Figure 1
Study flow.
ATG = anti-T-lymphocyte globulin; ECD = expanded-criteria donor; HLA-DSA = donor-specific HLA-antibodies; SCD = standard-criteria donor
DOI: https://doi.org/10.4414/smw.2013.13883
While the supply of organs cannot meet the rising need for donor kidneys, transplantation waiting lists are growing and a considerable number of patients die on the waiting list [1–3]. For this reason, from the end of the 1990s, deceased donor criteria have been extended to try to minimise the disparity between organ demand and supply [4, 5]. However, while in the USA the rate of kidneys from expanded criteria donors (ECDs) among all transplanted kidneys has slightly increased from 14.3% in 1999 to 17.1% in 2008, there is still an unaltered average discard rate of 40% for ECD kidneys which might be the consequence of an assumed inferior outcome [6, 7]. To assess the organ quality with respect to the outcome, several scoring systems considering a varying number of clinical, laboratory and histological parameters have been developed during the last 15 years [4, 8–11]. Based on these scores, most studies reported a substantial poorer graft survival [4, 7, 10, 15] and function [7, 9, 14, 16, 17] of ECD kidneys. Depending on the score applied and the populations studied, differences in one-year kidney graft survival between ECD and standard criteria donor (SCD) recipients remarkably varied between 1.8% and 31.9% [4, 7, 13, 14, 16, 18–20]. In previous studies, however, immunological factors such as sensitisation of the recipient, type of immunosuppression and episodes of rejection, which are still regarded as the most important determinants of long-term outcome in kidney transplantation, were poorly controlled [21]. So far, the immunological risk was assessed by the number of human leucocyte antigen (HLA) mismatches and the level of panel reactive antibodies, both variables are known to lack specificity and sensitivity in predicting the immunological risk [13, 16, 22–27]. During the last years, prediction of the immunological risk has dramatically improved with the advent of new techniques such as single-antigen flow-beads, which allow detection of donor-specific HLA-antibodies (HLA-DSA) with very high accuracy [25, 27]. Moreover, recent studies revealed that transplants in the absence of HLA-DSA were associated with a very low risk for early rejection and good long-term allograft survival [28–32]. However, the donor status was not taken into account in these studies and respective analyses were not performed. In our study, we therefore investigated risk factors and outcome of ECD kidneys in a well-defined, immunological low-risk population characterised by the absence of pre-transplant HLA-DSA.
We conducted a single centre cohort study including all patients without pretransplant HLA-DSA who received a deceased-donor kidney transplant at the University Hospital Basel between January 1999 and December 2010. Excluded were recipients of HLA-identical transplants and recipients treated with anti-T-lymphocyte globulin (see fig. 1). Follow-up was at least one year, and follow-up ended with death, graft failure, or the end of the observational period in December 2011. Donors were retrospectively categorised as standard or ECD donors according to the ECD score, as adopted by the OPTN/UNOS board of directors in 2001 [4]. Based on this score, donors were classified as ECD if their age was ≥60 years, or between 50 and 60 years and at least two of the following medical criteria were present: history of hypertension, serum creatinine >1.5 g/dl (132 µmol/l) or cerebrovascular accident (CVA) as cause of death. Recipients were stratified by their donor status (fig. 1). The study protocol was approved by the Institutional Review Board (IRB no. 170/11) and was carried out according to the principles of the Declaration of Istanbul.

Figure 1
Study flow.
ATG = anti-T-lymphocyte globulin; ECD = expanded-criteria donor; HLA-DSA = donor-specific HLA-antibodies; SCD = standard-criteria donor
Demographic and clinical data of all donors were collected in an anonymised donor information form. The following variables were extracted: age, sex, weight, history of hypertension, cause of death and serum creatinine.
All clinical, immunological and laboratory data of recipients were continuously collected in standardised flow sheets and medical records. The following co-morbidities were extracted: diabetes mellitus, coronary artery disease (CAD), and peripheral artery disease (PAD). Graft function was assessed using estimated creatinine clearance (eCrCl) in accordance with the formula by Cockcroft and Gault [33].
Pretransplant HLA-antibodies were detected and specified by single HLA-antigen flow beads on a Luminex platform (LabScreen single antigen, One Lambda Inc., Canoga Park, CA, USA). Donor-specificity was determined by comparison of the HLA-antibody specificities of the recipient with the HLA-typing of the donor (i.e., by virtual crossmatching). Evaluation of HLA-DSA by virtual cross-matching was performed retrospectively from January 1999 to October 2004 and prospectively from November 2004 on as described previously [29, 30].
Indication biopsies were performed when serum creatinine rose by more than 20%. Biopsy specimens consisted of two cores obtained by a 16-gauge needle. Histological findings were graded in accordance with the updated Banff 2009 classification [34]. All reported rejection episodes were biopsy-proven.
Delayed graft function (DGF) was defined as the need for haemodialysis during the first week post-transplant owing to inadequate allograft function. Patients who had only one post-transplant haemodialysis session to correct hyperkalaemia or fluid overload were not considered to have DGF [35].
Initially, all recipients received a triple therapy. Driven by the intention to introduce newly approved immunosuppressants, the immunosuppressive regimens changed over time. Importantly, there was no specific immunosuppression or protocol with respect to donor status, ischaemia time or DGF during the observation period. Tac-based immunosuppressive protocols consisted of tacrolimus (Tac; Prograf®, Astellas), mycophenolate-mofetil (MMF; CellCept®, Roche) and prednisone (P) or Tac, azathioprine (Imurek®, Pro Concepta Zug AG) and P, or of a steroid-free regimen consisting of Tac, mycophenolate-sodium (MPS; Myfortic®, Novartis), and sirolimus (Sir; Rapamune®, Pfizer, previously Wyeth) or everolimus (Ev; Certican®, Novartis). Non-Tac-based immunosuppression consisted of ciclosporin A (Sandimmun Neoral®, Novartis), MMF and P or of a calcineurin-inhibitor-free regimen with MMF-Sir-P. MMF and MPS, and Sir and Ev were regarded as equivalent and summarised as mycophenolic acid (MPA) and mTor-inhibitor, respectively. Induction therapy with interleukin-2 (IL-2) receptor antagonists (daclizumab, Zenapax®, Roche or basiliximab, Simulect®, Novartis) was implemented in 2002 and was made standard practice in 2005. As Tac-based immunosuppression became standard practice in 2005, 85% of patients in the Tac group received IL-2 receptor antagonists, compared with 48% in the non-Tac group.
Primary endpoint of the study was graft survival. Secondary endpoints were graft function at one and three year follow-up and cumulative rejection rate at one year. To analyse graft survival, patients were censored for lost-of-follow-up but not for death. For evaluation of graft function, eCrCl was defined as 5ml/min in patients with graft failure prior to the respective follow-up time point, while patients who died with functioning graft or were lost-of-follow-up were excluded from the respective analysis.
The statistical analyses were performed using SPSS/PC (IBM SPSS statistics 19). Discrete variables were expressed as counts (percentage), and comparison between groups was done with Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed or as median and range if not normally distributed, and t-test or Mann-Whitney test were used for comparison between groups, respectively. One-year and three year eCrCl was compared by Wilcoxon signed-rank test. Graft survival and risk of rejection were analysed by uni- and multi-variate Cox regression. To address the limitations of the data due to a decreasing number of patients in long-term follow up and to ensure a high validity of the analyses, graft survival was modelled with a follow-up period of six years. The six year follow-up period was the breakpoint when two thirds of all patients were censored or had had an event. Cumulative survival analyses were calculated using Kaplan-Meier analysis. Differences between curves in Kaplan-Meier graphs were evaluated by means of log-rank statistics. A p-value of <0.05 was considered statistically significant, significance levels are two-tailed, and confidence intervals (CI) are 95% CIs.
Three hundred and eighty-eight patients received a deceased-donor kidney transplant. Eighty-nine patients with pretransplant HLA-DSA, six patients with unknown HLA-DSA status, four patients with HLA-identical transplants and seventeen patients treated with anti-T-lymphocyte globulin (ATG; ATG-Fresenius, Fresenius Medical Care) in the absence of HLA-DSA were excluded. Further, four recipients were excluded because of missing clinical data, and three recipients due to a non-classifiable donor status, leaving 265 eligible patients (fig. 1). Median follow-up was 4.3 years (range 0.0–12.8 years). Five patients (2%) were lost to follow-up and remained in the study for 0.6, 1.0, 1.0, 4.9 and 9.0 years after transplantation.
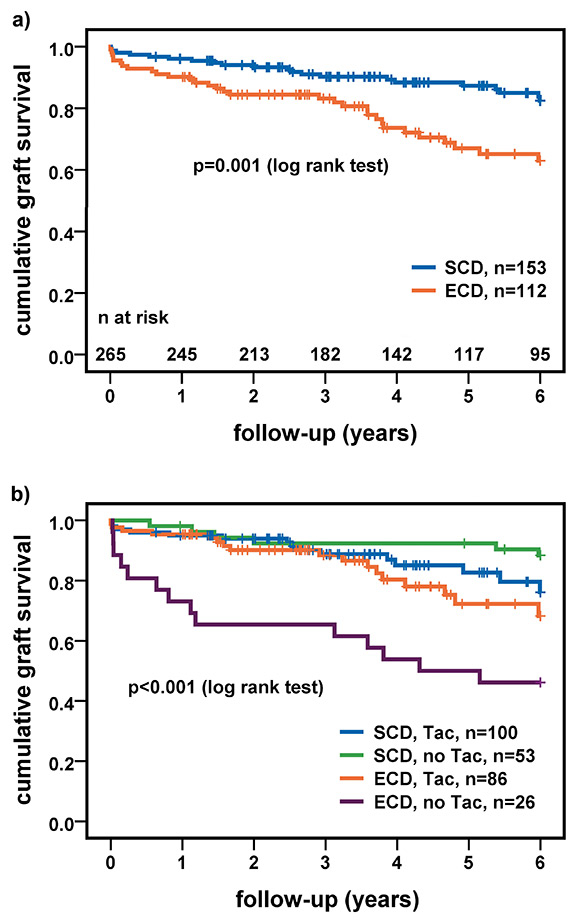
Figure 2
(a) Cumulative graft survival stratified by donor status. (b) Cumulative graft survival stratified by donor status and immunosuppression; p-values of difference between subgroups in figure 2b: ECD/Tac versus ECD/no Tac p = 0.008 (log rank test); SCD/Tac versus SCD/no Tac p = 0.1 (log rank test).
ECD = expanded-criteria donor; SCD = standard-criteria donor; Tac = tacrolimus
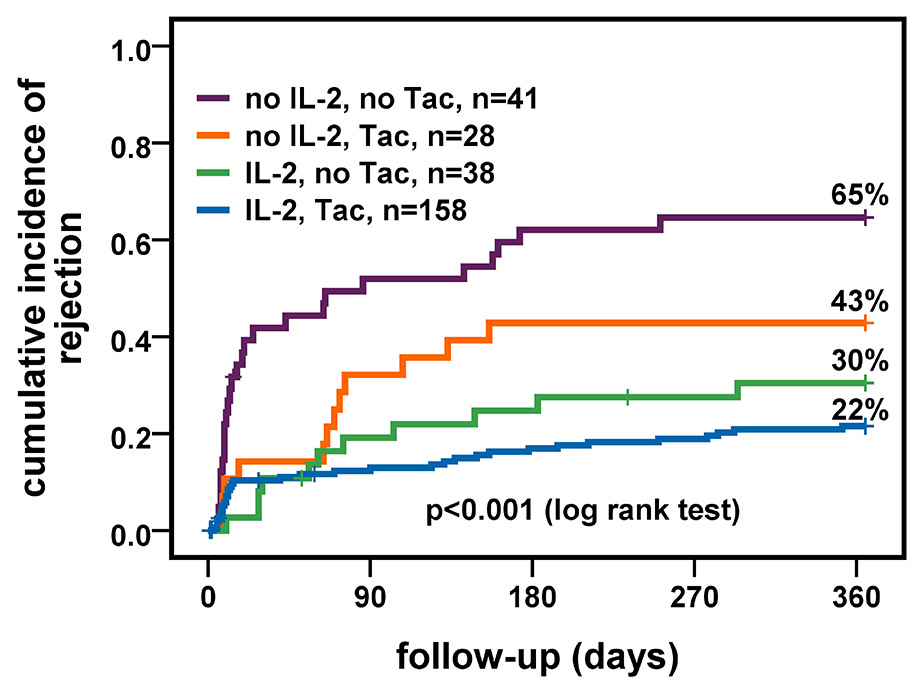
Figure 3
Cumulative incidence of clinical rejection stratified by immunosuppression and induction therapy.
IL-2 = interleukin-2 receptor antagonist; Tac = tacrolimus
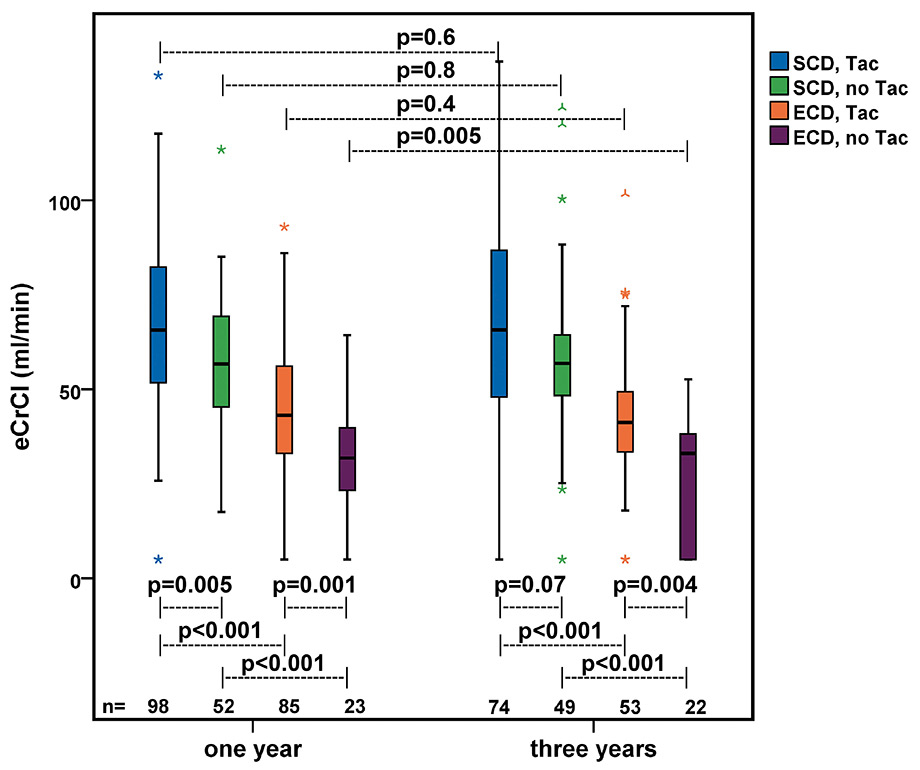
Figure 4
Renal function at one-year and three-year follow-up stratified by donor status and type of immunosuppression. An eCrCl of 5ml/min was assumed in patients who suffered from graft failure before the defined follow-up time points (one year n = 10 and three years n = 18); p-values of differences between groups were calculated by Mann-Whitney test; p-values of differences between one-year and three-year follow-up were calculated by Wilcoxon signed-rank test, considering only patients with values at one year and three year follow-up (n = 198).
ECD = expanded-criteria donor; eCrCl = estimated creatinine clearance in accordance with the formula by Cockcroft and Gault [33]; SCD = standard-criteria donor; Tac = tacrolimus
One hundred and twelve (42%) of 265 kidney transplants were classified as ECD organs. Characteristics by donor status are shown in table 1. ECDs were significantly older than SCDs (median age 68 vs 39 years, p <0.001), had a higher frequency of hypertension (59% vs 9%, p <0.001), died more often from a CVA (75% vs 36%, p <0.001) and had a significant lower eCrCl (median 75 vs 119 ml/min, p <0.001).
Recipients’ characteristics are shown in table 2. One hundred and eighty-four (69%) recipients were male. Median age at transplantation was 57 years (range 20–75 years). Recipients of ECD kidneys were significantly older (62 vs 51 years, p <0.001), and more likely to suffer from diabetes mellitus (33% vs 14%, p = 0.001) and vascular disease (35% vs 23%, p = 0.04) than recipients of SCD kidneys. Median cold ischaemia time of ECD kidneys was significantly longer compared with SCD kidneys (763 vs 585 min, p <0.001).
Of the 265 transplanted patients, 52 patients (20%) suffered death or graft failure. Overall, one, three and five-year graft survival rates were 94%, 87% and 79%, respectively. Overall, in multivariate Cox regression the ECD status was the only significant risk factor for graft failure (hazard ratio [HR] 2.31, CI 1.22-4.37; p = 0.01) (table 3). Subgroup analysis revealed an increased risk for graft failure with increasing recipients age in the uni- and multi-variate model in the SCD group (HR 1.05 per year, CI 1.01–1.10; p = 0.01 univariate and HR 1.06 per year, CI 1.01–1.10; p = 0.02 multivariate). In ECD recipients the number of HLA mismatches was a risk factor (HR 1.39, CI 1.01–1.91; p = 0.04) and immunosuppression with Tac protective (HR 0.39, CI 0.19‒0.80; p = 0.01) in the univariate model, whereas in the multivariate analysis only immunosuppression with Tac indicated a trend for risk reduction (HR 0.46, CI 0.20–1.06; p = 0.07). When grouping by donor status, one, three and five-year survival rates were 96%, 90% and 87%, respectively, for SCD and 90%, 83% and 67%, respectively, for ECD kidneys (p = 0.001) (fig. 2a). Figure 2b shows cumulative graft survival stratified by donor status and immunosuppressive regimen. There was no significant difference in one, three and five-year survival rates between ECD recipients treated with Tac (95%, 88% and 72%, respectively), SCD recipients treated with Tac (95%, 89% and 83%, respectively) or without Tac (98%, 92% and 92%, respectively) (p = 0.06). In contrast, one, three and five-year survival rates in recipients of ECD kidneys treated without Tac were significantly lower (73%, 65% and 50%, respectively; p <0.001).
Overall, the cumulative incidence of clinical rejection was 32% after one year. In multivariable Cox regression, higher recipients age (HR 0.97, CI 0.95–0.99; p = 0.001), induction therapy with an IL-2 antagonist (HR 0.41, CI 0.25‒0.69; p = 0.001) and immunosuppression with Tac (HR 0.60, CI 0.37-0.97; p = 0.04) were protective factors, whereas ECD status (HR 1.09, CI 0.66‒1.82; p = 0.7) had no effect on rejection-free graft survival (table 4). Figure 3 shows cumulative incidence of rejection stratified by immunosuppressive regimen and induction therapy with IL-2 receptor antagonists. Recipients treated with Tac-based immunosuppression and induction therapy had the lowest cumulative rejection rate (22%) compared with Tac-based immunosuppression without induction therapy (43%) and non-Tac-based immunosuppression with (30%) and without induction therapy (65%) (p-value <0.001). Rejection rates did not differ between ECD and SCD recipients, overall (27% and 35%) and stratified by immunosuppressive regimen (all p-values ≥0.2) (not shown in figure).
Two hundred and fifty-eight and 198 patients were included for graft function analysis at one and three year post-transplant, respectively. For the one-year follow-up, seven patients ( six patients who died with functioning graft and one patient who was lost to follow-up) and for the three-year follow-up 67 patients (16 patients who died with functioning graft, three who were lost to follow-up and 48 who had less than three years of follow-up) were excluded from analysis. At one and three years after transplantation, recipients of ECD kidneys had a significantly lower allograft function (median eCrCl 40 ml/min [range 5–93 ml/min] and 37 ml/min [range 5–102 ml/min], respectively) than recipients of SCD kidneys (62 ml/min [range 5–133 ml/min] and 58 ml/min [range 5–137 ml/min], respectively) (p-value <0.001) (not shown in figure). The difference in eCrCl between recipients of ECD and SCD kidneys was consistent throughout the subgroups stratified by immunosuppressive regimen in both follow up periods (all p-values <0.001) (fig. 4).
Recipients of ECD kidneys treated with Tac had a higher median eCrCl than those treated without Tac at one year (43 ml/min [range 5–93 ml/min] vs 32 ml/min [range 5–64 ml/min], p = 0.001) and at three years (41 ml/min [range 5–102 ml/min] vs 33 ml/min [range 5–53 ml/min], p = 0.004) after transplantation. In recipients of SCD kidneys there was a significant difference between those treated with Tac and those treated without Tac at one year (66 ml/min [range 5–133 ml/min] vs 57 ml/min [range 18–113 ml/min], p = 0.005), whereas at three years the difference was not significant (66 ml/min [range 5–137] vs 57 ml/min [range 5–124 ml/min], p = 0.07) (see fig. 4). Regarding the kidney function over time, recipients of ECD kidneys treated without Tac had a significant decrease in eCrCl from one to three years (median change –3.2 ml/min, p = 0.005). In recipients of ECD kidneys treated with Tac (median change 0.0 ml/min, p = 0.4) and in recipients of SCD kidneys treated with Tac (median change 0.3 ml/min, p = 0.6) or without Tac (median change –0.5 ml/min, p = 0.8) graft function remained stable over time (see fig. 4).
When stratified by donor status and clinical rejection, ECD recipients who suffered a rejection had a lower eCrCl at one-year and at three-year follow-up (37 ml/min and 33 ml/min) compared with ECD recipients who did not suffer a rejection (42 ml/min and 41 ml/min) (p = 0.06 and p = 0.03, respectively), whereas no such difference was detectable between SCD recipients with (60 ml/min and 59 ml/min) and without rejection (64 ml/min and 58 ml/min) (p = 0.4 and p = 0.8, respectively) (not shown in figure).
| Table 1: Characteristics of donors. | ||||
| All | SCD | ECD | p-value& | |
| Number of donors | 265 | 153 (58) | 112 (42) | |
| Male sex | 143 (54) | 84 (55) | 59 (53) | 0.8* |
| Age | 52 [0‒86] | 39 [0‒59] | 68 [52‒86] | <0.001° |
| Age below 10 yearsa | 28 (11) | 28 (18) | <0.001* | |
| Age over 60 years | 106 (40) | 106 (95) | <0.001* | |
| Creatinine (µmol/l) (n = 222/112/110)§,# | 75 [27‒813] | 71 [34‒813] | 79 [27‒315] | 0.3° |
| Creatinine over 132 µmol/l (n = 222/112/110)§, # | 30 (12) | 18 (12) | 12 (11) | 0.8* |
| eCrCl (n = 213/109/104)§,# | 88 [14‒308] | 119 [14‒308] | 75 [24‒212] | <0.001° |
| Hypertension§ (n = 235/136/99) | 70 (30) | 12 (9) | 58 (59) | <0.001* |
| Cause of death | ||||
| – CVA (ischaemia or haemorrhage) | 139 (52) | 55 (36) | 84 (75) | <0.001* |
| – Trauma/accident | 63 (24) | 47 (31) | 16 (14) | 0.002* |
| – Drowning | 5 (2) | 5 (3) | 0.08* | |
| – Suicide | 8 (3) | 5 (3) | 3 (3) | 1.0* |
| – Other | 50 (19) | 41 (27) | 9 (8) | <0.001* |
| Data are displayed as counts and (percent) or median and [range]. &p-value between SCD and ECD. °Mann-Whitney U-Test, *Fisher’s exact Test, §Due to missing data the number is below the total numbers of donors, first number reflects all donors, second SCD donors, third ECD donors. #Due to different reference values, donors under 16 years (n = 21, 11%) were not included in analysis of serum creatinine and eCrCl. aIn the orginal publications donors younger than 10 years were not considered [4, 18]. SCD: standard-criteria donor; ECD: expanded-criteria donor; eCrCl: estimated creatinine clearance according to the formula by Cockcroft and Gault [33]; CVA: cerebrovascular accident. To convert serum creatinine from µmol/l to mg/dl divide by 88.4. | ||||
| Table 2: Characteristics of recipients by donor status. | ||||
| All | Recipients of SCD kidneys | Recipients of ECD kidneys | p-value& | |
| Number of patients | 265 | 153 (58) | 112 (42) | |
| Male sex | 184 (69) | 106 (69) | 78 (70) | 1.0* |
| Age (n = 191) | 57 [20-75] | 51 [20-74] | 62 [28-75] | <0.001° |
| Vascular disease | 74 (28) | 35 (23) | 39 (35) | 0.04* |
| – CAD | 53 (20) | 24 (16) | 29 (26) | 0.04* |
| – PAD | 38 (14) | 19 (12) | 19 (17) | 0.4* |
| Diabetes mellitus | 59 (22) | 22 (14) | 37 (33) | 0.001* |
| Transplant, n with 1st, 2nd, 3rda | 238 (90), 22 (8), 5 (2) | 136 (89), 14 (9), 3 (2) | 102 (91), 8 (7), 2 (2) | 0.9* |
| HLA mismatches mean (±SD) | 4.2 (±1.2) | 4.3 (±1.2) | 4.1 (±1.2) | 0.1°° |
| Cold ischaemia time (min) | 660 [79-3540] | 585 [79-2220] | 763 [120-3540] | <0.001° |
| Induction therapy with IL-2-antagonists | 196 (74) | 114 (74) | 82 (73) | 0.9* |
| Immunosuppressive therapy | ||||
| – CyA-MPA-P | 54 (20) | 35 (23) | 19 (17) | 0.3* |
| – Sir-MPA-P | 25 (9) | 18 (12) | 7 (6) | 0.1* |
| – Tac-Aza-P | 37 (14) | 20 (13) | 17 (15) | 0.7* |
| – Tac-MPA-mTor | 51 (19) | 35 (23) | 16 (14) | 0.09* |
| – Tac-MPA-P | 97 (37) | 45 (29) | 52 (47) | 0.007* |
| – other Tac-MPA containing regimen | 1 (0) | 1 (1) | 0.4* | |
| Immunosuppression including Tac | 186 (70) | 100 (65) | 86 (77) | 0.06* |
| Data are displayed as counts and (percent) or median and [range]. &p-value between SCD and ECD. °Mann-Whitney-U-Test, °°Students t-Test, *Fisher’s exact Test. aTransplant, n with 1st, 2nd, 3rd: number (percent) of patients with first, second and third transplants. SCD: standard-criteria donor; ECD: expanded-criteria donor. Vascular disease: presence of CAD and/or PAD, CAD: coronary artery disease, PAD: peripheral artery disease. IL-2: interleukin-2 receptor; CyA: ciclosporin, MPA: mycophenolic acid, P: prednisone, Tac: tacrolimus, Aza: azathioprine, mTor (mTor-Inhibitor,): sirolimus or everolimus, Sir: sirolimus. | ||||
| Table 3: Cox regression of graft survival. | ||||||||||||
| Overall, n = 265 | SCD, n = 153 | ECD, n = 112 | ||||||||||
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR (CI) | p-value | HR (CI) | p-value | HR (CI) | p-value | HR (CI) | p-value | HR (CI) | p-value | HR (CI) | p-value | |
| ECD | 2.46 (1.41‒4.29) | 0.001 | 2.31 (1.22‒4.37) | 0.01 | ||||||||
| Male sex | 0.95 (0.53‒1.69) | 0.9 | 0.70 (0.38‒1.30) | 0.3 | 1.16 (0.45‒2.99) | 0.8 | 1.03 (0.38‒2.80) | 0.9 | 0.82 (0.39‒1.70) | 0.6 | 0.57 (0.26‒1.26) | 0.2 |
| Age | 1.04 (1.01‒1.07) | 0.005 | 1.02 (0.99‒1.06) | 0.1 | 1.05 (1.01‒1.10) | 0.01 | 1.06 (1.01‒1.10) | 0.02 | 1.00 (0.97‒1.04) | 0.9 | 0.99 (0.95‒1.04) | 0.7 |
| Vascular disease | 2.01 (1.15‒3.52) | 0.02 | 1.59 (0.85‒2.97) | 0.1 | 2.32 (0.96‒5.59) | 0.06 | 1.41 (0.51‒3.90) | 0.5 | 1.54 (0.75‒3.19) | 0.2 | 1.30 (0.57‒2.94) | 0.5 |
| Diabetes mellitus | 1.94 (1.09‒3.47) | 0.03 | 1.46 (0.77‒2.76) | 0.2 | 1.90 (0.70‒5.18) | 0.2 | 1.32 (0.44‒3.92) | 0.6 | 1.43 (0.70‒2.95) | 0.3 | 1.74 (0.76‒3.96) | 0.2 |
| Number of HLA mismatches | 1.26 (0.98‒1.61) | 0.07 | 1.29 (0.99‒1.68) | 0.06 | 1.19 (0.80‒1.77) | 0.4 | 1.33 (0.83‒2.11) | 0.2 | 1.39 (1.01‒1.91) | 0.04 | 1.26 (0.89‒1.77) | 0.2 |
| Cold ischaemia time (hours) | 1.02 (0.98‒1.05) | 0.3 | 0.99 (0.96‒1.03) | 0.7 | 0.97 (0.89‒1.05) | 0.4 | 0.94 (0.87‒1.03) | 0.2 | 1.01 (0.97‒1.05) | 0.7 | 1.01 (0.97‒1.04) | 0.8 |
| Induction therapy IL-2-antagonists | 0.75 (0.42‒1.32) | 0.3 | 0.85 (0.43‒1.70) | 0.6 | 1.22 (0.47‒3.20) | 0.7 | 0.94 (0.31‒2.85) | 0.9 | 0.56 (0.27‒1.14) | 0.1 | 1.12 (0.43‒2.88) | 0.8 |
| IS based on Tac | 0.92 (0.52‒1.63) | 0.8 | 0.84 (0.45‒1.56) | 0.6 | 2.07 (0.78‒5.46) | 0.1 | 1.74 (0.61‒4.97) | 0.3 | 0.39 (0.19‒0.80) | 0.01 | 0.46 (0.20‒1.06) | 0.07 |
| Delayed graft function | 0.85 (0.44‒1.65) | 0.6 | 0.73 (0.35‒1.51) | 0.4 | 0.96 (0.32‒2.86) | 0.9 | 1.16 (0.37‒3.59) | 0.8 | 0.65 (0.28‒1.51) | 0.3 | 0.59 (0.23‒1.56) | 0.3 |
| Clinical rejection in 1st year | 1.06 (0.60‒1.89) | 0.8 | 1.07 (0.55‒2.06) | 0.9 | 0.65 (0.25‒1.69) | 0.4 | 0.68 (0.21‒2.19) | 0.5 | 1.83 (0.89‒3.77) | 0.1 | 1.40 (0.61‒3.20) | 0.4 |
| Cox regression was modelled with a follow‒up period of 6 years. N of events until 6 year follow‒up: Overall n = 52, SCD n = 21, ECD n = 31. SCD: standard-criteria donor; ECD: expanded-criteria donor; IL‒2: interleukin‒2 receptor; IS: immunosuppression; Tac: tacrolimus. | ||||||||||||
| Table 4: Cox regression of clinical rejection. | ||||
| Overall, n = 265 | Univariate | Multivariate | ||
| HR (CI) | p-value | HR (CI) | p-value | |
| ECD | 0.72 (0.46–1.13) | 0.2 | 1.09 (0.66–1.82) | 0.7 |
| Male sex | 1.51 (0.90–2.52) | 0.1 | 1.31 (0.78–2.22) | 0.3 |
| Recipients age in years | 0.97 (0.95–0.99) | <0.001 | 0.97 (0.95–0.99) | 0.001 |
| Vascular disease | 1.06 (0.66–1.71) | 0.8 | 1.55 (0.90–2.67) | 0.1 |
| Diabetes mellitus | 0.86 (0.50–1.48) | 0.6 | 0.95 (0.53–1.73) | 0.9 |
| Number of HLA mismatches | 1.35 (1.10–1.66) | 0.004 | 1.22 (0.99–1.50) | 0.06 |
| Cold ischaemia time (hours) | 0.98 (0.95–1.01) | 0.3 | 0.98 (0.95–1.01) | 0.2 |
| Induction therapy IL-2-antagonists | 0.32 (0.21–0.49) | <0.001 | 0.41 (0.25–0.69) | 0.001 |
| Immunosuppression based on Tac | 0.43 (0.28–0.67) | <0.001 | 0.60 (0.37–0.97) | 0.04 |
| Delayed graft function | 0.78 (0.46–1.31) | 0.3 | 1.02 (0.58–1.80) | 0.9 |
| ECD: expanded-criteria donor; IL-2: interleukin-2 receptor; Tac: tacrolimus. | ||||
In our well-defined immunologically low-risk population, the overall outcome of kidney grafts was favourable, which is in line with recent observations showing that transplantations in absence of HLA-DSA result in good long-term graft survival [28, 30, 32]. When stratified by donor status, graft survival in recipients of ECD kidneys was still acceptable, but compared to SCD kidneys, one, three and five year graft survival rates were 6%, 7% and 20% lower. This finding is consistent with the vast majority of the literature reporting inferior outcome of ECD kidneys [7]. However, while overall ECD status was the only significant risk factor in multivariate analysis, risk factors for graft survival were different according to the donor status. In the SCD group, recipients’ age was a significant risk factor, which reflects the fact that graft survival was not censored for death. In the ECD group age was not a risk factor, which might be explained by the uneven age distribution in this group (75% of ECD recipients were more than 55 years old) and by the poorer outcome of ECD organs in very young recipients [36, 37]. The multivariate model showed a trend for Tac as a protective factor for graft survival in the ECD group. The beneficial effect on graft survival and graft function of immunosuppression based on low-dose Tac in combination with MPA or azathioprine was demonstrated in several studies [38–42]. Unfortunately, none of them stratified outcomes by donor status. Our study shows for the first time that recipients of ECD kidneys treated with Tac had a better graft survival and graft function compared with those treated without Tac. In our population and with a follow-up of 6 years, the beneficial effect of Tac-based immunosuppression resulted in ECD graft survival rates similar to SCD kidneys. This might be explained by a reduced rejection rate in combination with the fact that ECD grafts are more susceptible to additional injuries and therefore have a larger benefit from prevention by optimised immunosuppressive treatment [41, 43–45]. Recipients of ECD kidneys treated with Tac preserved their graft function, while a significant decrease was shown in ECD kidneys treated without Tac. In keeping with the results of the Symphony study, cumulative rejection rates in patients treated with Tac were significantly lower, and recipients of ECD kidneys without rejection had a better graft function than those with rejection after three years post-transplant. Beside the reduced rejection rate, recipients treated with low-dose Tac-based immunosuppression might also benefit from less calcineurin inhibitor nephrotoxicity, which is one of the most important nonimmunological causes of chronic allograft nephropathy [46]. As emphasised by Chapman, Tac in low doses is associated with a reduced chronic nephrotoxicity compared to ciclosporin [47]. Even compared with a calcineurin inhibitor-free immunosuppression with Sir, a low-dose Tac regimen was not associated with an increase in nephrotoxicity [48, 49] while concurrently revealing an equal or even better graft function [43, 48].
Interestingly, in our study population there was a difference in cold ischaemia time (CIT) between ECD and SCD, which disappeared with the introduction of the new Swiss transplant law in July 2007 (data not shown). The longer CIT in ECD kidneys prior to this date can probably be explained by the fact that organ allocation was less strictly coordinated nationally, leaving more autonomy to regional transplantation centres, and that decision processes and allocation took longer for ECD than for SCD organs in this setting. However, there was no significant difference in CIT between treatment groups in either SCD or ECD recipients (p = 0.1 and p = 0.4, respectively), and CIT was not identified as a risk factor for graft survival in multivariable Cox regression, which is consistent with data from a large registry based study [50].
Interestingly, the donor status was not a risk factor for rejection, and recipients of ECD kidneys did not suffer from more rejection compared with SCD. Recently, Diet et al. studied the recipient’s immunological risk and its potential effects on the outcome of kidney transplants from ECD and observed similar rejection rates in recipients of ECD and SCD kidneys after adjustment for the immunological risk [13]. Our findings underline the fact that the risk of rejection depends on the immunological risk, recipient’s age and immunosuppressive regimen rather than the donor status [17, 22–24, 31, 51–54]. Recipients of ECD kidneys should therefore be treated with an optimised immunosuppressive regimen, and allocation of ECD kidneys to young recipients should be avoided.
In our study, the pretransplant baseline eCrCl of ECD kidneys was 37% lower than that of SCD kidneys, and rejection had a significant negative impact on graft function in recipients of ECD kidneys. Therefore, we think that, particularly in kidneys with assumed reduced nephron mass such as ECD kidneys, the immunological risk should be kept as low as possible by accurate pretransplant risk assessment and risk-adjusted immunosuppression during the post-transplant period to avoid further damage.
Our study has some limitations. First, the sample size is modest and there is a risk of missing effects of smaller size. Given the higher pretransplant functional reserve of SCD compared with ECD kidneys, we expected a smaller impact of injuries on the outcome. This may partly explain why we missed an effect of the immunosuppressive regimen and rejection episodes on the outcome in our SCD population in contrast to the large population of the Symphony study [41]. Second, since our study is retrospective in nature we cannot properly establish relationships between cause and effect. Beside immunosuppression, influences of other factors in the management of renal transplant recipients such as blood pressure control or the use of statins cannot be excluded. Third, since the majority of the recipients with Tac-based immunosuppression received induction therapy with IL-2 receptor antagonists, we cannot properly separate the effect of each on graft survival in spite of a negative finding in the Cox regression model. Finally, since we have not analysed the histological findings of reperfusion biopsies we cannot evaluate their prognostic impact on graft outcome.
In summary, graft survival of ECD kidneys in patients without pretransplant HLA-DSA is excellent when treated with Tac-based immunosuppression and comparable to SCD kidneys during the first six years. Tac-based immunosuppression improves and preserves graft function of ECD kidneys in the mid-term. We conclude that kidney transplants with reduced baseline nephron mass, such as ECD kidneys, which are highly vulnerable to additional hits, should be allocated to patients with low immunological risk and preferably treated with Tac-based immunosuppression in order to achieve optimal graft survival and function.
Acknowledgment: The authors thank Thomas Voegele, head of transplant coordination of the University Hospital Basel, for his great support and assistance in data collection. Further, we owe a huge debt of gratitude our nursing team and the staff of our typing laboratory for their enthusiastic work and personal dedication for our patients.
1 Wynn JJ, Alexander CE. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transpl Int. 2011;24(4):324–32.
2 Wolfe RA, Merion RM, Roys EC, Port FK. Trends in organ donation and transplantation in the United States, 1998–2007. Am J Transplant. 2009;9(4 Pt 2):869–78.
3 Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12(3):589–97.
4 Port FK, Bragg-Gresham JL, Metzger RA, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74(9):1281–6.
5 Rosengard BR, Feng S, Alfrey EJ, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2(8):701–11.
6 OPTN/SRTR 2009 Annual Report, available at http://optn.transplant.hrsa.gov/ar2009, accessed on 27.5.2011
7 Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008;52(3):553–86.
8 Nyberg SL, Matas AJ, Kremers WK, et al. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant. 2003;3(6):715–21.
9 Anglicheau D, Loupy A, Lefaucheur C, et al. A simple clinico-histopathological composite scoring system is highly predictive of graft outcomes in marginal donors. Am J Transplant. 2008;8(11):2325–34.
10 Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–6.
11 Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5(4 Pt 1):757–65.
12 Nyberg SL, Baskin-Bey ES, Kremers W, Prieto M, Henry ML, Stegall MD. Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation. 2005;80(7):925–9.
13 Diet C, Audard V, Roudot-Thoraval F, Matignon M, Lang P, Grimbert P. Immunological risk in recipients of kidney transplants from extended criteria donors. Nephrol Dial Transplant. 2010;25(8):2745–53.
14 Domagala P, Kwiatkowski A, Perkowska-Ptasinska A, et al. Assessment of kidneys procured from expanded criteria donors before transplantation. Transplant Proc. 2009;41(8):2966–9.
15 Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354(4):343–52.
16 Gallego Valcarce E, Ortega Cerrato A, Llamas Fuentes F, et al. Short cold ischaemia time optimises transplant results for kidneys from expanded criteria donors. Nefrologia. 2009;29(5):456–63.
17 Galeano C, Marcen R, Jimenez S, et al. Utilization of elderly kidney donors (>70 years) does not affect graft survival in the medium term. Transplant Proc. 2010;42(10):3935–7.
18 Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–25.
19 Collini A, De Bartolomeis C, Ruggieri G, Barni R, Bernini M, Carmellini M. Long-term outcome of renal transplantation from marginal donors. Transplant Proc. 2006;38(10):3398–9.
20 Goldberg RJ, Smits G, Wiseman AC. Long-term impact of donor-recipient size mismatching in deceased donor kidney transplantation and in expanded criteria donor recipients. Transplantation. 2010;90(8):867–74.
21 Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346(8):580–90.
22 Stratta RJ, Rohr MS, Sundberg AK, et al. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Ann Surg. 2004;239(5):688–95; discussion 695-687.
23 Chavalitdhamrong D, Gill J, Takemoto S, et al. Patient and graft outcomes from deceased kidney donors age 70 years and older: an analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database. Transplantation. 2008;85(11):1573–9.
24 Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010;252(4):662–74.
25 Amico P, Honger G, Steiger J, Schaub S. Utility of the virtual crossmatch in solid organ transplantation. Curr Opin Organ Transplant. 2009;14(6):656–61.
26 Dunn TB, Noreen H, Gillingham K, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011;11(10):2132-2143.
27 Laperrousaz S, Tiercy S, Villard J, Ferrari-Lacraz S. HLA and non-HLA polymorphisms in renal transplantation. Swiss Med Wkly. 2012;142:w13668.
28 Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8):1398–406.
29 Amico P, Hirt-Minkowski P, Honger G, et al. Risk stratification by the virtual crossmatch: a prospective study in 233 renal transplantations. Transpl Int. 2011.
30 Amico P, Honger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87(11):1681–8.
31 Patel AM, Pancoska C, Mulgaonkar S, Weng FL. Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant. 2007;7(10):2371–7.
32 Otten HG, Verhaar MC, Borst HP, Hene RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2012;12(6):1618–23.
33 Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
34 Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464–71.
35 Hirt-Minkowski P, Amico P, Honger G, et al. Delayed graft function is not associated with an increased incidence of renal allograft rejection. Clin Transplant. 2012;26(6):E624–633.
36 Stallone G, Infante B, Gesualdo L. Older donors and older recipients in kidney transplantation. Journal of nephrology. 2010;23(Suppl 15):S98–103.
37 Segoloni GP, Messina M, Squiccimarro G, et al. Preferential allocation of marginal kidney allografts to elderly recipients combined with modified immunosuppression gives good results. Transplantation. 2005;80(7):953–8.
38 Srinivas TR, Schold JD, Guerra G, Eagan A, Bucci CM, Meier-Kriesche HU. Mycophenolate mofetil/sirolimus compared to other common immunosuppressive regimens in kidney transplantation. Am J Transplant. 2007;7(3):586–94.
39 Gonwa T, Johnson C, Ahsan N, et al. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 2003;75(12):2048–53.
40 Mucha K, Foroncewicz B, Paczek L, et al. 36-month follow-up of 75 renal allograft recipients treated with steroids, tacrolimus, and azathioprine or mycophenolate mofetil. Transplant Proc. 2003;35(6):2176–8.
41 Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–75.
42 Wlodarczyk Z, Walaszewski J, Perner F, et al. Freedom from rejection and stable kidney function are excellent criteria for steroid withdrawal in tacrolimus-treated kidney transplant recipients. Ann Transplant. 2002;7(3):28–31.
43 Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9(8):1876–85.
44 Lee JP, Heo NJ, Joo KW, et al. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25(8):2772–85.
45 de Fijter JW. The impact of age on rejection in kidney transplantation. Drugs & aging. 2005;22(5):433–49.
46 Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33.
47 Chapman JR. Chronic calcineurin inhibitor nephrotoxicity-lest we forget. Am J Transplant. 2011;11(4):693–7.
48 Pankewycz O, Leca N, Kohli R, et al. Conversion to low-dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy-guided study. Transplant Proc. 2011;43(2):519–23.
49 Stegall MD, Park WD, Larson TS, et al. The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant. 2011;11(4):698–707.
50 Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11(12):2647–56.
51 Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC. Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: are they relevant? Transplantation. 2008;85(8):1200–4.
52 van den Berg-Loonen EM, Billen EV, Voorter CE, et al. Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation. 2008;85(8):1086–90.
53 Bielmann D, Honger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7(3):626–32.
54 Heldal K, Thorarinsdottir S, Hartmann A, et al. Induction with interleukin-2 antagonist for transplantation of kidneys from older deceased donors: an observational study. Transplantation research. 2013;2(1):11.
Funding / potential competing interests: The study was supported by a project fund from Amgen AG Switzerland. The sponsor did not influence either study design, collection, analysis and interpretation of data, writing of the report or the decision to submit the report for publication. The authors of this manuscript have no conflicts of interest to disclose as described by Swiss Medical Weekly.
Authors’ contribution: Claudia Praehauser and Patricia Hirt-Minkowski contributed equally.