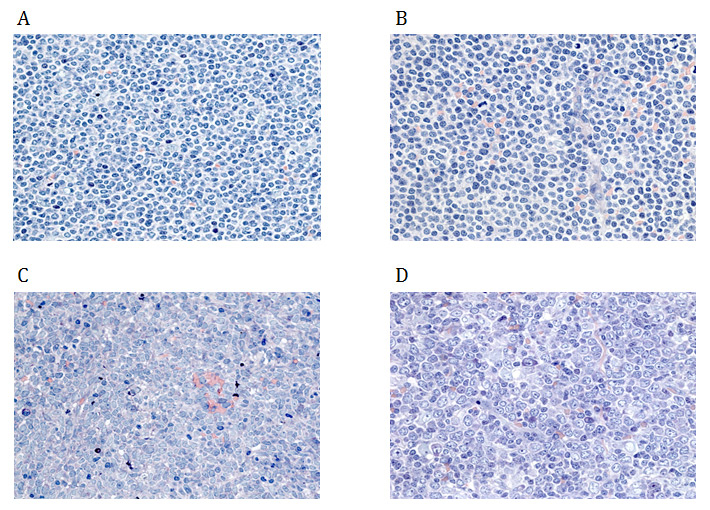
Figure 1
Cytomorphologic subtypes of mantle cell lymphoma: (A) classical, (B) small cell variant, (C) blastoid variant, (D) pleomorphic variant.
DOI: https://doi.org/10.4414/smw.2013.13868
Abbreviations
Ara-C: cytarabine, cytosine arabinoside
ASCT: autologous stem cell transplantation
BTK: Bruton’s tyrosine kinase
CDK: cyclin-dependent kinase
CLL: chronic lymphocytic leukaemia
CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone
CNS: central nervous system
CR: complete response
CRu: unconfirmed complete response
CT: computed tomography
DLBCL: diffuse large B-cell lymphoma
ECOG: Eastern Cooperative Oncology Group
EFS: event-free survival
ESMO: European Society for Medical Oncology
FDG-PET: 18F-fluorodeoxyglucose positron emission tomography
FL: follicular lymphoma
FL I: follicular lymphoma Grade I
GELA: Groupe d'Etude des Lymphomes de l'Adulte
GHS: global health status
GI: gastrointestinal
GLSG: German Low Grade Lymphoma Study Group
HDT: high-dose chemotherapy
HCL: hairy cell leukaemia
HIV: human immunodeficiency virus
HR: hazard ratio
HyperCVAD: hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone
IFN-α: interferon α
Ig: immunoglobulin
MCL: mantle cell lymphoma
MIPI: mantle cell international prognostic index
mTOR: mammalian target of rapamycin
MZL: marginal zone B-cell lymphoma
NCCN: National Comprehensive Cancer Network
NHL: non-Hodgkin’s lymphoma
ORR: overall response rate
OS: overall survival
PBLL/L: precursor B lymphoblastic leukaemia/lymphoma
PCM: plasma cell myeloma
QOL: quality of life
R: rituximab
R-BEAM: rituximab plus carmustine, etoposide, cytarabine and melphalan
R-CVP: rituximab plus cyclophosphamide, vincristine and prednisone
R-FC: rituximab plus fludarabine and cyclophosphamide
R-FCM: rituximab plus fludarabine, cyclophosphamide and mitoxantrone
R-DHAP: rituximab plus dexamethasone, Ara-C and cisplatin
R-MCP: rituximab plus mitoxantrone, chlorambucil and prednisolone
RT: radiation therapy
SCT: stem cell transplantation
SLL: small lymphocytic lymphoma
TBI: total body irradiation
TTF: time to treatment failure
Mantle cell lymphoma (MCL) accounts for an estimated 3%–6% of all non-Hodgkin’s lymphoma (NHL) cases [1–3]. This relatively rare lymphoma entity is characterised by an unfavourable clinical course with median overall survival (OS) of only 4–5 years [1, 3, 4, 5]. Indeed, MCL comprises the worst traits of both indolent and aggressive lymphomas, because it combines incurability with aggressive growth. Most patients present with an advanced stage of disease at diagnosis, with involvement of multiple lymph nodes, blood, spleen, bone marrow and the gastrointestinal (GI) tract. Although the disease demonstrates an initially encouraging response to treatment, its clinical course is usually marked by recurrent relapses, resulting in a dismal long-term outcome [6].
Standard therapy for MCL consisted of chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone). The addition of the monoclonal antibody rituximab to the standard CHOP regimen improved outcome parameters such as overall response rate (ORR), complete response (CR) and OS [7–9]. The evidence for the improvement in OS is based on a systematic review and on SEER-Medicare data [7, 9]. Response duration has been further improved with the application of more aggressive chemotherapy regimens including high-dose cytarabine (Ara-C) alongside high-dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT). But the outcome for those who relapse after first-line treatment or ASCT is poor, and salvage therapy is usually followed by only a short duration of response. An additional obstacle to successful treatment is the fact that many MCL patients are elderly (over 65 years of age) and are not candidates for either high-dose AraC-containing regimens or HDT.
This review will discuss the key factors that drive clinical practice with respect to diagnosis, treatment, and follow-up of MCL patients.
MCL patients are predominantly male (ratio of 2:1 or greater), with a median age at diagnosis of 60–65 years [10, 11]. Most patients initially present with stage III-IV generalised, nonbulky lymphadenopathy, often with extranodal involvement. The bone marrow, tonsils, spleen, liver and GI tract are among the most common extranodal sites. Leukaemic spread can also be detected in many cases [12]. Although common, GI involvement may not be clinically obvious during presentation and is often missed unless endoscopy studies are performed [13]. Data from a prospective study in 13 untreated MCL patients at diagnosis revealed that 92% of this group had upper or lower GI tract infiltration by MCL [14], suggesting that the actual incidence of GI involvement may be much higher than the estimated 30% who present with obvious GI symptoms. The incidence of central nervous system (CNS) manifestation in MCL patients has been reported to range from 4%‒26% [15]. In most cases, CNS infiltration is a late event in the course of the disease, particularly in patients with blastoid histology [15].
The diagnosis of MCL is made on biopsies of lymph nodes, bone marrow or other affected tissues, or through blood examinations [11]. Classical MCL exhibits a typical morphology of small- to medium-sized monomorphic lymphoid cells with indented nuclear contours, dispersed chromatin, scant cytoplasm and inconspicuous nucleoli [16]. In contrast to follicular lymphoma (FL), marginal zone B-cell lymphoma (MZL), lymphoplasmacytic lymphoma or small lymphocytic lymphoma (SLL) / chronic lymphocytic leukaemia (CLL), blastic cells such as centroblasts, immunoblasts, or paraimmunoblasts, respectively, are not found in MCL [12]. Besides classical MCL, there are four cytological variants, namely the small cell variant, the marginal zone-like variant, the pleomorphic variant and the blastoid variant (fig. 1) [17]. Of these, the pleomorphic and blastoid variants are usually associated with an even poorer prognosis [17, 18].

Figure 1
Cytomorphologic subtypes of mantle cell lymphoma: (A) classical, (B) small cell variant, (C) blastoid variant, (D) pleomorphic variant.
As these variants morphologically overlap, in particular with SLL/CLL, MZL, precursor B-cell lymphoblastic leukaemia/lymphoma (PBLL/L) and DLBCL, additional immunophenotyping is mandatory for a more comprehensive definition of MCL. Phenotypically, MCLs are positive for the B-cell markers CD20 and CD79a as well as for lineage-unspecific CD5. In addition, they exhibit immunoglobulin (Ig) expression, preferably with lambda light-chain restriction, and in most cases overexpress cyclin D1 (CCND1) and SOX-11 in the nuclei. Although the latter two are highly characteristic of MCL, both may also be found in other subtypes of lymphomas, such as plasma cell myeloma (PCM), hairy cell leukaemia (HCL), Burkitt’s lymphoma and lymphoblastic lymphomas [19, 20]. More detailed differential diagnostic immunophenotypic profiling is summarised in table 1.
The ectopic and deregulated expression of cyclin D1 in MCL is due to the chromosomal translocation t(11;14)(q13;q32), which fuses the enhancer-promoter of the Ig heavy chain gene with the transcription unit of the CCND1 proto-oncogene [21]. However, a few cases of MCL have been reported with alternative overexpression of cyclin D2 or D3 instead of D1, though additional confirmatory testing is needed in these cases [22, 23]. The most sensitive technique for detecting the classical t(11;14)(q13;q32) translocation is dual-colour fluorescence in-situ hybridisation [24].
Initial work-up typically includes a complete blood count, chemistry tests, screening for human immunodeficiency virus (HIV) and hepatitis B and C. Staging procedures are performed with the evaluation of blood and bone marrow by flow cytometry, and computed tomography (CT) of the neck, chest, abdomen and pelvis [11]. CT with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET – CT) may be useful in patients with limited-stage disease in order to confirm that the extent of their disease has not been underestimated, and there is also preliminary evidence that post-treatment PET-CT results may predict clinical outcome as demonstrated in a subset of MCL patients who received first-line treatment with high-dose chemotherapy [25].
The involvement of the GI tract should be assessed with endoscopy if the patient exhibits GI symptoms or prior to starting treatment with a dose-intensive regimen [11]. However, the relevance of GI tract staging to prognosis remains undefined. The cerebrospinal fluid is examined only when neurological symptoms are evident or if the patient has a blastoid variant of the disease [15], but there are no formal recommendations for CNS prophylaxis at either diagnosis or relapse. Although CNS prophylaxis is not routinely justified for all patients, Gill et al. [26] suggest that it may benefit patients with blastoid MCL or those with systemic relapse after first-line therapy. In general, however, we do not recommend CNS prophylaxis.
The first prognostic index for MCL was recently developed by the European MCL Network (mantle cell international prognostic index; MIPI) [27]. The MIPI stratifies patients into low, intermediate and high risk groups, based on four independent prognostic factors: age, performance status, lactate dehydrogenase level and leukocyte count. A simplified version of the MIPI has also been described by the same group [27]. Although it has been independently validated for use in MCL [28], use of the MIPI is limited. This index has prognostic value only for predicting OS, but cannot predict response to chemotherapy or progression-free survival (PFS) [23].
Another important prognostic factor for MCL not included in the MIPI is proliferation measured by the nuclear immunoreactivity of Ki-67 [29]. The Ki-67 index is the most powerful single prognostic factor for OS, retaining its prognostic relevance in patients treated with rituximab-containing regimens [30]. Although MCL may present as aggressive disease, it can take an indolent course in approximately 30% of patients [31, 32]. Survival is longer in this subset (up to 7–10 years), and these patients may be observed for months to years before needing chemotherapy. Observational data suggest that delayed therapy has no apparent negative impact on their overall survival [31]. Although there is consensus on those patients who require immediate therapy, the identification of patients with indolent MCL who may benefit from delayed therapy remains a challenge; however, these cases are clinically leukaemic, phenotypically SOX-11 negative and genetically hypermutated with respect to the variable Ig heavy chain genes. Nevertheless, once chemotherapy is warranted in these patients, their outcomes appear to be similar to those who initially present with more aggressive disease [23].
| Table 1: Differential diagnostic immunophenotypic profiles of mantle cell lymphoma and other B-cell lymphomas. | |||||||
| Immunophenotypic profile | |||||||
| Disease entity | CD20 | CD79a | CD5 | CD23 | CD10 | CCND1 | SOX11 |
| MCL | + | + | + | – | – | + | + |
| SLL/CLL | (+) | + | + | + | – | – | – |
| MZL | + | + | – | +/- | – | – | – |
| FL I | + | + | – | – | + | – | – |
| DLBCL | + | + | – | – | +/– | – | – |
| PBLL/L | (+)/– | + | – | – | –/+ | – | –/+ |
| HCL | + | + | – | – | – | -/+ | –/+ |
| PCM | –/+ | + | – | – | – | -/+ | – |
| DLBCL = diffuse large B-cell lymphoma; FL I = follicular lymphoma Grade I; HCL = hairy cell leukaemia; MCL = mantle cell lymphoma; MZL = marginal zone B-cell lymphoma; PBLL/L = precursor B lymphoblastic leukaemia/lymphoma; PCM = plasma cell myeloma; SLL/CLL = small lymphocytic lymphoma / chronic lymphocytic leukaemia | |||||||
| Table 2: Summary of the management of the subgroups of patients with mantle cell lymphoma. | ||
| Patient subgroup | First-line treatment | Level of evidence |
| Limited-stage MCL | Multiagent (immuno)chemotherapy plus involved-field RT | IV |
| Younger, eligible for transplant | Aggressive Ara-C-containing chemotherapy in combination with rituximab | I |
| HDT followed by ASCT to consolidate clinical response | I | |
| Fit patients (younger or elderly), not eligible for transplant | R-CHOP induction with rituximab maintenance | I |
| R-bendamustine induction | II | |
| R-HyperCVAD | III | |
| Elderly unfit patients, not eligible for transplant | Less aggressive regimens, i.e. rituximab with low-dose bendamustine or oral chlorambucil, with or without rituximab maintenance | II |
| Relapse | ||
| Eligible for transplant | HDT followed by ASCT, in patients who did not receive this first-line | III |
| Allogeneic SCT, in young, fit patients | III | |
| Not eligible for transplant | Rechallenge with same induction regimen, if duration of remission >12 months, preferably using a non-cross-resistant regimen | II |
| Treatment with new agents in clinical trials | V | |
| AraC = cytarabine; ASCT = autologous stem cell transplantation; HDT = high-dose therapy; MCL = mantle cell lymphoma; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone; R-HyperCVAD = rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone; RT = radiation therapy Levels of evidence: I – Available evidence from at least one high quality, large, randomised, controlled trial or meta-analyses of several high quality randomised trials. II – Smaller randomised studies or subanalyses of larger randomised trials with possible bias, or meta-analyses of such trials or meta-analyses of heterogeneous trials. III – Prospective cohort studies. IV– Retrospective cohort studies, case-control studies. V – Uncontrolled studies, individual case reports, expert opinions. | ||
Because of the rarity of MCL, there is a paucity of large randomised studies and most of the available data are based on smaller phase II trials. In general, therapeutic approaches can be divided into two clinical contexts: the treatment of patients who are eligible for SCT, and those who are unable to undergo SCT (table 2).
Approximately 6%–8% of MCL patients present with limited-stage disease [33], but few data are available regarding the disease course in these patients. Leitch et al. [34] retrospectively described the clinicopathological features and outcomes of 26 patients who were identified with limited-stage MCL at the British Columbia Cancer Agency since 1984. Patients with stage IA or IIA, nonbulky (<10 cm) disease were treated according to standard protocols, namely with single- or multiple-agent chemotherapy and/or involved-field radiation therapy (RT). Median age at diagnosis was 68 years, and the median OS for the whole group was 6.8 years. The authors noted that initial treatment with RT was the most important factor for improved PFS rates (5-year PFS 68% vs 11%; p = 0.002) with a trend towards improved OS in patients receiving RT (6-year OS 71% vs 25%; p = 0.13). Of particular importance, a plateau in the PFS curve at 60% was seen in patients receiving RT and 6 of 17 irradiated patients were free of progression after >5 years. Similar results were reported in a more recent study, which retrospectively assessed 26 patients with stage I and II MCL [35]. The authors focused their analysis on the 21 patients who were treated with curative intent (chemotherapy and/or RT, followed by HDT with ASCT in two patients). An ORR of 95% as well as a local control rate of 95% was achieved in this subset of patients, leading to a median PFS and OS of 3.2 and 6.4 years, respectively. Systemic relapse was more common in patients with stage II disease and in those with the blastoid variant of MCL. Owing to the small number of patients in all published trials [36, 37], the power of any comparisons is very limited. Although limited-stage MCL is associated with better outcomes, systemic relapse is still a significant problem.
On the basis of these scant data, outside the context of clinical trials, a treatment strategy using brief multiagent (immuno)chemotherapy in combination with involved field RT is recommended for patients with limited-stage MCL.
In younger, fit patients with few or no comorbidities, the therapeutic approach places emphasis on more intense regimens which combine aggressive chemotherapy with rituximab, with or without HDT followed by ASCT. In this clinical context, dose-intensified high-dose Ara-C-containing induction regimens in combination with rituximab are considered as the current standard of care [38].
In a randomised trial of the German Low Grade Lymphoma Study Group (GLSG), the addition of rituximab to CHOP significantly improved ORR and CR rates [8]. In an update of this trial after a median follow-up of 65 months, median time to treatment failure (TTF) was prolonged from 14 months for CHOP to 28 months for R-CHOP (p = 0.0003). However, in contrast to other lymphoma entities, no clear improvements of OS were observed, with 5-year OS rates of 59% for R-CHOP and 46% for CHOP (p = 0.27). However, with only 122 patients randomised, the trial was underpowered to detect clinically relevant differences in this secondary endpoint [39].
Owing to the failure of R-CHOP-like regimens alone to prolong OS, more aggressive treatment regimens with HDT followed by ASCT have been explored. More recent studies have shown promising survival data with dose-intensified induction regimens that incorporate high-dose Ara-C [40]. The combination of rituximab alongside six to eight cycles of fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (HyperCVAD) alternating with high doses of Ara-C and methotrexate yielded an 87% CR/CRu and an impressive 3-year failure-free survival of 73% in the subgroup of patients younger than 65 years of age [40]. Another rituximab and high-dose Ara-C-containing treatment schedule alternating with a dose-intensified R-CHOP regimen (“maxi-R-CHOP”) followed by HDT with ASCT (MCL-2 trial) was tested by the Nordic Lymphoma Group in a large nonrandomised phase II study [41]. In comparison with their previous study (MCL-1 trial), which did not contain high-dose Ara-C, results with respect to EFS, PFS and OS were significantly improved. A phase II study by the French Groupe d’Etudes des Lymphomes de l’Adulte (GELA) has also shown promising results using a rituximab and high-dose Ara-C-containing induction regimen before HDT followed by ASCT [42].
Recently, the presentation of the results from the randomised European MCL Network Younger trial has further strengthened the role of Ara-C in front-line therapy for MCL [43]. In this large trial, a total of 497 patients up to the age of 65 years with previously untreated MCL were randomised to receive either six courses of CHOP plus rituximab followed by myeloablative radiochemotherapy (12 Gray total body irradiation [TBI], 2x60 mg/kg cyclophosphamide) and ASCT (control arm A) versus alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high-dose Ara-C-containing myeloablative regimen (10 Gray TBI, 4x1.5 g/m2 Ara-C, 140 mg/m2 melphalan) and ASCT (experimental arm B). CR and CR/CRu rates were significantly higher in arm B (25% vs 36%; p = 0.012 and 40% vs 54%; p = 0.0003). After a median follow up of 51 months, a lower number of relapses occurred in the high-dose Ara-C-containing treatment arm, leading to a significantly longer TTF (46 months vs 88 months; p = 0.0382, hazard ratio [HR] 0.68). In addition, OS was improved (82 months vs median not reached; p = 0.045). The authors concluded that an induction regimen containing high dose Ara-C followed by HDT followed by ASCT should become the new standard of care of MCL patients up to 65 years of age [43].
Although HDT followed by ASCT had been shown to improve PFS after CHOP-like induction chemotherapy versus conventional chemotherapy alone [44], no prospective comparisons are available with regard to its use after the above-mentioned more intensive Ara-C- and/or rituximab-containing induction regimens. Recently, a large retrospective analysis of 167 younger MCL patients (<65 years) from the National Comprehensive Cancer Network (NCCN) NHL database compared the outcome of patients treated with either the R-HyperCVAD regimen or the R-CHOP regimen with or without HDT/ASCT as first-line therapy [45]. Interestingly, outcomes were comparable between patients treated with R-HyperCVAD, with or without HDT/ASCT, and R-CHOP followed by HDT/ASCT ‒ 3-year PFS rates were 58% (95% confidence interval [CI] 44%–69%) for the R-HyperCVAD arm, 55% (95% CI 22%–79%) for the R-HyperCVAD + HDT/ASCT and 56% (95% CI 33%–74%) for the R-CHOP + HDT/ASCT arm, respectively) ‒ thereby challenging the role of ASCT in patients treated with intensive Ara-C-containing induction regimens. In contrast, the R-CHOP only arm (without HDT/ASCT consolidation) demonstrated an inferior 3-year PFS of 18% (95% CI 6%–36%) compared with all the other treatment regimens. The rate of treatment-related complications was higher for patients treated with R-HyperCVAD with or without HDT/ASCT.
Though there is no consensus on the optimal pretransplant induction regimen, results so far point towards intensive combinations that include high-dose Ara-C. At present, HDT followed by ASCT plays a role as part of the front-line treatment and consolidates the best possible clinical response following upfront intensive immunochemotherapy.
The management of elderly patients with no significant comorbidities and younger patients who are not eligible for transplant follows similar strategies. There are two main features of the treatment of this patient population: the identification of an effective induction regimen, and the use of remission consolidating and maintenance therapy to prolong the duration of response. A recent phase III trial conducted by the European MCL Network had the two-fold objective of comparing R-CHOP with R-FC (rituximab plus fludarabine and cyclophosphamide) as induction treatment, and comparing maintenance with rituximab versus interferon-α (IFN-α) in elderly MCL patients [46]. Patients were either ≥66 years of age, or 60–65 years if ineligible for HDT with ASCT, with an Eastern Cooperative Oncology Group (ECOG) status of ≤2. The key findings from this trial showed that R-CHOP was superior to R-FC in terms of efficacy and tolerability (4-year OS 62% for R-CHOP vs 47% for R-FC; p = 0.005), and that rituximab maintenance was able to prolong the duration of remission. Maintenance rituximab not only conferred a survival advantage over maintenance IFN-α, but was especially beneficial to those who had been successfully pretreated with R-CHOP (4-year OS 87% vs 63% in favour of rituximab; p = 0.005). The duration of remission and OS after a response to R-CHOP were significantly shorter amongst patients who were not assigned to any maintenance therapy, as compared with those who were randomly assigned to IFN-α (p = 0.002 and p <0.001, respectively). As an alternative to the R-CHOP induction / rituximab maintenance regimen, a consolidation strategy with radioimmunotherapy can be considered [47].
Other induction regimens have been explored in this clinical setting, including R-HyperCVAD and R-bendamustine. Ten-year follow-up of a prospective phase II trial conducted by the MD Anderson Cancer Centre in 97 elderly patients treated with R-HyperCVAD alternating with rituximab plus high-dose methotrexate and Ara-C without HDT and ASCT, showed that the median OS for all patients had not been reached and the median TTF for all patients was 4.6 years [48]. These promising results, however, have been tempered by the significant mucosal and haematological toxicities as well as the 8% treatment-related death rate associated with this regimen [40, 49]. This regimen is therefore not recommended for elderly patients with comorbidities. R-bendamustine front-line therapy has shown encouraging results in a randomised phase III trial in elderly patients with indolent and mantle cell lymphoma. At a median follow-up of 45 months, PFS was significantly prolonged with R-bendamustine compared with R-CHOP (median PFS 69.5 vs 31.2 months in favour of R-bendamustine; HR 0.58, 95% CI 0.44–0.74; p <0.0001) [50]. The superior response of R-bendamustine over R-CHOP and R-CVP was confirmed in the Bright study [51]. Furthermore, patients receiving R-bendamustine reported improved quality of life (QOL) parameters, as measured by the global health status (GHS)/QOL scale and the QLQ-C30 questionnaire [52]. Thus, R-bendamustine may provide an effective alternative to R-CHOP in the front-line treatment of elderly patients and those who are not eligible for transplant. The feasibility of combining this regimen with rituximab maintenance is an attractive option, and is currently under investigation.
There are only limited treatment options for elderly, unfit patients with multiple comorbidities. In the case of asymptomatic patients with nonbulky disease, low Ki-67 levels and normal blood counts, initial therapy may be deferred until the onset of disease symptoms [53]. For symptomatic patients, less aggressive treatment regimens may be used, such as rituximab in combination with low-dose bendamustine [54] or oral chlorambucil [10]. Rituximab monotherapy may be another option for elderly patients unable to tolerate chemotherapy. However, in contrast to the findings in FL, patients receiving prolonged treatment with single-agent rituximab did not show an improved clinical response [55]. Maintaining the balance between durable remission and treatment-induced toxicity is particularly challenging, and patients are strongly encouraged to obtain treatment within the context of a clinical trial.
| Table 3. Novel agents for the treatment of relapsed/refractory MCL [79]. | |||
| Drug | Clinical development phase | Drug class | Mode of action |
| Temsirolimus [74] | Phase III | Inhibitor of mammalian target of rapamycin (mTOR) | Derivative of rapamycin, an agent exhibiting antifungal, immunosuppressant and anti-tumour activities. Inhibits the effects of mTOR, an important cell cycle-regulatory protein. |
| Everolimus (RAD001) [75, 80] | Phase II | ||
| Deforolimus [81] | Phase II | ||
| Bortezomib [72, 82, 83, 84] | Phase II | Proteasome inhibitors | Inhibition of proteasome actions, cell cycle arrest, induction of apoptosis. |
| Carfilzomib (PR-171) [85, 86] | Phase I | ||
| Lenalidomide [87, 88, 89, 90] | Phase II | Immune modulatory drugs | Immune modulator, affecting both cellular and humoral immunity. Also shown to have anti-angiogenic properties. |
| Flavopiridol [91, 92] | Phase II | Cyclin-dependent kinase (CDK) inhibitors | Inhibitor of cyclin-dependent kinases leading to cell cycle arrest. |
| PD0332991 [93] | Phase I | ||
| Ibrutinib [78] | Phase II | Bruton’s tyrosine kinase (BTK) inhibitor | Induces apoptosis and inhibits cellular migration and adhesion in malignant B-cells. |
| CAL-101 (GS-1101) [94, 95, 96] | Phase I | PI3-kinase inhibitor | Induces apoptosis and reduces cell viability. |
Despite the improved activity of first-line treatment, nearly all MCL patients experience relapse. There is a lack of randomised trials comparing the relative survival advantages of various second-line options. In general, patients with relapsed disease can be broadly classified into those who are transplant-eligible, and those for whom transplant is not an option.
Regardless of the induction regimen, consolidation therapy with SCT is a key option for patients who experience relapse [56]. HDT followed by ASCT is recommended for eligible patients who did not undergo this as part of their first-line treatment. Allogeneic SCT is a possibility for young, fit patients with no signs of CNS disease and who have a compatible donor [57]. An analysis of the long-term outcomes (median follow-up 56 months) of 35 patients with relapsed/refractory MCL who underwent nonmyeloablative allogeneic SCT revealed a median PFS duration of 60 months, while the median OS was not yet reached. The 6-year actuarial PFS and OS rates were 46% and 53%, respectively [58]. Nevertheless, mortality due to graft-versus-host disease and secondary infections are major limiting factors, and careful patient selection remains a key factor in the outcome of this treatment modality [53, 59].
Relapsing patients who are not eligible for SCT have very few options, aside from receiving treatment with new agents in a clinical trial. In the cases where the duration of remission was longer than 12 months, the patient may be rechallenged with the same induction regimen. Generally, non-cross-resistant regimens are used. Outside of clinical trials, the combination of rituximab plus chemotherapy according to the patient’s performance status forms the basis of treatment, supplemented by rituximab maintenance therapy where possible [60, 61]. There is no regimen that has been shown to be superior, although several combinations have been tested in this patient subset, including bendamustine [62], R-bendamustine [63–65], R-FC [66] and R-FCM [67]. Treatment with single-agent radioimmunotherapy is another option in the relapsed setting [68].
The proteasome inhibitor bortezomib has been tested as a monotherapy, resulting in ORR of around 30% [69, 70], including 8% CR/CRu [70]. Bortezomib has also been tested in combination with other agents, including rituximab plus bendamustine [71] and rituximab plus dexamethasone [72]. A second novel class of agents are inhibitors of the mammalian target of rapamycin (mTOR), such as temsirolimus and everolimus. They act via the PI3 kinase/Akt/mTOR signalling pathway implicated in the pathogenic deregulation of cyclin D1 in MCL [73]. In a randomised open-label phase III trial in 162 relapsed/refractory MCL patients, temsirolimus was evaluated in comparison with investigator’s choice of therapy. The high-dose temsirolimus regimen (175 mg/75 mg) resulted in an ORR of 22% and a significantly longer PFS compared with investigator’s choice therapy (median PFS 4.8 vs 1.9 months; p = 0.0009; HR = 0.44) [74]. A number of ongoing trials are currently exploring the feasibility of combining temsirolimus with various chemotherapy regimens in the salvage treatment setting [73]. Everolimus has been tested in a couple of small phase II trials so far and demonstrated similar response and PFS rates [75].
The immunomodulatory agent lenalidomide has also shown encouraging activity. Subgroup analysis of 15 relapsed or refractory MCL patients from an open-label phase II NHL trial resulted in a 53% ORR and a median duration of response of 13.7 months [76]. Lenalidomide has also been effectively combined with rituximab, yielding 57% ORR, 36% CR and a median response duration of around 19 months with a reasonable tolerability profile [77]. A multicentre phase II trial by the European MCL Network is currently underway evaluating the activity of lenalidomide with bendamustine plus rituximab in MCL patients in first relapse or with primary refractory disease [38].
Encouraging data has been obtained for ibrutinib, an orally administered inhibitor of Bruton’s tyrosine kinase (BTK) [78]. Preliminary results from a phase II trial in 110 evaluable patients with relapsed or refractory MCL (subdivided into bortezomib-naïve or bortezomib-exposed groups) showed ORR, CR and PR rates of 68%, 22% and 46%, respectively [78]. Median duration of response, PFS and OS have not been reached. The durability of the responses, alongside the drug’s tolerability profile, suggest that ibrutinib is a strong candidate for the treatment of relapsed/refractory MCL, warranting further investigation.
In addition to the above agents, other new drugs are being actively explored for the treatment of relapsed/refractory MCL. A complete overview of these novel therapies is beyond the scope of this review, but a summary of new agents under investigation is provided in table 3.
The primary purpose of patient follow-up in MCL is to identify relapse or progression. The majority of relapses occur within 2 years after first-line therapy. Although there is no data to support a specific follow-up strategy in MCL, patients are usually seen every 3 months for the first 2 years after treatment, every 4–6 months for an additional 3 years, and subsequently once a year.
The choice of strategy for approaching the treatment of MCL will remain a complex problem that still requires evidence-based guidance. Yet achieving cure for MCL remains elusive; in the absence of cure, the treatment goal is to prolong survival while maintaining the patient’s quality of life. Increasing the intensity of therapeutic regimens can be a double-edged sword, prolonging a patient’s survival but compromising on quality of life. Until we achieve cure, we must continue to pursue novel therapies that enable us to enhance not only the duration of the response but also the quality of the extended survival conferred to the patient.
Acknowledgment:The authors would like to thank Dr Karen Yeow (Biomedicomm Ltd), who provided editorial assistance with the manuscript, and Dr Oliver Meier (Roche Pharma Schweiz AG) who organized the authors' meeting to discuss the manuscript.
1 Andersen NS, Jensen MK, de Nully BP, et al. A Danish population-based analysis of 105 mantle cell lymphoma patients: incidences, clinical features, response, survival and prognostic factors. Eur J Cancer. 2002;38:401–8.
2 The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–18.
3 Velders GA, Kluin-Nelemans JC, De Boer CJ, et al. Mantle-cell lymphoma: a population-based clinical study. J Clin Oncol. 1996;14:1269–74.
4 Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89:2067–78.
5 Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–8.
6 Dreyling M and Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009;542–51.
7 Griffiths R, Mikhael J, Gleeson M, et al. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood. 2011;118:4808–16.
8 Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23:1984–92.
9 Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–14.
10 Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114:1469–76.
11 Vose JM. Mantle cell lymphoma: 2012 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2012;87:604–9.
12 Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94:1488–92.
13 Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003;97:586–91.
14 Salar A, Juanpere N, Bellosillo B, et al. Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol. 2006;30:1274–80.
15 Ferrer A, Bosch F, Villamor N, et al. Central nervous system involvement in mantle cell lymphoma. Ann Oncol. 2008;19:135–41.
16 Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue (IARC WHO Classification of Tumours). World Health Organization. 2008; 4th edition.
17 Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–53.
18 Cogliatti SB, Bertoni F, Zimmermann DR, et al. IgV H mutations in blastoid mantle cell lymphoma characterize a subgroup with a tendency to more favourable clinical outcome. J Pathol. 2005;206:320–7.
19 Chen YH, Gao J, Fan G, et al. Nuclear expression of sox11 is highly associated with mantle cell lymphoma but is independent of t(11;14)(q13;q32) in non-mantle cell B-cell neoplasms. Mod Pathol. 2010;23:105–12.
20 Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94:1555–62.
21 Bertoni F, Rinaldi A, Zucca E, et al. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22–7.
22 Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–97.
23 Shah BD, Martin P, Sotomayor EM. Mantle cell lymphoma: a clinically heterogeneous disease in need of tailored approaches. Cancer Control. 2012;19:227–35.
24 Belaud-Rotureau MA, Parrens M, Dubus P, et al. A comparative analysis of FISH, RT-PCR, PCR, and immunohistochemistry for the diagnosis of mantle cell lymphomas. Mod Pathol. 2002;15:517–25.
25 Mato AR, Svoboda J, Feldman T, et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer. 2012;118:3565–70.
26 Gill S, Herbert KE, Prince HM, et al. Mantle cell lymphoma with central nervous system involvement: frequency and clinical features. Br J Haematol. 2009;147:83–8.
27 Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65.
28 Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010;115:1530–3.
29 Klapper W, Hoster E, Determann O, et al. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop. 2009;2:103–11.
30 Determann O, Hoster E, Ott G, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111:2385–7.
31 Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–13.
32 Martin P and Leonard J. Is there a role for “watch and wait” in patients with mantle cell lymphoma? Semin Hematol. 2011;48:189–93.
33 Chandran R, Gardiner SK, Simon M, et al. Survival trends in mantle cell lymphoma in the United States over 16 years 1992–2007. Leuk Lymphoma. 2012;53:1488–93.
34 Leitch HA, Gascoyne RD, Chhanabhai M, et al. Limited-stage mantle-cell lymphoma. Ann Oncol. 2003;14:1555–61.
35 Bernard M, Tsang RW, Le LW, et al. Limited-stage mantle-cell lymphoma: Treatment outcomes at the Princess Margaret Hospital. Leuk Lymphoma. 2012; epub before print.
36 Samaha H, Dumontet C, Ketterer N, et al. Mantle cell lymphoma: a retrospective study of 121 cases. Leukemia. 1998;12:1281–7.
37 Vandenberghe E, De Wolf-Peeters C, Vaughan HG, et al. The clinical outcome of 65 cases of mantle cell lymphoma initially treated with non-intensive therapy by the British National Lymphoma Investigation Group. Br J Haematol. 1997;99:842–7.
38 Dreyling M, Kluin-Nelemans HC, Bea S, et al. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: report of the 10th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2011;52:2226–36.
39 Hoster E, Unterhalt M, Wormann B, et al. The Addition of Rituximab to First-Line Chemotherapy (R-CHOP) Results in Superior Response Rates, Time to Treatment Failure and Response Duration in Patients with Advanced Stage Mantle Cell Lymphoma: Long Term Results of a Randomized GLSG Trial. Blood (ASH Annual Meeting Abstracts). 2008;112:Abstract 3049.
40 Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–23.
41 Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93.
42 Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53.
43 Hermine O, Hoster E, Walewski J, et al. Alternating Courses of 3x CHOP and 3x DHAP Plus Rituximab Followed by a High Dose ARA-C Containing Myeloablative Regimen and Autologous Stem Cell Transplantation (ASCT) Increases Overall Survival When Compared to 6 Courses of CHOP Plus Rituximab Followed by Myeloablative Radiochemotherapy and ASCT in Mantle Cell Lymphoma: Final Analysis of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net). Blood (ASH Annual Meeting Abstracts). 2012;120:Abstract 151.
44 Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–84.
45 LaCasce AS, Vandergrift JL, Rodriguez MA, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: an analysis from the NCCN NHL Database. Blood. 2012;119:2093–9.
46 Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–31.
47 Smith MR, Li H, Gordon L, et al. Phase II Study of Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Immunochemotherapy Followed by Yttrium-90-Ibritumomab Tiuxetan in Untreated Mantle-Cell Lymphoma: Eastern Cooperative Oncology Group Study E1499. J Clin Oncol. 2012;30:3119–26.
48 Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–8.
49 Merli F, Luminari S, Ilariucci F, et al. Rituximab plus HyperCVAD alternating with high dose cytarabine and methotrexate for the initial treatment of patients with mantle cell lymphoma, a multicentre trial from Gruppo Italiano Studio Linfomi. Br J Haematol. 2012;156:346–53.
50 Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10.
51 Flinn IW, van der Jagt RH, Kahl BS, et al. An Open-Label, Randomized Study of Bendamustine and Rituximab (BR) Compared with Rituximab, Cyclophosphamide, Vincristine, and Prednisone (R-CVP) or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in First-Line Treatment of Patients with Advanced Indolent Non-Hodgkin's Lymphoma (NHL) or Mantle Cell Lymphoma (MCL): The Bright Study. Blood (ASH Annual Meeting Abstracts). 2012;120:Abstract 902.
52 Burke JM, van der Jagt RH, Kahl B, et al. Differences in Quality of Life Between Bendamustine Plus Rituximab Compared with Standard First-Line Treatments in Patients with Previously Untreated Advanced Indolent Non-Hodgkin’s Lymphoma or Mantle Cell Lymphoma. Blood (ASH Annual Meeting Abstracts). 2012; 120: Abstract 155.
53 Martin P, Smith M, and Till B. Management of mantle cell lymphoma in the elderly. Best Pract Res Clin Haematol. 2012;25:221–31.
54 Weidmann E, Neumann A, Fauth F, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22:1839–44.
55 Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol. 2005;23:705–11.
56 Reddy N, Greer JP, Goodman S, et al. Consolidative therapy with stem cell transplantation improves survival of patients with mantle cell lymphoma after any induction regimen. Exp Hematol. 2012;40:359–66.
57 Cassaday RD and Gopal AK. Allogeneic hematopoietic cell transplantation in mantle cell lymphoma. Best Pract Res Clin Haematol. 2012;25:165–74.
58 Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–52.
59 Ruan J, Coleman M, and Leonard JP. Management of relapsed mantle cell lymphoma: still a treatment challenge. Oncology (Williston Park). 2009;23:683–90.
60 Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood. 2006;108:4003–8.
61 Kenkre VP, Long WL, Eickhoff JC, et al. Maintenance rituximab following induction chemo-immunotherapy for mantle cell lymphoma: long-term follow-up of a pilot study from the Wisconsin Oncology Network. Leuk Lymphoma. 2011;52:1675–80.
62 Moosmann P, Heizmann M, Kotrubczik N, et al. Weekly treatment with a combination of bortezomib and bendamustine in relapsed or refractory indolent non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:149–52.
63 Rigacci L, Puccini B, Cortelazzo S, et al. Bendamustine with or without rituximab for the treatment of heavily pretreated non-Hodgkin's lymphoma patients : A multicenter retrospective study on behalf of the Italian Lymphoma Foundation (FIL). Ann Hematol. 2012;91:1013–22.
64 Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4473–9.
65 Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–9.
66 Thomas DW, Owen RG, Johnson SA, et al. Superior quality and duration of responses among patients with mantle-cell lymphoma treated with fludarabine and cyclophosphamide with or without rituximab compared with prior responses to CHOP. Leuk Lymphoma. 2005;46:549–52.
67 Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–71.
68 Wang M, Oki Y, Pro B, et al. Phase II study of yttrium-90-ibritumomab tiuxetan in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:5213–8.
69 O’Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–84.
70 Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74.
71 Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–12.
72 Lamm W, Kaufmann H, Raderer M, et al. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica. 2011;96:1008–14.
73 Kirschey S, Wagner S, and Hess G. Relapsed and/or Refractory Mantle Cell Lymphoma: What Role for Temsirolimus? Clin Med Insights Oncol. 2012;6:153–64.
74 Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–9.
75 Renner C, Zinzani PL, Gressin R, et al. A multicenter phase II trial (SAKK 36/06) of single-agent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica. 2012;97:1085–91.
76 Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–9.
77 Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–23.
78 Wang M, Rule SA, Martin P, et al. Interim Results of an International, Multicenter, Phase 2 Study of Bruton’s Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), in Relapsed or Refractory Mantle Cell Lymphoma (MCL): Durable Efficacy and Tolerability with Longer Follow-up. Blood (ASH Annual Meeting Abstracts). 2012;120:Abstract 904.
79 Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38.
80 Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–7.
81 Rizzieri DA, Feldman E, Dipersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756-2762.
82 Houot R, Le GS, Ojeda UM, et al. Combination of rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil (RiPAD+C) as first-line therapy for elderly mantle cell lymphoma patients: results of a phase II trial from the GOELAMS. Ann Oncol. 2012;23:1555–61.
83 Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–5.
84 O’Connor OA, Moskowitz C, Portlock C, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: results of a multicentre Phase 2 clinical trial. Br J Haematol. 2009;145:34–9.
85 Ruschak AM, Slassi M, Kay LE, et al. Novel proteasome inhibitors to overcome bortezomib resistance. J Natl Cancer Inst. 2011;103:1007–17.
86 O’Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–91.
87 Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36.
88 Zaja F, De LS, Vitolo U, et al. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: clinical results and effects on microenvironment and neo-angiogenic biomarkers. Haematologica. 2012;97:416–22.
89 Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol. 2012;159:154–63.
90 Gunnellini M, Falchi L. Therapeutic Activity of Lenalidomide in Mantle Cell Lymphoma and Indolent Non-Hodgkin’s Lymphomas. Adv Hematol. 2012; epub.
91 Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–23.
92 Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8.
93 Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607.
94 Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88.
95 Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4.
96 Iyengar S, Clear A, Bodor C, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–84.
Funding / potential competing interests: This work was supported by Roche Pharma (Schweiz) AG, Reinach, Switzerland.