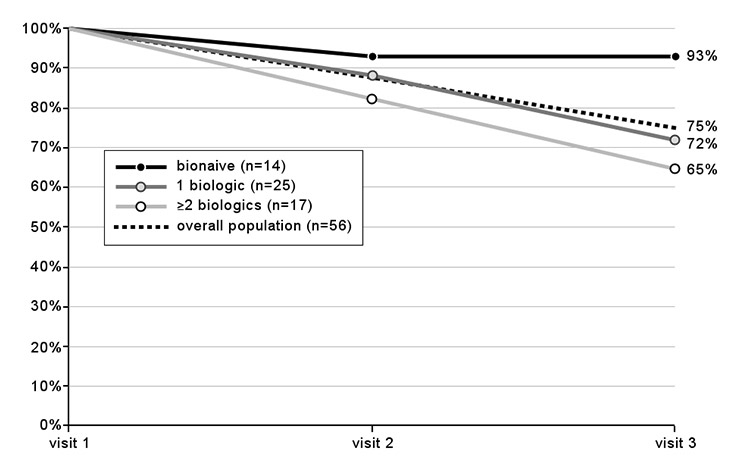
Figure 1
Drug retention over time: percentages of patients still on treatment at visits 2 and 3 (numbers in brackets indicate the population size at visit 1).
DOI: https://doi.org/10.4414/smw.2013.13849
Rheumatoid arthritis (RA), with a prevalence of 0.5%–1.0%, is the most widespread autoimmune inflammatory rheumatic disease [1]. Non biologic and biologic disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX) and TNFα-inhibitors (infliximab, etanercept, adalimumab), and biologics with different modes of actions such as abatacept, tocilizumab or rituximab are the mainstay of therapy in patients with RA. The efficacy and the tolerability of these agents have been demonstrated in numerous clinical trials [2, 3]. However, the setting of randomised controlled clinical studies does not necessarily reflect daily clinical practice and results may differ from those obtained in routine clinical practice [4].
A non-interventional observational study over 12 months, in collaboration with office-based Swiss rheumatologists on the use of biologics in RA patients in routine clinical practice was performed. The study aim was to investigate various treatment aspects such as physicians’ expectations, as well as efficacy and tolerability of biologic treatments in a real-world setting.
This was a non-interventional observational survey on the use of biologics in RA patients in routine clinical practice, approved by the responsible cantonal ethics committees. All adult patients with a confirmed diagnosis of moderate to severe RA could be included in the study at initiation of a new biologic, and all patients gave written informed consent prior to enrollment. Diagnosis of RA was made by the treating rheumatologist according to the 1987 ACR criteria [5]. There were no specific exclusion criteria, in particular regarding disease activity as current scores do not encompass all dimensions of an insufficient treatment in real-life setting.

Figure 1
Drug retention over time: percentages of patients still on treatment at visits 2 and 3 (numbers in brackets indicate the population size at visit 1).
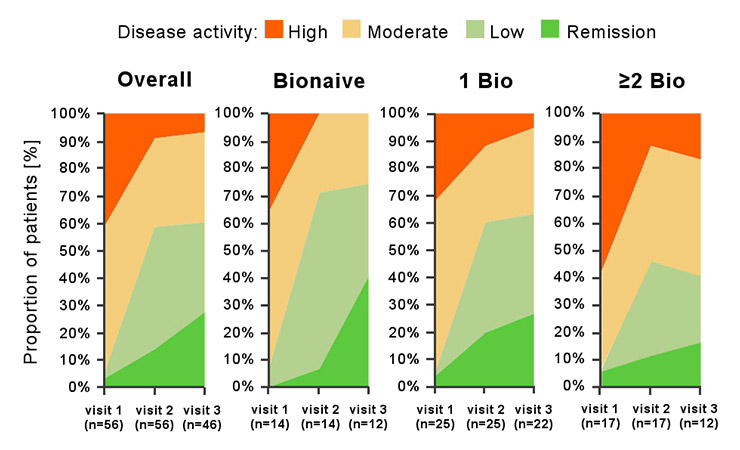
Figure 2
Disease state over time as assessed by physicians shown for the overall population, for biologic-naïve patients, and for patients with one (1 Bio) or at least two (≥2 Bio) previous biologics. Numbers in brackets indicate the numbers of patients (n) with available physicians’ assessment at each visit.
Participating physicians used standardised questionnaires to collect data on the use of biologics at inclusion (visit 1) and 2 follow up visits around 6 and 12 months after inclusion. Due to the nature of this observational survey, only data generated in routine clinical assessment were collected, without the introduction of any new interventions. All treatment decisions were at the discretion of the participating rheumatologists.
At inclusion (visit 1), the following baseline data was documented where available: baseline characteristics including gender, age, height, bodyweight; rheumatoid factor (RF) and antibodies against cyclic citrullinated peptide (anti-CCP) status; type and number of biologic pre-treatments, reasons for discontinuation and switching to current biologic treatment, disease parameters such as disease activity score with 28 joint count [6] (original DAS28-ESR or DAS28), C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR); disease activity state classified at the discretion of the rheumatologists as high disease activity (HDA), moderate disease activity (MDA), low disease activity (LDA), or remission; current biologic treatment, concomitant DMARD therapy, and glucocorticoid treatment; physicians’ expectations for the initiated biologic treatment; impairment in daily activities using a scale ranging from 1 to 10 (1 = no impairment; 10 = severe impairment). For the documentation of physicians’ expectations at baseline, a series of pre-defined statements (achieving remission, good tolerability, satisfaction of the patient, reduction of the DAS28 score, improvement of physical performance/function, inhibition of radiographic progression) could be selected and additional expectations could be documented in a free text field. For every choice that has been made, physicians had to indicate the relative importance by ranking their options. Physicians also had to indicate the time they expect a meaningful improvement after initiation of therapy by using four pre-defined tick boxes labelled with “immediately”, “≤3 months”, “≤6 months” and “>6 months”.
At visits 2 and 3, the physicians re-assessed disease activity state (HDA, MDA, LDA, and remission), disease activity parameters (DAS28, CRP, ESR), current biologic treatment, concomitant DMARD therapy, glucocorticoid treatment, and impairment in daily activities. Efficacy as well as safety/tolerability of the current biologic treatment were assessed using six pre-defined, numbered and labelled tick boxes ranging from 1 to 6 (1 = insufficient; 2 = moderate; 3 = satisfactory; 4 = good; 5 = very good; 6 = excellent).
Patient data was considered for a descriptive statistical analysis if a valid data set was available for visit 1 and at least 1 follow-up visit (mostly visit 2). Due to the vast majority of patients being treated with abatacept, other biologics were not included in the treatment outcome analysis. Data are presented as-observed.
Side effects were reported following the Swiss pharmacovigilance regulations.
No formal statistical testing was performed. The collected data was analysed using the SPSS 19 (Windows 7) in a descriptive statistical manner. Data are simple tabulations of the observed data allowing for the calculations of mean and median values, standard deviations, quantiles and confidence intervals.
| Table 1:Baseline characteristics of the study population. If not indicated otherwise, number of valid assessments for the abatacept and the “other treatments” group were 56 and 13, respectively. | ||||
| Patients receiving abatacept | Patients receiving other treatments | |||
| Patients, n | 56 | 13 | ||
| Gender, % female | 76.8% | 76.9% | ||
| Mean Age (SD), years | 56.9 (13.4) | 58.7 (14.7) | ||
| Disease duration, mean (SD), years | 7.7 (7.8) | 6.9 (5.4) | ||
| Disease duration, median (range), years | 5.1 (0.3–37.5) | 6.7 (0.3–18.5) | ||
| Disease status [# valid assessments] | ||||
| % RF positive | [n = 52] | 71.2% | [n = 12] | 75.0% |
| % anti-CCP positive | [n = 45] | 62.2% | [n = 10] | 60.0% |
| DAS28 (SD) | [n = 36] | 4.4 (1.2) | [n = 6] | 4.83 (0.3) |
| CRP (SD), mg/l | [n = 52] | 17.6 (17.2) | [n = 12] | 17.1 (10.3) |
| ESR (SD), mm/h | [n = 53] | 26.6 (19.1) | [n = 12] | 31.7 (11.5) |
| Concomitant DMARD, n (%) | 44 (78.6%) | 8 (61.5%) | ||
| None, n (%) | 12 (21.4%) | 5 (38.5%) | ||
| Methotrexate, n (%) | 36 (81.8%) | 6 (46.2%) | ||
| Leflunomide, n (%) | 9 (20.5%) | 2 (15.4%) | ||
| Sulfasalazine and/or hydroxychloroquine, n (%) | 5 (11.4%) | |||
| Glucocorticoid, n (%) | 42 (75%) | 4 (30.8%) | ||
| Previous biologic treatment | ||||
| No biologic (bio-naïve), n (%) | 14 (25%) | 10 (76.9%) | ||
| ≥1 biologic, n (%) | 42 (75%) | 3 (23.1%) | ||
| Number of previous biologics, mean | 1.5 | 2.3 | ||
From April 2009 until the end of March 2011, 70 RA patients were included by 23 office-based rheumatologists. One patient was lost to follow-up after the first visit and was therefore excluded from analysis. The other 69 patients had valid documentation available for baseline visit and at least 1 follow-up visit. Of the 69 patients, 13 received other biologics than abatacept and were not included in the current analysis due to the small sample size. The baseline characteristics of the excluded patients appeared similar to those in the abatacept subpopulation, with the exception that more patients seemed to have been bionaive (table 1). Therefore, a data set of 56 patients treated with the intravenous formulation of abatacept was eligible for analysis.
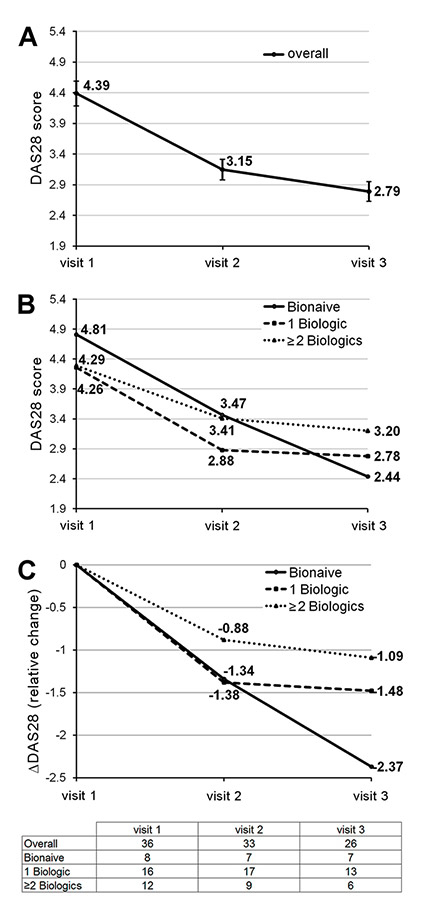
Figure 3
Change in DAS28 over time in the overall population and in the different subgroups. Data are mean values and, where indicated, with 95% confidence intervals. The table shows the numbers of patients with available DAS28-assessment at each visit. A) overall population; B) subgroup analysis showing absolute change in DAS28; C) subgroup analysis showing relative change in DAS28.
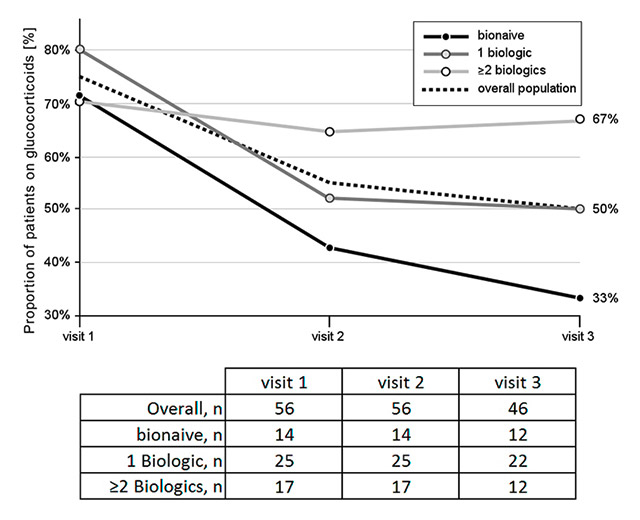
Figure 4
Glucocorticoid use over time: percentages of patients regularly taking glucocorticoids at the time of each visit. The numbers of patients with available information on glucocorticoid use at each visit are listed in the table.
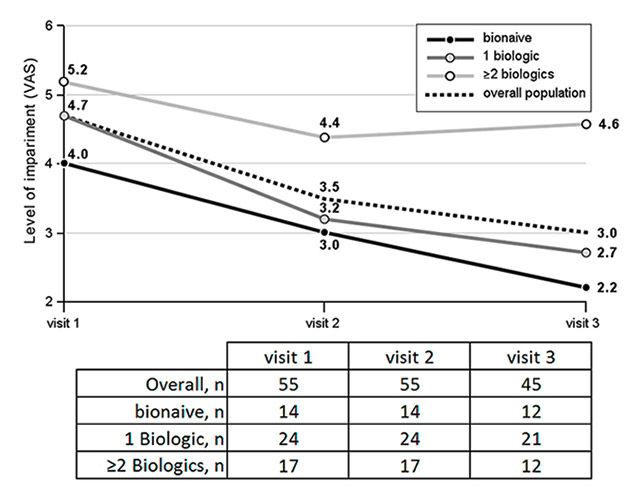
Figure 5
Impairment in daily activities: average level of patients’ impairment measured on a visual analogue scale (VAS): 1 = no impairment; 10 = severe impairment. The numbers of patients with available assessments at each visit are listed in the table.
At baseline, the most frequent expectations of physicians for the initiated abatacept treatments were good tolerability (91.1%), patient’s satisfaction (87.6%), and improvement of physical performance/function (80.4%). However, in terms of importance, the physicians rated achieving remission and improvement of physical performance/function as the most relevant treatment outcomes. The time span between the initiation of abatacept treatment and a meaningful improvement of the disease activity was expected to be 3 months for 37 patients (66.1%), 3 to 6 months for 18 patients (32%), and more than 6 months for 1 patient (1.8%).
The baseline characteristics of the abatacept population are presented in table 1. Most patients had an established disease and were seropositive for RF or anti-CCP. With a mean DAS28 of 4.4 at baseline, disease was active at initiation of the abatacept therapy. Twenty-five percent of patients were biologic-naïve. Almost half of the patients (44.6%) only had one previous biologic treatment, while the remaining 30.4% had received at least 2 previous biologics. Tumour necrosis factor (TNF) inhibitors were the predominantly used previous biologics with a median treatment duration of 7 months. In the vast majority of patients switched to abatacept, the previous biologic treatment was discontinued for inadequate response (table 2).
The mean observation time was 10.6 months (SD, 3.5 months). Ten patients (17.9%) had only one follow-up visit, while 46 patients (82.1%) completed visit 3. The mean intervals between visit 1 and visit 2 and between visit 1 and visit 3 were 5.9 months (median 6 months) and 11.7 months (median 12 months), respectively.
Three quarters of the patients (75%) in the abatacept population were still on treatment at the final visit. In biologic-naïve patients, the retention rate was markedly higher than in pretreated patients (fig. 1). Overall, abatacept treatment was discontinued in 7 patients (12.5%) at visit 2 and in an additional 7 patients (12.5%) at visit 3. Reasons for discontinuation or switching were inadequate response for all 7 patients at visit 2, and side effects (n = 1), inadequate response (n = 3), comorbidity (n = 1), achieving remission (n = 1), and “unpaid bills” (n = 1) at visit 3.
At baseline (visit 1), 44 patients (78.6%) received concomitant DMARD, 36 patients were on methotrexate (MTX), 9 patients received leflunomide, and 5 patients had sulfasalazine and/or hydroxychloroquine. Furthermore, 42 patients (75%) were on glucocorticoids at visit 1.
The evolution of disease activity state over time is presented in figure 2 for the overall population and for the subgroups of patients. At visit 1, most patients had high or moderate disease activity (23 patients with HDA [41.1%], and 30 patients with MDA [53.6%]). Only 1 patient had LDA (1.8%), while 2 patients were in remission (3.6%). Radiographic progression and insufficient state of physical function motivated the initiation of a new biologic therapy in the latter two patients. At the end of the observation period, we observed a clear overall improvement with 15 patients in LDA (32.6%), 13 patients in remission (28.3%), and 15 patients in MDA (32.6%). Only 3 patients were still judged as in HDA (6.5%).
In the biologic-naïve group, none of the patients remained in HDA at visit 2 or visit 3. The proportion of patients in MDA decreased between visit 1 and visit 2, and remained stable up to visit 3. On the other hand, the proportion of patients in LDA/remission substantially increased from visit 1 to visit 3 (7% and 75%, respectively).
The subpopulation with prior biologic treatment also demonstrated a clinically meaningful response to abatacept, i.e., a positive change of at least one disease activity category. However, the response was less pronounced than in the biologic-naïve subpopulation. There appears to be a trend for a negative correlation between treatment response and number of prior biologic treatments (fig. 2).
The mean DAS28 declined in the overall population from 4.39 (visit 1) to 2.79 (visit 3). Although baseline DAS28 was higher in the biologic-naïve subpopulation than in the groups with prior biologic treatments, biologic-naïve patients tended to demonstrate a better DAS28 response (fig. 3). The relative DAS28 reductions were –1.09 for the group pre-treated with ≥2 biologics, –1.48 for the group pretreated with 1 biologic, and –2.37 in biologic-naïve patients (fig. 3).
In parallel, there was a decrease in the concomitant use of glucocorticoids (fig. 4). This effect was more marked in the biologic-naïve patients as compared to the patients previously treated with 1 biologic. In contrast, no reduction on concomitant glucocorticoid use was observed in patients pre-treated with ≥2 biologics.
On a scale from 1 (least) to 10 (highest) the average impairment in daily activities was 4.7 at visit 1, decreasing to 3.0 at visit 3 (fig. 5). Baseline impairment was higher in patients previously treated with ≥2 biologics, than in patients with only 1 prior biologic treatment (5.2 and 4.7, respectively). Biologic-naïve patients showed the least impairment at baseline (4.0). Again, while the biologic-naïve patients and the ones treated with 1 previous biologic showed a marked improvement, impairment in daily activities was only slightly improved over time in the patients pretreated with ≥2 biologics.
The physicians assessed the long term efficacy and safety/tolerability of abatacept at visit 3 as follows: 95.6% of physicians rated the safety/tolerability as “good”, “very good” or “excellent”, while for 4.4%, safety was rated as modest to insufficient. The efficacy was assessed as “good” or better by 66.7%, as “sufficient” or “modest” by for 26.6% and as “insufficient” by 6.7% of physicians. There was a numerical trend towards a slightly better efficacy rating for biologic-naïve patients, with 83.3% of physicians rating it as “good” or better in this subpopulation.
CRP levels and ESR decreased between visit 1 and visit 3 from 17.5 mg/dl to 11.9 mg/dl, and from 26.5 mm/h to 17.8 mm/h, respectively. The clinical responses in function of baseline disease activity parameters are presented in figure 6. Patients with low disease activity or remission (DAS28 ≤3.2) at baseline remained well controlled under abatacept treatment, while patients with moderate (3.2 < DAS28 ≤5.1) or high disease activity (DAS28 >5.1) at baseline showed a marked treatment response and achieved a similar good disease control at visit 3, independent of baseline (fig. 6A).
Likewise, patients seemed to respond to abatacept treatment irrespective of baseline ESR or CRP, achieving a comparable mean DAS28 at visit 3 within an individual category (fig. 6B and 6C).
| Table 2:Pre-treatments and reasons for switching to abatacept (AE: adverse event; IR: inadequate response). | ||
| Pre-treatments before Abatacept initiation (n = 56) | ||
| Biologic-naïve, n (%) | 14 (25%) | |
| 1 biologic, n (%) | 25 (44.6%) | |
| 2 biologics, n (%) | 14 (25%) | |
| ≥3 biologics, n (%) | 3 (5.4%) | |
| Infliximab (n = 14) | ||
| Treatment duration, median (range) | 7 (3–57) | months |
| Reason for discontinuation | 14.3% | AE |
| 85.7% | IR | |
| Etanercept (n = 12) | ||
| Treatment duration, median (range) | 8 (2–63) | months |
| Reason for discontinuation | 25.0% | AE |
| 75.0% | IR | |
| Adalimumab (n = 33) | ||
| Treatment duration, median (range) | 6 (0–28) | months |
| Reason for discontinuation | 18.2% | AE |
| 78.8% | IR | |
| 3% | AE & IR | |
| Rituximab (n = 3) | ||
| Treatment duration, median (range) | 12 (1–15) | months |
| Reason for discontinuation | 100.0% | IR |
| Tocilizumab (n = 0) | ||
The present non-interventional observational study evaluated the use of abatacept in RA patients in routine clinical practice in Switzerland. Participating rheumatologists were office-based and each individual RA patient was evaluated and followed by a single physician. Importantly, the baseline characteristics of the abatacept patients in this study were generally similar to the general population of RA patients in Switzerland [7].
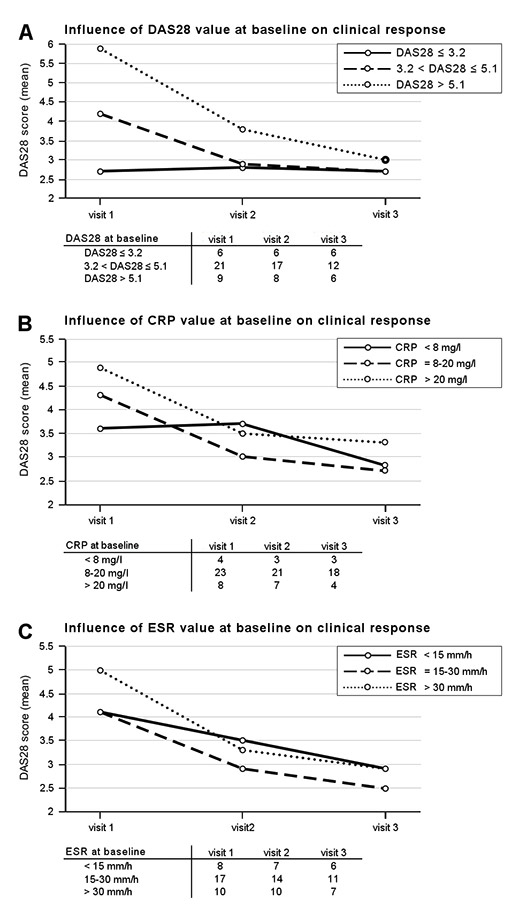
Figure 6
Clinical response over time depending on A) disease activity, B) CRP, and C) ESR values at baseline. The numbers of patients with available assessments at baseline and subsequent visits are listed in the tables. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
The efficacy of abatacept in combination with MTX has been demonstrated in different controlled clinical trials in MTX-naïve patients [8], in biologic-naïve patients [9–11], and in RA patients with insufficient response to prior therapy with TNF inhibitors [12]. The present non-interventional observational study supports these findings demonstrating efficacy in a broad spectrum of RA patients in a real-life setting. Abatacept was effective in the overall population, and in all subgroups of patients, ranging from biologic-naïve patients to “treatment refractory” patients with at least 2 previous biologic treatments. However, there was a definite trend for a better response in biologic-naïve patients as compared to biologic non responders, an observation valid for clinical response determined either by physician-rated disease activity state or by DAS28 (figs 2 and 3).
Glucocorticoids are effective in relieving signs and symptoms of RA and inhibiting radiographic progression, either as monotherapy or in combination with synthetic DMARD monotherapy or combination therapy [13], though they are well known for numerous adverse effects [14]. Reduction in the proportion of patients using glucocorticoids is highly desirable, and may also be considered as a surrogate marker of efficacy. The present observational report could demonstrate a numerical reduction in the proportion of patients using glucocorticoids after initiation of abatacept treatment in a routine clinical practice setting. However, the observed reduction was again more pronounced in the biologic-naïve patients, while concomitant glucocorticoid use remained unchanged in patients pretreated with ≥2 biologics. This might be at least partly explained with the higher proportions of patients demonstrating persistently high disease activity throughout the observation period in the latter group.
While certain biologics appear mainly efficacious in patients with very severe inflammation, response to abatacept was similar regardless of baseline CRP or ESR in our cohort, a finding resembling the observations made in clinical trials. In a post-hoc analysis of the AIM data, baseline CRP levels did not seem to influence the outcome of abatacept treatment at 1 year [15].
Drug retention, a surrogate marker of efficacy and safety, was high. At the end of the observation period with a mean duration of 10.6 months, 75% of patients were still on abatacept (fig. 1). Drug retention rate was higher (92%) in the biologic-naïve subpopulation, which might reflect the more favourable clinical response observed in this subpopulation and a higher treatment satisfaction. The high drug retention rate in biologic-naïve patients confirms comparable rates observed in various randomised controlled trials in a real-life setting. In the AGREE trial on abatacept in MTX-naïve patients with early erosive RA, the retention rate was still 90.6% one year after treatment initiation [16]. In the AIM study, a similar retention rate of 89% was seen in biologic-naïve patients [17]. In the ATTAIN trial in RA patients refractory to anti-TNF treatment, the retention rate in patients being newly treated with abatacept was 86.4% after 6 months of treatment [18], which compares well to the retention rates observed in the present survey after 6 months (88% in patients with 1 previous biologic treatment, and 82% in patients with at least 2 previous biologic treatments).
Participating physicians expected a meaningful drug response 3 months after initiation of abatacept treatment for the majority of their patients (66.1%). This expectation concurs with the findings in the AGREE trial [19], where the median duration to achieve 50% improvement in the physicians’ assessment of disease activity was 2 months. In the same clinical trial, the predictive value of achieving a particular disease state with abatacept after 3 months regarding its respective outcome after 1 year was also investigated [20]. The majority of patients in remission at month 3 remained in remission at month 12 (89.7%), while two-thirds of the patients with low disease activity score (LDAS) at month 3 achieved remission at month 12 (67.9%), and 61.4% of patients with a moderate disease activity score (MDAS) at month 3 achieved LDAS or remission at month 12. Thus, although a treatment response can be expected within the first 3 months of abatacept treatment, there is a fair chance for further improvement thereafter, an observation that also applies to the population of the present study.
Our study has some limitations. The short follow-up duration of one year does not allow for long-term conclusions. Also, the total number of enrolled patients and consequently the number of patients analysed in the subgroups is limited. The data analysis was performed in a descriptive way and is therefore not suitable to demonstrate significance. Importantly, results should be interpreted with some caution, given that the applied as-observed analysis tends to overestimate the true treatment effects. Furthermore, due to the non-interventional and observational design of the survey, data assessment was not standardised, which reflected routine clinical practice but increased the potential risk for assessment bias. Lastly, the study was conducted with intravenous abatacept. This is attributable to the fact that abatacept was not yet registered for subcutaneous injection in Switzerland when our study was performed. However, a recently published randomised clinical trial demonstrated similar efficacy and safety of intravenous and subcutaneous abatacept [21].
The results presented herein suggest that abatacept is an effective and well tolerated treatment in RA patients in routine clinical practice, irrespective of baseline disease activity and CRP or ESR levels.
Acknowledgement:We would like to thank the 23 office-based physicians who participated in this study in such an active and motivated manner and Nicoleta Gosoniu and Lorenzo Hess from Brunner & Hess Co. for their support in data management and analysis.
1 Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–81.
2 Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69(6):976–86.
3 Gaujoux-Viala C, Smolen JS, Landewé R, Dougados M, Kvien TK, Mola EM, et al. Current evidence for the management of rheumatoid arthritis with synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69(6):1004–9.
4 Kvien TK, Mikkelsen K, Nordvåg BY. Results from controlled clinical trials: how relevant for clinical practice? J Rheumatol. 2003;30(6):1135–7.
5 Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
6 Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
7 Zufferey P, Dudler J, Scherer A, Finckh A. Disease activity in rheumatoid arthritis patients at initiation of biologic agents and 1 year of treatment: Results from the Swiss SCQM registry. Joint Bone Spine. 2012 Jul 4. [Epub ahead of print]
8 Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–7.
9 Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–76.
10 Dougados M, Kremer JM, Le Bars M, Reed D, Park S, Vatsanos G, et al. Abatacept improves disease activity status over time in patients with rheumatoid arthritis and an inadequate response to methotrexate [abstract]. Ann Rheum Dis. 2009;68:573.
11 Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103.
12 Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
13 Gorter SL, Bijlsma JW, Cutolo M, Gomez-Reino J, Kouloumas M, Smolen JS, Landewé R. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69(6):1010–4.
14 McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131–7.
15 Sibilia J, Flipo RM, Combe B, Gaillez C, Le Bars M, Poncet C, Elegbe A, Westhovens R. L’abatacept Confère une Efficacité Clinique Indépendamment du Niveau de CRP à l’Inclusion Chez les Patients Présentant une Polyarthrite Rhumatoïde et une Réponse Inadaptée au Methotrexate (MTX) dans l’Étude AIM. Congrès SFR; Paris, France; 11–14 Dec 2011. Poster Presentation: Me 115
16 Bathon J, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis. 2011;70(11):1949–56.
17 Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–76.
18 Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
19 Durez P, Bathon J, Becker JC, Covucci A, Moniz Reed D, Westhovens R. Time course of improvement in ACR core components in patients with early rheumatoid arthritis treated with abatacept plus methotrexate. Poster FRI0201, EULAR Annual Congress, June 16–19, 2010.
20 Westhovens R, Moniz Reed D, Becker J-C, Vratsanos G, Yazici Y. Early improvements in disease activity with abatacept continue to increase or are maintained over time in methotrexate-naïve patients with early rheumatoid arthritis and poor prognostic factors. Poster SAT0109. EULAR Congress, 10–13 June, 2009.
21 Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63(10):2854–64.
Funding / potential competing interests:This study was sponsored by the Bristol-Myers Squibb affiliate Switzerland. JD is a member of Bristol-Myers Squibb Swiss Advisory Board and has received speaker honoraries from Bristol-Myers Squibb. AF has received speaker honoraries from Bristol-Myers Squibb. TH has received consulting fees from Bristol-Myers Squibb. RT is a full-time employee of, and stockholder in, Bristol-Myers Squibb.