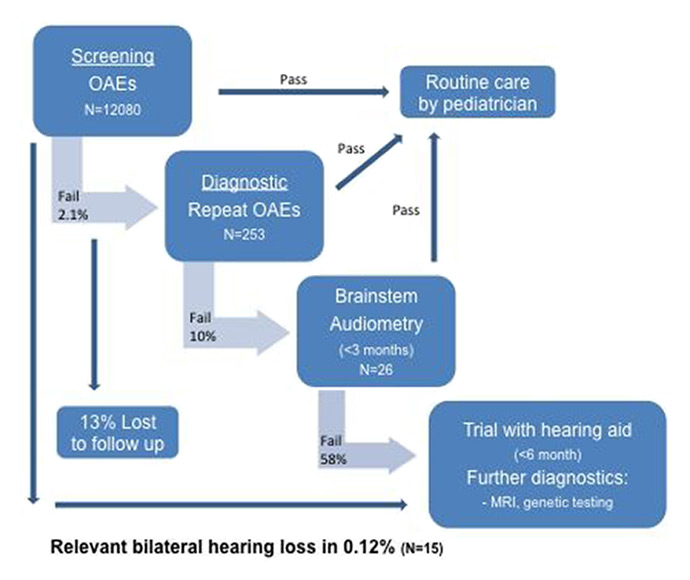
Figure 2
Follow-up of the cohort born in University Hospital Zurich 2005‒2010.
OAE = otoacoustic emission
All institutions performed UNHS by measuring otoacoustic emissions (OAEs). Fifty percent of institutions only test one ear if this passes the screening; the remaining institutions routinely test both ears.
In 88% of the institutions the test is made by nurses/midwifes and in the remainder by doctors or audiometrists. Costs in 81.6% of institutions are covered by the institutions themselves, in the others the parents must pay. (We did not collect data on how much.)
A striking result was that only one of the 14 midwife led birth-centres performed routine hearing screening, although so few births happen in these institutions that the impact overall is fairly negligible. All birth-centres that did not themselves offer routine screening recommended it to the parents.
The postal questionnaire showed that follow-up of newborns who failed UNHS is performed by ENT doctors (54.4%), paediatricians (29.1%) or again in the birth clinic (13.6%).
In the retrospective cohort study, 12,080 newborns born in the years 2005–2010 at the University Hospital Zurich were analysed. To our best knowledge, 100% of these cases had screening. A total of 253/12,080 (2.1%) failed the UNHS and were followed up in our paediatric audiology unit. The mean age at follow-up was 2.4 months (range 0.2–6.2 months). Of note, the same audiologists perform the hearing screening and the follow-up in our hospital.
All infants underwent repeat OAEs and 10% had further investigation with brainstem electrical response audiometry (BERA). A relevant bilateral hearing loss (of 40dB or more) was found in 15/253 (5.9%). One of those received a cochlear implant, the others hearing aids. Figure 2 shows the care pathway together with patient numbers.
In summary, a total of 15/12,080 (0.12%) of patients were found to have a relevant bilateral hearing loss. Unfortunately, 33/253 (13%) children failing UNHS did attend their follow-up appointment and were not contactable owing to out-of-date details. Given the figures outlined above, we estimate that perhaps two of these children would have had a relevant hearing loss. Whether or not these children were followed up at another institution is unclear.
| Table 2:Universal newborn hearing screening (UNHS) in Switzerland 2012. | |||
| UNHS | No UNHS | Number of births | |
| Maternity clinics (n = 119) | 102 93.7% | 8 | 80363 |
| Birth-centres (n = 14) | 1 7.1% | 13 | 1074 |
| Number of births | 79 721 | 1716 | 81437 |
| Screening % | 97.9% | 2.1% | 100% |
The medical evidence supporting the use of otoacoustic emissions to screen for congenital hearing loss is well established. It is worth noting, however, that only small number of children who fail their initial screen will ultimately have a relevant bilateral hearing loss. By finding these children early, appropriate further investigation and intervention can help to minimise the impact of hearing loss on speech/language and social development.
Hearing loss is one of the most common congenital disorders, with an estimated incidence of 1–3/1000 live births [3–6]. Most neonatal hearing loss is sensorineural and a known genetic cause is found in 50% of children [7]. Of these children, approximately 70% have nonsyndromic deafness, most often related to cochlear hair cell dysfunction because of errors in production of the gap junction protein connexin 26 [8]. Other causes of neonatal sensorineural hearing loss include congenital infections such as cytomegalovirus, hyperbilirubinaemia and ototoxic medications [3]. Overall, recognised risk factors are present in only 50% of infants born with hearing loss [3, 6, 9].
Hearing impairments are not easily detectable on clinical examination and the newborn generally shows no particular problems. The infants can behave normally, especially at the beginning. Without appropriate investigations, congenital hearing loss is often only first uncovered years later, as a result of lack of language development [2]. Indeed, the average age of detection of hearing loss without screening is 2.5–3 years of age [2]. Furthermore, without early intervention, children with hearing loss demonstrate predictable irreversible deficits in communication and psychosocial skills, cognition and literacy [3]. The positive effect of early intervention and early care with hearing aids compared with a later onset of intervention is proven [10]. Furthermore, screening has been shown to be cost effective, greatly reducing future treatment and loss of productivity costs [11, 12].
The characteristics of a good screening programme are described elsewhere [13]. Specifically in terms of UNHS, a German paediatric audiology consensus in 2001 recommended a screening rate of >95%, a screen-fail <4% and appropriate follow-up of >95%. In addition, the audiological diagnosis should be confirmed within an appropriate time-frame, namely within the first 3 months of life, and the intervention should begin in the first 6 months of life [13].
In Switzerland, as in many European countries and the USA, UNHS is either recommended or already established and regulated by law [14]. In some countries, however, despite being recommended, UNHS is not reimbursed by standard health insurance.
Despite a successful pilot project [2] and the recommendations of various societies, UNHS is not reimbursed by the standard health insurance in Switzerland. Internally, in the University Hospital Zurich, we estimate that the actual cost of performing screening is roughly 25–30 CHF per patient. This takes into account the set-up costs of the equipment and training, as well as the ongoing costs of maintenance and personnel time. The patient’s health insurance, however, is charged a fixed rate for the birth and stay on the maternity ward, and there exists no specific possibility to charge for the screening. Once a screening test has been failed, however, the patient is then followed up as an outpatient and the hospital can charge the health insurer via the Tarmed system.
Screening costs are, therefore, generally carried by the hospitals. Despite this financial drawback, a nationwide survey in 2008 found that more than 80% of newborns received screening [15] and our current study estimates 97.9% of newborns are being screened. This compares to published data from other countries with, for example, the United Kingdom having a screening rate of 97.4% in 2006 and 99.7% in 2011 [16]. In Italy the rate in 2003 was 29.3% and in 2006 was 48.4% [17] and in the USA the rate 2010 was 95% [18].
However, measured against the criteria of the German audiological consensus, the Swiss UNHS programme can be considered only a qualified success. Firstly, it must be emphasised that our data are extrapolated from “clinic-level” information and not from individual patient records. If a clinic said that they routinely performed hearing screening, we have assumed a 100% screening rate. This is probably not true, so the actual screening rate probably lies slightly below the 97.9% we have calculated. This may well mean a screening rate of <95%. More worryingly, only 87% of patients present for follow-up evaluation, well below the recommended 95%. Though this is an improvement over the 83% previously reported in a Switzerland [15], there is still considerable room for improvement. Again, this result is similar to other countries, with Italy reporting a follow-up rate of 75%, New York state 89% and Korea 80% [19–21]. As a result, many children who should have further evaluation or treatment are not receiving these services.
The reasons for poor follow-up are probably multiple. A USA study [22] identified four areas in which there were barriers to follow-up, namely: lack of service-system capacity, lack of provider knowledge, challenges to families in obtaining services and information gaps. They also identified five key areas for future programme improvements: improving data systems to support surveillance and follow-up activities, ensuring that all infants have a medical “home”, building capacity beyond identified providers, developing family support services and promoting the importance of early detection.
Better communication between healthcare providers and patient tracking would clearly help. Some states of Germany run centralised databases so that children who have not passed or, indeed, have not been screened, can be identified and followed up [23].
In the University Hospital Zurich, follow-up takes place at an average age of 2.4 months. The screening fail rate of 2.1%, with an ultimate relevant bilateral hearing impairment in 0.12% is consistent with published results [6].
Each country will have specific barriers to the uptake of such screening programmes and their appropriate follow-up. In Switzerland, UNHS is not regulated by law and, despite what would seem to be an excellent screening rate, our follow-up still falls short of the recommended quality criteria. This is especially frustrating given our fairly static population and generally easy access to high quality care. A particularly striking result from our study is that midwife-run “birth-centres”, despite having knowledge of hearing screening, do not offer the service. Instead they inform the parents of the recommendation and rely on the parents seeking out a paediatrician/ENT doctor who can perform the test within a reasonable time-frame.
Whether a centralised tracking system of all newborns would improve follow-up is debatable and this clearly has data protection implications. Appropriate dissemination of information through relevant specialist societies, public health campaigns and the like may ultimately prove more efficacious.
Ideally, we would have performed a study looking at the records of each and every child born in Switzerland, but this was not feasible. Lack of information on how many patients were not screened despite being in an institution which claims to screen, how many children were born at home without medical supervision and so on are all limitations of our study. It would also be of great interest to understand better what happened to the children who did not attend follow-up: were they followed-up in another institution? Did they have hearing problems?
Another aspect which we have not addressed in this study is the possibility of passing the hearing screening, but still having a relevant hearing loss. There is very little literature as to the likelihood of a false positive OAE, although, anecdotally, we do occasionally see children who present as toddlers with relevant hearing loss despite a supposed normal screening. These children usually present because of poor language development or behavioural issues. The reason for this failure of the screening procedure could theoretically be a false positive screen, a retrocochlear pathology with intact hair cell function, or a progressive/delayed hearing loss.
The data presented in this article provide an estimate of the number of newborns undergoing hearing screening in Switzerland. The results show an improvement over the 5 years since the latest similar data were published. There is, however, still room for improvement. Exactly how this can be achieved is not clear, although it will probably involve close co-operation between parents, midwives, paediatricians, ENT doctors and, ultimately, the cost-carriers, be they the insurance companies, government or other. We hope, however, with this study to have raised the general awareness of UNHS and intend to revisit the topic in due course.
1 Grandori F, Lutman M. The European Consensus Development Conference on Neonatal Hearing Screening (Milan, May 15–16, 1998). Am J Audiol. 1999;8(1):19–20.
2 Probst R, Schmid N, Zehnder A. Otorhinolaryngologie 2001: Allgemeines Hörscreening für Neugeborene. Schweiz Med Forum. 2002;1:13-5. German.
3 Patel H, Feldman M. Universal newborn hearing screening. Paediatr Child Health. 2011;16(5):301–10.
4 Hyde ML. Newborn hearing screening programs: overview. J Otolaryngol. 2005;34(Suppl 2):S70–8.
5 Nelson HD, Bougatsos C, Nygren P, and U.S.P.S.T. Force, Universal newborn hearing screening: systematic review to update the 2001 US Preventive Services Task Force Recommendation. Pediatrics. 2008;122(1):e266–76.
6 Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening: summary of evidence. JAMA. 2001;286(16):2000–10.
7 Smith RJ, Bale JF, Jr., White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–90.
8 Nickel R, Forge A. Gap junctions and connexins in the inner ear: their roles in homeostasis and deafness. Curr Opin Otolaryngol Head Neck Surg. 2008;16(5):452–7.
9 American Academy of Pediatrics, Joint Committee on Infant Hearing., Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921.
10 Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol Clin North Am. 1999;32(6):1089–102.
11 Huang LH, Zhang L, Tobe RY, Qi FH, Sun L, Teng Y, et al. Cost-effectiveness analysis of neonatal hearing screening program in China: should universal screening be prioritized? BMC Health Serv Res. 2012;12:97.
12 Colgan S, Gold L, Wirth K, Ching T, Poulakis Z, Rickards F, et al. The cost-effectiveness of universal newborn screening for bilateral permanent congenital hearing impairment: systematic review. Acad Pediatr. 2012;12(3):171–80.
13 Gross M, Buser K, Freitag U, Hess M, Hesse V, Hildmann A, et al. Universelles Hörscreening bei Neugeborenen – Empfehlungen zu Organisation und Durchführung des universellen Neugeborenen-Screenings auf angeborene Hörstörungen in Deutschland. Z Geburtshilfe Neonatol. 2004;208(6):239–45. German.
14 Ptok M. Early detection of hearing impairment in newborns and infants. Dtsch Arztebl Int. 2011;108(25):426–31.
15 Veraguth D, Meyer A, Bohlender J. Aktueller Stand der Neugeborenen-Hörscreenings in der Schweiz. Aktuelle phoniatrisch-pädaudiologische Aspekte. 2008;16:41–3. German.
16 Davis A, Daniels S. Annual Report NHS Newborn Hearing Screening Programme 2010-11. UK National Screening Committee. Medical Research Council http://hearing.screening.nhs.uk/publications.
17 Bubbico L, Tognola G, Greco A, Grandori F. Universal newborn hearing screening programs in Italy: survey of year 2006. Acta Otolaryngol. 2008;128(12):1329–36.
18 Russ SA, Hanna D, DesGeorges D, Forsman I. Improving follow-up to newborn hearing screening: a learning-collaborative experience. Pediatrics. 2010;126(Suppl 1):S59–69.
19 Pisacane A, Auletta G, Toscano F, Errichiello M, Barrier F, Riccardi P, et al. Feasibility and effectiveness of a population-based newborn hearing screening in an economically deprived region of Italy. Int J Pediatr Otorhinolaryngol. 2013;77(3):329–33.
20 Liu CL, Farrell J, MacNeil JR, Stone S, Barfield W. Evaluating loss to follow-up in newborn hearing screening in Massachusetts. Pediatrics. 2008;121(2):e335–43.
21 Lim HW, Kim EA, Chung JW. Audiological Follow-up Results after Newborn Hearing Screening Program. Clin Exp Otorhinolaryngol. 2012;5(2):57–61.
22 Shulman S, Besculides M, Saltzman A, Ireys H, White KR, Forsman I. Evaluation of the universal newborn hearing screening and intervention program. Pediatrics. 2010;126(Suppl 1):S19–27.
23 Neumann K, Nawka T, Wiesner T, Hess M, Böttcher P, Gross M. Qualitätssicherung eines universellen Neugeborenen-Hörscreenings. HNO 2009;57:17-20.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.