Expression of somatostatin receptors, angiogenesis and proliferation markers in pituitary adenomas: an immunohistochemical study with diagnostic and therapeutic implications
DOI: https://doi.org/10.4414/smw.2013.13895
Alejandra
Magagna-Poveda, Henning
Leske, Christoph
Schmid, René
Bernays, Elisabeth
Rushing
Summary
PRINCIPLES: Pituitary adenomas are common intracranial neoplasms that generate symptoms as a result of either mass effect or the increased production of pituitary hormones. Although mostly benign, these tumours can be associated with considerable morbidity. We investigated a panel of immunohistochemical preparations to identify potential therapeutic targets and surrogate markers of clinical outcome.
METHODS: Tumour tissue from 25 patients was evaluated for immunohistochemical expression of somatostatin receptors 1‒5, von Willebrand-factor (vWF), interleukin-8 (IL-8), vascular endothelial growth factor receptor 2 (VEGFR-2), kinesin spindle protein (Eg5) and MIB-1 (Ki-67), and its relationship with clinical features was analysed.
RESULTS: The proliferation marker MIB-1 (Ki-67) was the only marker predictive of adenoma recurrence. Of note, 67% of all relapses were associated with tumours showing luteinising hormone expression. All pituitary adenomas showed variable somatostatin receptor, IL-8, Eg5, vWF and VEGFR-2 expression; a relationship between these parameters and clinical outcome could not be demonstrated in this cohort.
CONCLUSIONS: This study validates MIB-1 (Ki-67) as a reliable marker of tumour recurrence in pituitary adenomas. Considering the consistently increased expression of Eg5, IL-8, VEGFR-2, somatostatin receptors and vWF in these tumours, further investigation as potential therapeutic targets is warranted.
Introduction
Pituitary adenomas represent common, almost always benign intracranial neoplasms that are primarily found in adults during the 3rd‒6th decades [1]. Although pituitary adenomas have been studied extensively, the relationship between histopathological features and clinical outcome remains imprecise [1–5]. Even benign tumours may recur and cause significant morbidity and, rarely, mortality. Atypical adenomas show a greater degree of invasion of surrounding structures and higher recurrence rates. These tumours, comprising about 15% of adenomas, possess increased proliferative activity characterised by more than occasional mitoses, MIB-1 (Ki-67) labelling exceeding 3%, and significant p53 immunoreactivity [2–5, 43]. Although most carcinomas show elevated mitoses, their morphological features are not always overtly malignant [1–5]. Thus, there is a critical need for new therapies for aggressive tumours and biomarkers that reliably predict the biological behaviour of pituitary adenomas.
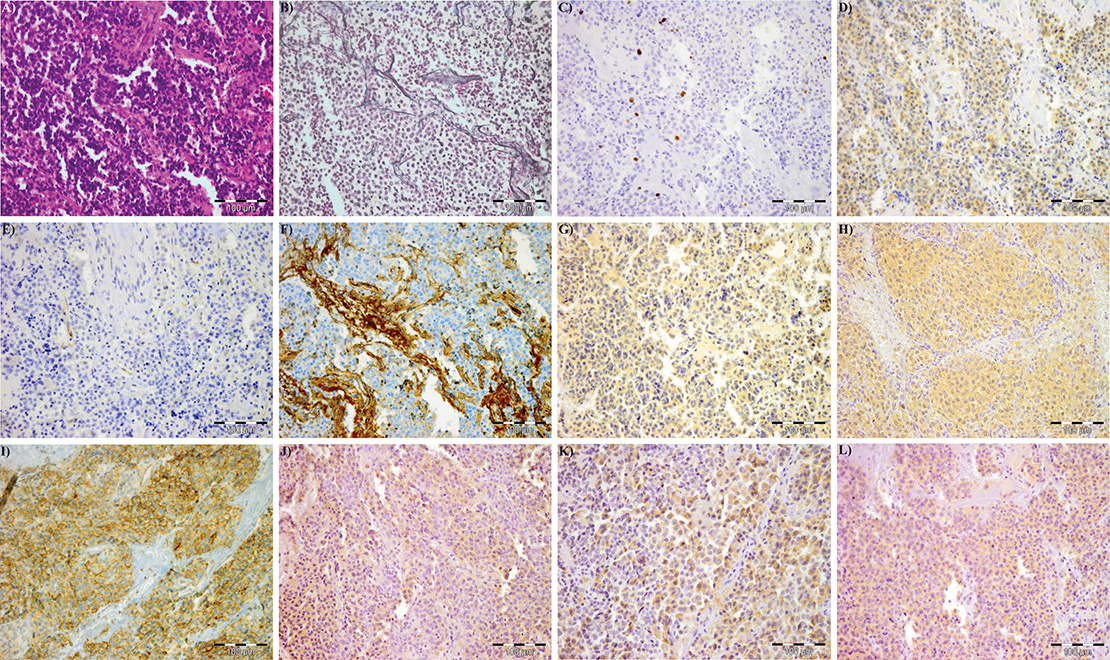
Figure 1
Immunohistochemical expression of the different markers‘ expression in a pituitary adenoma: (A) hematoxilin-eosin, (B) reticulin, (C) MIB-1 (Ki-67), (D) Eg5, (E) VEGFR-2, (F) vWF, (G) IL-8, (H) SSTR-1, (I) SSTR-2, (J) SSTR-3, (K) SSTR-4, (L) SSTR-5.
Eg5 = kinesin spindle protein; IL-8 = interleukin-8; SSTR = somatostatin receptor; VEGFR-2 = vascular endothelial growth factor receptor-2; vWF = von Willebrand-factor
The optimal treatment of pituitary adenomas encompasses invasive and noninvasive options and depends on multiple factors, including size, hormone production and the degree of invasion into surrounding structures. Minimally invasive, endoscopic transsphenoidal surgery is now firmly established as the gold-standard of treatment. Although surgery remains the first-line choice, invasive and/or refractory pituitary adenomas often present therapeutic challenges. For example, dopamine agonists have proven effective in prolactinomas, and most of these tumours can be managed exclusively with conservative therapy. Somatostatin (somatotrophin release-inhibiting factor, SRIF) is a polypeptide hormone highly expressed in corticotroph adenomas [7, 23]. According to their affinity profile for somatostatin analogues (SSAs), there are two subfamilies of somatostatin receptors (SSTRs), which translate into specific pharmacological properties: (1.) SRIF-1, comprising SSTR-2, SSTR-3 and SSTR-5, and (2.) SRIF-2, including SSTR-1 and SSTR-4 [7–9]. Octreotide and lanreotide bind with a high affinity to SSTR-2, moderate affinity to SSTR-3 and SSTR-5, and have low or absent affinity to SSTR-1 and SSTR-4 [7, 10]. Currently, SSAs are the mainstay of medical therapy for somatotroph adenomas, although they lack efficacy in patients with certain genetic forms of the disease [42]. Furthermore, although incompletely understood, data regarding resistance and the underlying mechanisms of certain pituitary adenomas are accruing[42]. SOM230 (pasireotide), a new multiligand SSA that binds to all SSTRs except SSTR-4, has been investigated in clinical trials for pituitary adenomas [11, 12, 15, 16] and is now registered for treatment of patients with Cushing’s disease in most European countries. DG3173 is a heptapeptide SSA with a novel amino acid sequence and unique cyclic backbone. In addition to its affinity to SSTR-2 and SSTR-5, it is the only SSA that binds to SSTR-4 [13]. Preliminary studies with SSAs support further investigation as treatment for relapsed/refractory pituitary adenomas [7, 14].
Inhibition of angiogenesis has shown at least partial efficacy in a variety of cancers and has prompted interest as an alternative therapy in aggressive pituitary tumours. The first case of bevacizumab-treated pituitary carcinoma with long-term control was recently reported, providing a possible new option in the management of aggressive pituitary tumours [17]. Another novel antineoplastic strategy is the targeted destruction of cells undergoing mitosis with kinesin spindle protein (KSP) inhibitors. In a recent study, patients with relapsed/refractory solid tumours and lymphoma were enrolled in a Phase I trial with AZD4877 (Eg5), a KSP inhibitor. The agent was generally well-tolerated and showed therapeutic promise [24], thus providing a potential non-classical approach to chemotherapy. The aims of this study were to investigate SSTR, proliferation and angiogenesis markers to better characterise their expression in different subtypes of pituitary adenoma and to ascertain their potential clinical and biological role.
Materials and methods
Patients
We conducted a retrospective study of pituitary adenomas in 25 patients treated and followed up at the University Hospital of Zurich between 1994 and 2011. Medical records were reviewed to extract patient characteristics (gender, age at initial diagnosis, symptoms at presentation, hormone production clinically, tumour size, type and nature of therapy). For the purposes of this study, recurrence was defined as the emergence of a new radiologically defined lesion, with the date of recurrence corresponding to the date of the radiological study. Information on the extent of resection was obtained from the neurosurgical and imaging records. In patients with subtotal surgical resection, recurrence was defined as radiological evidence of growth (increase in size) of the residual lesion.

Figure 2
Kaplan-Meier curve showing age and recurrence free survival.
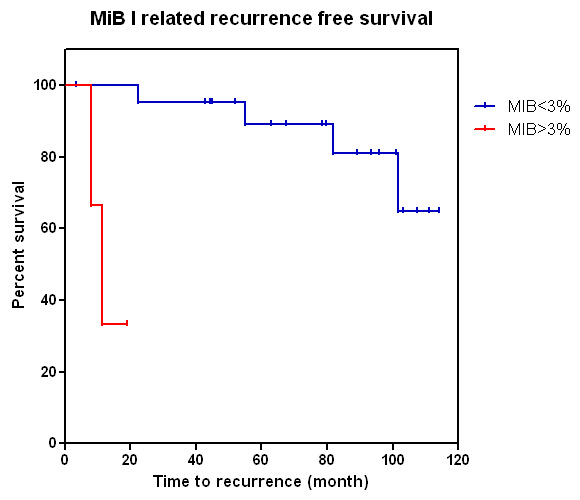
Figure 3
Kaplan-Meier curve showing MIB-1 (Ki-67) proliferation index and time to recurrence: cases with higher MIB-1 proliferation index are more likely to recur (p <0.0001).
Endoscopic transsphenoidal surgery was performed as the first-line treatment, sometimes followed by medical therapy or combined radiation-medical therapy. In all cases, efforts were made to resect as much tumour tissue as possible, while preserving the function of the surrounding normal pituitary gland. The study design complied with the criteria established by the local ethics committee.
Tumour samples
All pathology materials, consisting in most cases of haematoxylin-eosin and reticulin-stained sections, anterior pituitary immunohistochemistry and the MIB-1 (Ki-67) proliferation marker, were reviewed to confirm the diagnosis and to select appropriate blocks for additional immunohistochemical studies. Only cases with sufficient tissue remaining in the paraffin block were selected. Pituitary adenoma subtypes were classified in accordance with World Health Organisation (WHO) 2004 criteria on the basis of their hormone expression profile (immunophenotype) [18]. Additional clinical classification is shown in table 1 [41].
Immunohistochemistry
Anti-SSTR-1, -3, -4 and -5 antibodies were obtained from Novartis Pharma AG (Basel, Switzerland). Other antibodies used included anti-SSTR-2 (Gramsch Lab, 1:300), MIB-1 (KI-67, VentanaRoche, prediluted) and von Willebrand-factor (vWF, Dako, 1:1000). Antibodies against Eg5, interleukin-8 (IL-8) and vascular endothelial growth factor receptor-2 (VEGFR-2) were purchased from Abcam (Cambridge, UK, 1:100), R&D Systems Europe Ltd. (Abingdon, UK, 1:10) and Cell Signaling (BioConcept, Allschwil, Switzerland, 1:400), respectively. A Leica Bond-Max automated immunostainer employing 4 μm-thick, formalin fixed, paraffin embedded sections was used, selecting a single representative block from each tumour. Immunohistochemistry conditions were optimised for each antibody using manufacturers’ recommendations (fig. 1). Appropriate positive and negative controls were used as recommended by the manufacturer.
Immunohistochemical evaluation
For screening of antibodies, the staining results were scored for immunolabelling using a semiquantitative scale. Specific criteria for evaluating each antibody are detailed below.
SSTR-1, SSTR-2, SSTR-3, SSTR-4, SSTR-5, Eg5, IL-8
An immunoreactivity score (IRS) was recorded for all sections, noting the intensity of the colour as well as the percentage of cells showing a positive cytoplasmic or membranous staining. The percentage of positive cells was calculated as follows: no positive cells (0); <10% of positive cells (1); 10%–50% of positive cells (2); 51%–80% of positive cells (3); >80% of positive cells (4); and the intensity of staining: no staining (0); mild staining (1); moderate staining (2); strong staining (3). The overall IRS was calculated as [percentage of positive cells] x [intensity of staining], and classified as negative (“-“; IRS 0 and 1), weak expression (positive “+”; IRS 2 and 3), moderate expression (double positive “++”; IRS 4 to 8) or strong expression (triple positive “+++”; IRS 9 to 12) [7, 19].
VEGFR-2 and vWF
VEGFR-2 and vWF were found only in vessel endothelial cells. Immunostaining intensity (vessel density) was assessed with a modified IRS [7, 19], scoring the number of positive vessels instead: no immunoreactivity (0), <10% positive vessels (1), 10%–50% positive vessels (2); 51%–80% positive vessels (3); >80% positive vessels (4).
MIB-1 (Ki-67)
The proportion of immunoreactive cells for the nuclear antibody MIB-1 (Ki-67) was determined by counting the number of positive cells out of 500 tumour cells in the region of maximum staining.
Statistical analysis
Difference in the rate of recurrence between groups defined by receptor expression (e.g. SSTR-1) was tested using the log-rank (Mantel-Cox) test. For expression of each receptor type, the mean-value of the expression-level (classified by the observer) per group was calculated. The results are summarised in fig. 5. All calculations were performed using Graph Pad Prism 5. A p-value <0.05 was considered of statistical significance.
Results
Patient characteristics
The study comprised 9 females and 16 males with a mean age of 55 years at presentation (range 23–77). The presenting complaints are listed in table 1. The mean duration of follow-up was 67.7 months (range 3.47–114.13). All patients were treated with either surgery alone (n = 18, 72%), surgery and medical therapy (n = 5, 20%) or surgery and radiotherapy (n = 2, 8%). Patients with prolactinomas received dopamine agonists as medical therapy, including a single patient who received it prior to surgery, and the patients with growth hormone (GH) producing adenomas received somatostatin analogues. One patient died of oesophageal carcinoma. Of the remaining patients (n = 24), 4 men and 2 women (24%) underwent a second operation owing to recurrence. During follow-up, 52% of patients (n = 13) were tumour free after treatment, whereas 48% (n = 12) showed a stationary residual lesion on imaging.
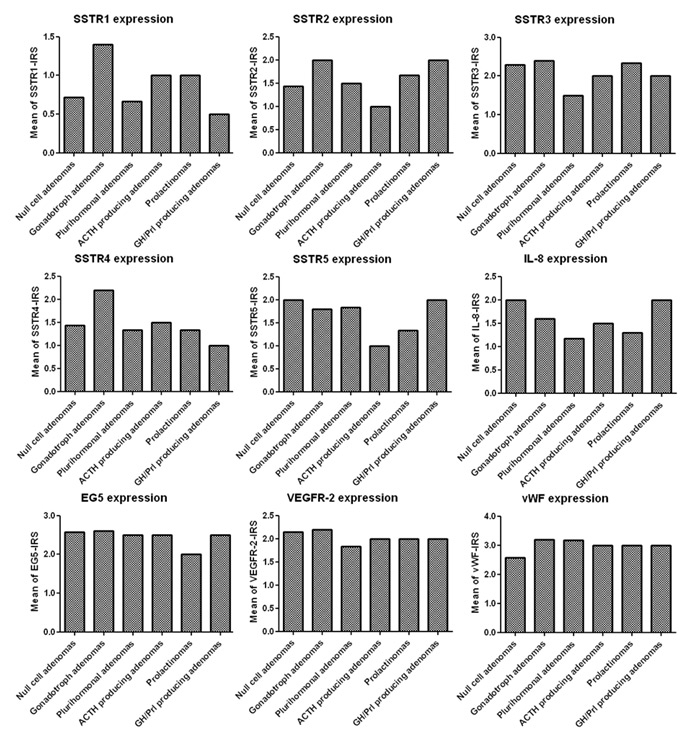
Figure 4
SSTR-, IL8-, Eg5-, VEGFR-2- and vWF-expression of the different pituitary adenoma subtypes. Note that the y-axes represent the mean immunoreactivity score.
ACTH = adrenocorticotrophic hormone; Eg5 = kinesin spindle protein; GH = growth hormone; IL-8 = interleukin-8; Prl = prolactin; SSTR = somatostatin receptor; VEGFR-2 = vascular endothelial growth facto receptorr-2; vWF = von Willebrand-factor
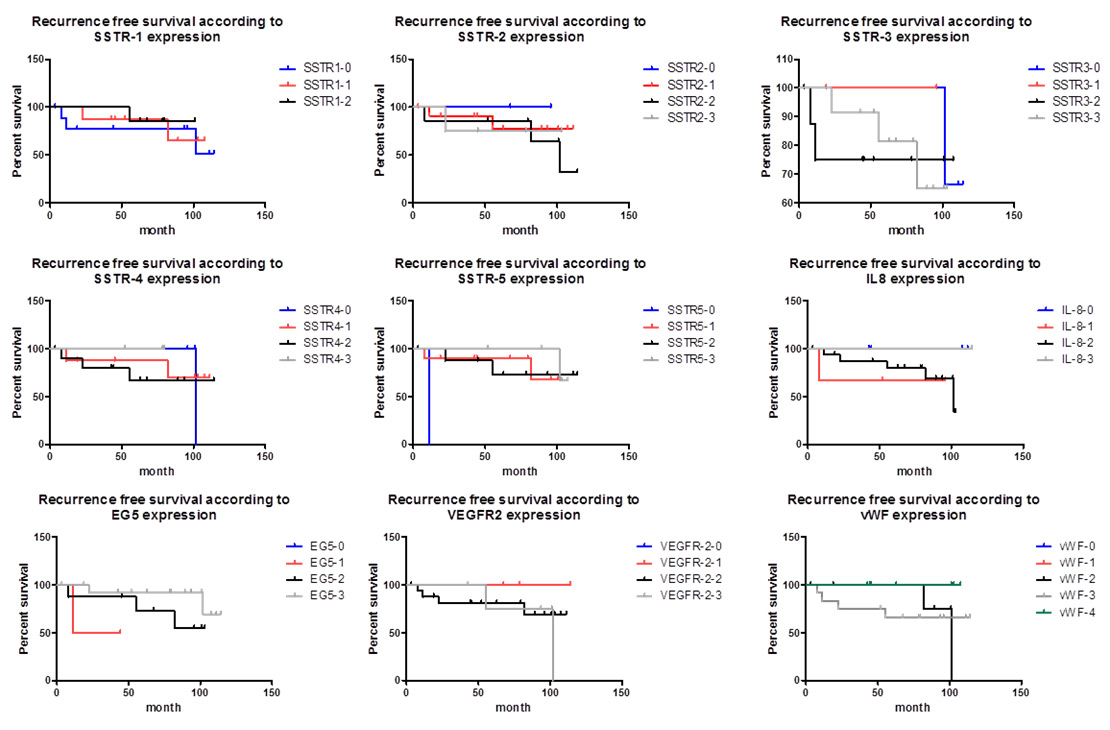
Figure 5
Kaplan-Meier curve showing the different markers and recurrence-free survival.
Eg5 = kinesin spindle protein; IL-8 = interleukin-8; SSTR = somatostatin receptor; VEGFR-2 = vascular endothelial growth factor receptor-2; vWF = von Willebrand-factor
There was no significant gender difference (p = 0.836) or relationship with patient age (p = 0.268) and recurrence rate (fig. 2) regarding the likelihood of recurrence.
Pathological features
Tumours ranged in size from 0.5 to 7 cm in greatest single dimension, with a mean size of 1.6 cm. There was no significant difference in mean size between females (2.14 cm) and males (1.20 cm). A variety of nonspecific histopathological growth patterns were seen. Mitotic activity was not significantly elevated. PAs were classified according to WHO 2004 classification [18] as detailed in table 1. Recurrent disease was shown in 24% (6/25) of cases, including one each of prolactinoma, adrenocorticotrophic hormone (ACTH) adenoma, mixed GH/prolactin cell adenoma, gonadotroph adenoma and two null cell adenomas.
Relationship between marker expression and recurrence
There was substantial variation in MIB-1 labelling among the different tumours and also within the same tumour. In the majority of cases (n = 22, 88%), the MIB-1-labelling index was <3%; the proliferation index exceeded 3% in only 12% of cases (n = 3), with one case in each of the following groups: null cell adenoma, ACTH adenoma and mixed GH/prolactin cell adenoma. Of note, cases with an increased proliferation index were more likely to recur during the follow-up period (p <0.0001, fig. 3). In the highly proliferating tumours, 2 of 3 adenomas recurred within the first year after the initial operation: the ACTH adenoma and mixed GH/prolactin cell adenoma. After two surgeries and postoperative radiotherapy, the latter example showed a stationary residual lesion during follow-up. There was no significant difference in outcome between patients who received standard therapy or standard plus adjuvant therapy (medical therapy or radiotherapy). Variation in SSTR expression was seen in the different pituitary adenoma subtypes (fig. 4); however, as shown in fig. 5, SSTR expression (SSTR-1, p = 0.409; SSTR-2, p = 0.958; SSTR-3, p = 0.852; SSTR-4, p = 0.246; SSTR-5, p = 0.912) and expression of IL-8 (p = 0.741), vVW (p = 0.937) and VEGFR-2 (p = 0.197) failed to correlate with recurrence. Eg5 was not superior to MIB-1-labelling index with respect to predicting recurrence-free survival (p = 0.733, fig. 4).
Hormone expression
Overall, 24% (n = 6) of all tumour cases recurred. Interestingly, there was a tendency for recurrent tumours to express luteinising hormone (LH). A total of 67% of the recurrent tumours were immunoreactive for LH, with 50% showing new positive LH expression or persistent LH expression in contrast to 2 LH-negative relapses. LH-negative recurrent tumours were either pure ACTH- or GH-positive adenomas.
|
Table 1: Patient characteristics. |
|
Patient characteristics (n = 25)
|
|
Gender
|
|
Histological adenoma subtypes
|
|
| Males |
16 |
ACTH cell adenomas |
2 (8%) |
| Females |
9 |
Prolactinomas |
3 (12%) |
|
Age (years)
|
|
Mixed bicellular GH/prolactin cell adenomas |
2 (8%) |
| Range |
23–77 |
Gonadotroph adenomas |
6 (24%) |
| Mean |
55 |
Plurihormonal adenomas |
7 (28%) |
| Males |
62 |
Null cell adenomas |
5 (20%) |
| Females |
44 |
Clinical adenoma subtypes
|
|
|
Symptoms at presentation
|
|
Functioning adenomas |
8 (32%) |
| Headache |
3 (12%) |
PRL-secreting adenomas |
3 (12%) |
| Acromegaly |
3 (12%) |
GH-secreting adenomas |
2 (8%) |
| Visual disturbances |
9 (36%) |
Gonadotropin secreting adenomas |
3 (12%) |
| Amenorrhoea, galactorrhoea |
1 (4%) |
Non-functioning adenomas |
12 (48%) |
| Loss of libido |
3 (12%) |
Silent adenomas |
5 (20%) |
| Tiredness |
2 (8%) |
Treatment
|
|
| Dizziness |
3 (12%) |
Surgery alone |
18 (72%) |
| Gynecomastia |
1 (4%) |
Surgery + postoperative medical therapy |
4 (16%) |
|
Size (cm)
|
|
Preoperative medical therapy + surgery |
1 (4%) |
| Range |
0.5–7 |
Surgery + radiotherapy |
2 (8%) |
| Mean |
1.6 |
|
|
| ACTH = adrenocorticotrophic hormone; GH = growth hormone; PRL = prolactin |
Discussion
Recent advances in understanding the molecular pathomechanisms of pituitary adenomas have proven useful for developing novel therapeutic approaches. The major signalling pathways affected by SSAs have been characterised providing additional potential targets for therapeutic intervention in refractory cases. In fact, therapy with SSAs is now considered the most important adjunct to surgery and potential combination therapy with other medical approaches [42]. In the present study, we investigated the expression of SSTR, proliferation and angiogenesis markers to ascertain their potential clinical and biological role in different subtypes of pituitary adenoma. Not surprisingly, pituitary adenomas generally showed proliferating indices below 3%, with a higher labelling index considered a defining feature of atypical adenomas [18]. Confirming previous studies, we found that the MIB-1 proliferation index was related with recurrence (p <0.0001). Among the highly proliferative tumours, recurrences were noted in 2 of 3 adenomas within the first year after the initial surgery.
SSTR-expression appeared highly variable within and among different tumour subtypes. Ramírez et al. [20] found significant levels of SSTR-2 and SSTR-5 expression in null cell adenomas. In our series, variable SSTR-expression was seen in the different PA subtypes (see fig. 5). Similar results were observed by Plöckinger et al. in GH- and GH/prolactin-secreting adenomas [7].
With the exception of the MIB-1 proliferation index (see fig. 3), none of the markers analysed were predictive of relapse. Interestingly, a tendency was seen for recurrent tumours to express LH. A total of 67% of recurrent tumours were immunoreactive for LH, with 50% showing new LH positivity or persistent LH expression in contrast to 2 LH-negative relapses. As a rule, these tumours present as macroadenomas and incomplete tumour removal may explain the relapses.
Eg5 is a microtubule-dependent motor protein (KSP) encoded by the KIF11 gene located at 10q24.1, with a primary function in mitotic spindle formation. Liu et al. [21] suggested a role for Eg5 in pancreatic tumourigenesis, with adenosine triphosphatase- (ATPase-) dependent up-regulation and enhanced cell proliferation seen in pancreatic cancer cell lines. In our study, we were unable to demonstrate a relationship between Eg5 expression and recurrence (see fig. 4, p = 0.1145). More specifically, the 3 cases with greater than 3% MIB-1 index (atypical adenomas) showed no significant higher Eg5 expression compared with typical adenomas.
Angiogenesis, an interactive process between tumour and host cells, is required for tumour growth, progression and metastasis [21, 25–27]. VEGF employs primarily VEGFR-2, a receptor tyrosine kinase, to induce vascular endothelial survival, proliferation and vessel permeability [28, 29]. In 1999, Lloyd et al. reported lower VEGF immunostaining in normal pituitary glands compared with adenomas, with even greater expression in pituitary carcinomas [30]. Subsequently, VEGFR-2 messenger ribonucleic acid (mRNA) and protein over-expression was identified in a series of adenomas, with highest expression in nonfunctioning adenomas and GH-producing adenomas [31]. However, no difference was observed between VEGF expression and its receptors and tumour progression parameters, such as grade, proliferative index and vessel density [22]. More recently, Western blot analysis of 56 pituitary adenomas revealed that VEGF protein expression was higher in prolactinomas compared with nonfunctioning, GH- and ACTH-secreting adenomas [32]. Other studies have observed absent or reduced immunostaining of vascular VEGFR-2 in pituitary adenomas [33–36]. Nevertheless, McCabe et al. reported a strong over-expression of VEGFR-2 mRNA (average 14-fold, maximum 233-fold) in more than 100 human pituitary adenomas using quantitative polymerase chain reaction (PCR) [31]. In the same study, enhanced VEGFR-2 protein expression was also shown (by Western blotting) in several adenomas investigated. Only oestrogen-induced prolactinomas in rats exhibited over-expression of both VEGFR-2 and neuropilin-1 (NRP-1) [37, 38]. In our study, endothelial VEGFR-2 expression was expressed both in normal and pituitary adenomas tissue. In contrast, Kuczynski et al. [39] found low or undetectable VEGFR-2 in normal tissue and the proportion of VEGFR2-positive vessels increased with tumour grade. The survival rate of patients in one series correlated with higher levels of IL-8 and VEGF [40]. We did not observe any relationship between IL-8 and VEGFR-2 expression (see fig. 3), indicating the expression of these angiogenic factors is likely to be independent. The contradictory findings regarding VEGFR-2 expression in human pituitary adenomas need to be clarified in future studies.
Although we failed to observe a relationship between different SSTR subtypes, angiogenesis and proliferation markers (except MIB-1 [Ki-67]) and disease progression, there are several factors that may have interfered with the interpretation of the data. As noted, the overall number of tumours is rather low, and the tumour cohort consists of different adenoma subtypes. These different pituitary adenoma subtypes are likely to possess differences in biological behaviour, which failed to emerge in the limited cases available for study.
In conclusion, our study confirms previous observations that the MIB-1 (Ki-67) labelling index represents a useful prognostic marker, superior to Eg5, in patients with pituitary adenomas. In addition, all adenomas analysed showed expression of SSTR, Eg5, VEGFR-2, IL-8 and vWF, which supports further investigation of these markers as targets for non-invasive treatment. Although other small studies have been published, additional large-scale studies need to be performed to better understand the different roles of the SSTR in pituitary adenomas and further investigate their role as candidates for therapeutic targeting.
References
1 Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, QuabbeHJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor. Registry. Eur J Endocrinol. 2007;156:203–16.
2 Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. Neurosurgery. 2011;114:336–44.
3 Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery. 2006;59:341–53.
4 Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Ki-67 in pituitary neoplasms: a review – part I. Neurosurgery. 2009;65:429–37.
5 Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Biomarkers of pituitary neoplasms: a review (Part II). Neurosurgery. 2010;67:1790–8.
6 Horvath E, Kovacs K, Singer W, Smyth HS, Killinger DW, Erzin C, et al. Acidophil stem cell adenoma of the human pituitary: clinicopathologic analysis of 15 cases. Cancer. 1981;47:761–71.
7 Plöckinger U, Hoffmann U, Geese M, Lupp A, Buchfelder M, Flitsch J, et al. DG3173 (Somatoprim), a unique Somatostatin receptor subtype 2-, 4- and 5-selective analogue, effectively reduces GH-secretion in human growth hormone secreting pituitary adenomas even in Octreotide non-responsive tumours. Eur J Endocrinol. 2011;166:223–34.
8 Duran-Prado M, Malagon MM, Gracia-Navarro F, Castano JP. Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functionning. Mol Cell Endocrinol. 2008;286:169–79.
9 Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–91.
10 Poll F, Lehmann D, Illing S, Ginj M, Jacobs S, Lupp A, et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24:436–46.
11 Petersenn S, Schopohl J, Barkan A, Mohideen P, Colao A, Abs R, et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010;95:2781–9.
12 Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74.
13 Afargan M, Janson ET, Gelerman G, Rosenfeld R, Ziv O, Karpov O, et al. Novel long-acting somatostatin analog with endocrine selectivity: potent suppression of growth hormone but not of insulin. Endocrinology. 2001;142:477–86.
14 Culler MD. Somatostatin-Dopamine Chimeras: A Novel Approach to Treatment of Neuroendocrine Tumors. Horm Metab Res. 2011;43:854–7.
15 Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366(10):914–24.
16 Ben-Shlomo A, Melmed S. Pasireotide – a somatostatin analog for the potential treatment of acromegaly, neuroendocrine tumors and Cushing’s disease. IDrugs. 2007;10:885–95.
17 Ortiz LD, Syro LV, Scheithauer BW, Ersen A, Uribe H, Fadul CE, et al. Anti-VEGF therapy in pituitary carcinoma. Pituitary. 2012;15(3):445–9.
18 Lloyd RV, Kovacs K, Young Jr WF, et al. Pituitary tumours: introduction. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C (eds) World Health Organization Classification of Tumours: Pathology and Genetics – Tumours of Endocrine Organs. Lyon: IARC Press; 2004.
19 Remmele W, Stegner H Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40.
20 Ramírez C, Cheng S, Vargas G, Asa SL, Ezzat S, González B, et al. Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J Clin Endocrinol Metab. 2012;97(5):1745–51.
21 Liu M, Wang X, Yang Y, Li D, Ren H, Zhu Q, et al. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol. 2010;221:221–8.
22 Onofri C, Theodoropoulou M, Losa M, Uhl E, Lange M, Arzt E, et al. Localization of vascular endothelial growth factor (VEGF) receptors in normal and adenomatous pituitaries: detection of a non-endothelial function of VEGF in pituitary tumours. J Endocrinol. 2006;191:249–61.
23 Ben-Shlomo A, Melmed S. Somatostatin agonists for treatment of acromegaly. Mol cell Endocrinol. 2008;286:192–8.
24 Gerecitano JF, Stephenson JJ, Lewis NL, Osmukhina A, Li J, Wu K, et al. A Phase I trial of the kinesin spindle protein (Eg5) inhibitor AZD4877 in patients with solid and lymphoid malignancies. Invest New Drugs.2012;doi: 10.1007/s10637-012-9821-y.
25 Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61.
26 Weidner N, Folkman J, Pozza F et al. Tumor angiogenesis: a new significant and independent prognostic indicator early stage breast cancer. J Natl Cancer Inst. 1992;84:1875–87.
27 Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–8.
28 Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14.
29 Olsson AK, Dimberg A, Kreuger J, Claesson- Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71.
30 Lloyd Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. Vascular Endothelial Growth Factor (VEGF) Expression in Human Pituitary Adenomas and Carcinomas. Endocr Pathol. 1999;10:229–35.
31 McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, et al. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumours. J Clin Endocr Metab. 2002;87:4238–44.
32 Christina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, et al. VEGF and CD31 association in pituitary adenomas. Endocr Pathol. 2010;21:154–60.
33 Gorczyca W, Hardy J. Microadenomas of the human pituitary and their vascularization. Neurosurgery. 1988;22:1–6.
34 Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocr Metab. 2000;85:1159–62.
35 Vidal S, Kovacs K, Horvath E, Scheithauer BW, Kuroki T, Lloyd RV. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 2001;438:595–602.
36 De la Torre NG, Turner HE, Wass JA. Angiogenesis in prolactinomas: regulation and relationship with tumour behaviour. Pituitary. 2005;8:17–23.
37 Banerjee SK, Sarkar DK, Weston AP, De A, Campbell DR. Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumours may mediate estrogen-initiated tumour angiogenesis. Carcinogenesis. 1997;18:1155–61.
38 Banerjee SK, Zoubine MN, Tran TM, Weston AP, Campbell DR. Over-expression of vascular endothelial growth factor164 and its co-receptor neuropilin-1 in estrogen-induced rat pituitary tumours and GH3 rat pituitary tumour cells. Int J Oncol. 2000;16:253–60.
39 Kuczynski EA, Patten SG, Coomber BL. VEGFR2 expression and TGF-β signaling in initial and recurrent high-grade human glioma. Oncology. 2011;81(2):126–34.
40 Kitadai Y, Haruma K, Sumii K et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100.
41 Mete O, Asa SL. Clinicopathological correlations in pituitary adenomas. Brain Pathol. 2012;22(4):443–53.
42 Gadelha MR, Kasuki L, Korbonits M. Novel pathway for somatostatin analogs in patients with acromegaly. Trends Endocrinol Metab. 2013;24(5):238–46.
43 Thoma CR, Toso A, Meraldi P, Krek W. Mechanisms of aneuploidy and its suppression by tumour suppressor proteins. Swiss Med Wkly. 2011;141:w13170.




