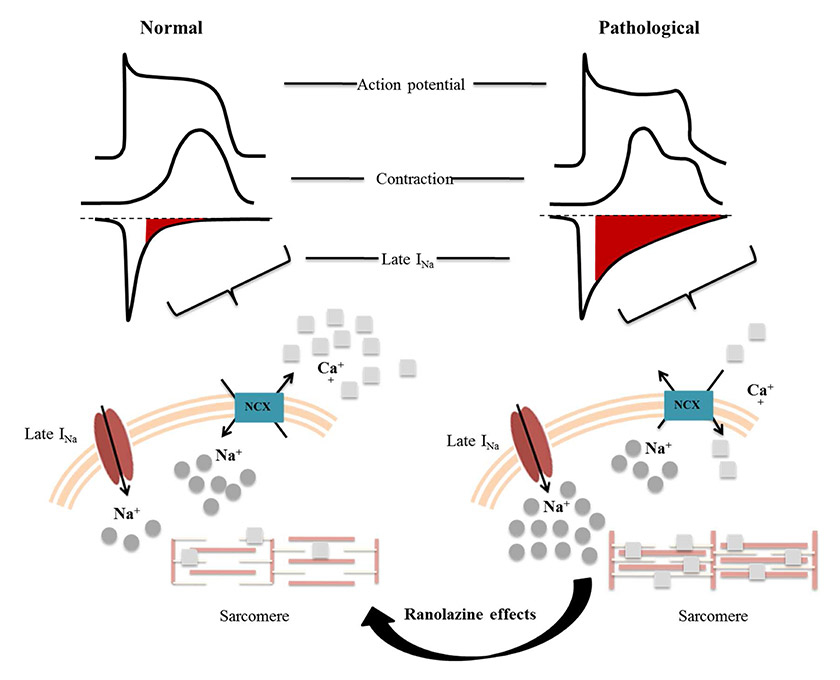
Figure 1
Abnormal function of the myocardium during ischaemia and the mechanism of action of ranolazine.
INa= late Na current; NCX = Na-Ca exchange
DOI: https://doi.org/10.4414/smw.2013.13874
Chronic angina represents a major burden for public health systems because of its poor prognosis and its high treatment costs. Ranolazine is an emerging drug recently approved for the treatment of this disease. The main molecular mechanism underlying ranolazine-mediated beneficial effects has been identified as inhibition of the late Na+ current during the action potential, which potentially improves oxygen consumption, diastolic dysfunction and coronary blood flow. Moreover, this particular mechanism of action also confers on ranolazine a potential antiarrhythmic effect. The aim of this review is to update the evidence for ranolazine treatment in chronic angina and discuss its therapeutic perspectives based on the most recent clinical and experimental studies.
Stable angina (SA) is one of the most common manifestations of coronary artery disease (CAD) [1]. It is characterised by elevated medical care costs [1]. Despite advances in cardiovascular disease prevention, the incidence of SA is expected to increase in the near future in developed countries. In clinical practice, the treatment goals set by guidelines are often utopian. In fact, it is recommended to (at the same time): (i.) reduce premature cardiovascular death, (ii.) prevent complications that impair the patient’s functional well-being, (iii.) maintain or restore a quality of life that is satisfactory to the patient, (iv.) eliminate ischaemic symptoms and (v.) minimise costs of health care [1]. Current pharmacological approaches include: short- or long-acting nitrates, calcium-channel blockers and β-blockers [1]. However, even their intensive use is not highly effective in preventing SA [2]. Currently, N-(2-6-dimethylphenyl)-4(2-hydroxy-3-[2-methoxyphenoxy]-propyl)-1-piperazineacetamidededihydrochloride (also named ranolazine), is the most potent clinical Na+-channel blocker. It was first approved in 2006 by the US Food and Drug Administration (FDA), and in the European Union ranolazine is approved (at a maximum dosage of 750 mg twice daily) for the symptomatic treatment of patients with inadequately controlled SA in addition to other therapies, or alone in the case of intolerance to first-line therapies [3]. The aim of this review is to explore the pathophysiological mechanisms of action of ranolazine and to update evidence from recent clinical trials on its efficacy and safety in SA.
SA is defined as substernal chest discomfort with a characteristic quality and duration that is provoked by exertion or emotional stress and relieved by rest or nitroglycerin [1]. Chronic SA is caused by myocardial ischaemia generally due to one or more significant obstructive lesions in the coronary arteries. During ischaemia the imbalance between the oxygen supplied and required by the myocardium leads to a dramatic reduction in myocardial contractility and impaired activity of ion pumps involved in myocardial contraction-relaxation processes. Ischaemia disrupts action potential physiology. An early event is the increase in intracellular [Na+] [4], mainly induced by an increase in the late Na+ current (INa) [5], but also by Na+/H+exchanger activation and Na+/K+ adenosine triphosphatase (ATPase) inhibition. The late INa delays repolarisation by increasing action potential duration. Moreover, an elevated [Na+] adversely affects cellular pathways of Ca++ transportation. The [Ca++] is mainly regulated by the Na+/Ca++exchanger, which exchanges one Ca++ ion for three Na+ ions per cycle and can work in both directions: in forward mode, eliminating Ca++ outside the cell, or in reverse mode. Ca++-carrier activity and direction depend on several factors, such as membrane potential (during the action potential it usually works in reverse mode), [Na+] and [Ca++]. When hypoxia is established, an elevated [Na+] triggers the reverse mode Na+/Ca++exchanger [6], impairing [Ca++] removal from the cell [7, 8]. High [Ca++] keeps contractile proteins active, increasing energy consumption and diastolic tone and then impairing ventricular relaxation. These pathophysiological events are major determinants of increased ventricular wall tension [9]. This process might create a vicious circle, potentially increasing coronary vessel resistance and decreasing coronary blood flow [10].
Ranolazine is a potent late INa inhibitor. Indeed, in the therapeutic range (2–8 µmol/l), ranolazine has an almost selective action on the late INa (about 38-fold higher than on peak INa) with concentration-, frequency-, and voltage-dependent inhibitory effects [11, 12]. The mechanism of action of ranolazine is markedly different from other antianginal drugs, such as calcium-channel blockers, β-blockers and nitrates (fig. 1). The late INa inhibition by ranolazine has been observed in myocardial models exposed to lipid peroxidation [13, 14], ischaemia-reperfusion injury [15] and palmitoyl-L-carnitine [16], and in heart failure [17, 18].

Figure 1
Abnormal function of the myocardium during ischaemia and the mechanism of action of ranolazine.
INa= late Na current; NCX = Na-Ca exchange
Moreover, ranolazine has been shown to decrease the variability of the action potential duration in single myocytes exposed to Anemonia sulcata toxin (ATX-II, known to increase late INa magnitude) [19]. Ranolazine might also interfere with Ca++-dependent pathways. As mentioned above, ranolazine-induced blockade of INa keeps the Na++/Ca++ exchange in forward mode, thus preventing Ca++ overload [20]. Lowering the intracellular [Ca++] promotes diastolic relaxation and then reduces O2 consumption and ATP utilisation. In addition, since ranolazine improves diastolic function and reduces wall tension, this drug might indirectly increase blood flow within the ischaemic myocardial [21, 22]. Hale and coworkers showed a protective role of ranolazine in a rabbit model of ischaemia/reperfusion injury, associated with an improvement in ejection fraction and stroke volume, and less wall motion abnormality, after reperfusion [23]. Finally, Sossalla and colleagues showed that ranolazine improves diastolic function as a result of altered [Na+] and [Ca++] [18] (see fig. 1).
In agreement with basic research studies, recent studies using myocardial perfusion imaging techniques confirmed that ranolazine improved coronary perfusion and oxygen supply in humans [24, 25]. Ranolazine has been extensively studied in human ischaemic heart disease, in a wide range of dosages and clinical presentations, from SA to acute coronary syndromes (ACS). The first studies, dating back to the 1990s, gave conflicting results for the small numbers of patients and low doses tested (30–60–120 mg three times daily) [26, 27]. Even the first randomised trial failed to provide definitive results, partly because the immediate-release formulation of ranolazine (at the time the only available form) was used [28, 29]. The sustained-release (SR) form of ranolazine, produced later, has been studied in several randomised, double-blind, placebo-controlled trials. These studies provided the evidence that supported the registration of ranolazine SR (Ranexa®) for clinical use in chronic ischaemic heart disease (table 1).
In the MARISA (Monotheraphy Assessment of Ranolazine in Stable Angina) trial, 191 patients with effort angina for at least 3 months (but responsive to calcium-channel blockers, β-blockers and/or nitrates) were randomised to treatment with ranolazine SR (500, 1000 or 1500 mg twice daily) or placebo. Ranolazine SR was administered as monotherapy after interruption of all other antianginal medications. After a week, the treated group reached primary endpoints: improved total exercise duration, time to onset of angina and time to ≥1 mm ST-segment depression (p <0.005) at peak treadmill exercise. However, it should be noted that treatment with the higher dose of ranolazine was stopped before study end because of an increased incidence of adverse effects (such as bradycardia, hypotension and lengthening of QTc) [35].
The CARISA (Combination Assessment of Ranolazine in Stable Angina) trial investigated the effects of 12 weeks of ranolazine treatment (750 or 1000 mg twice daily), in association with other classical antianginal therapy. This placebo-controlled study enrolled 823 patients. Treatment with Ranolazine SR met the primary endpoints (i.e., increase of total exercise duration at both trough [p = 0.03 for 750 mg] and peak levels [p = 0.001 for 750 mg], increase of time to onset of angina and increase of time to ≥1 mm ST-segment depression at peak exercise [p = 0.02 and p <0.001, respectively, for 750 mg]). A prolonged treatment duration was associated with a reduction in angina episodes and nitrate consumption (p <0.02) [36]. A recent post-hoc analysis of the CARISA trial confirmed the effectiveness of ranolazine in symptomatic patients with SA who were also on background therapy with maximally-tolerated doses of first-line antianginal therapies [37].
The ERICA (Efficacy of Ranolazine in Chronic Angina) trial compared treatment with ranolazine with placebo in 565 patients persistently symptomatic (>3 angina attacks per week) despite a maximal dose of amlodipine. Patients were randomised to receive ranolazine SR 1000 mg twice daily or placebo for 6 weeks. The primary endpoints of this study were: decrease in angina attacks (p = 0.02) and concurrently in nitrate consumption (p= 0.01) as compared with placebo [38].
The MERLIN (Metabolic Efficiency with Ranolazine for Less Ischaemia in Non-ST-Elevation Acute Coronary Syndrome) TIMI-36 trial was designed to prove the efficacy of ranolazine in unstable angina / non-ST-elevation acute coronary syndrome (NSTEMI-ACS). In contrast to the CARISA and ERICA trials, this study was designed to assess clinical outcomes over 1 year. The patients (n = 6,560) were randomised, within 48 hours of angina onset, to ranolazine (intravenously for the first 96 hours and then orally 1000 mg SR twice daily) or placebo treatment. Treatment was continued for a median of 348 days, in addition to standard antiangina therapy. This study did not demonstrate a beneficial effect of ranolizine on its primary composite endpoint of cardiovascular death, myocardial infarction or recurrent ischaemia. However, the incidence of recurrent ischaemia was significantly lower in the ranolazine group (p= 0.03) [30]. Subsequent post-hoc analyses confirmed these results. In a subgroup of patients with a previous history of chronic angina, the primary endpoints were observed less frequently in the ranolazine-treated group (hazard ratio [HR] 0.86, confidence interval [CI] 0.75–0.97; p = 0.01) compared with placebo. This effect was placed in relation to the reduction in recurrent ischaemia (HR 0.78, CI 0.67–0.91; p = 0.002). Moreover, in the ranolazine-treated group, worsening angina (HR 0.77, CI 0.59–1.00; p = 0.04) and the intensification of antianginal therapy (HR 0.77, CI 64–0.92; p = 0.005) were also reduced. In addition, treadmill exercise tests at 8 months (total exercise duration, mean time to angina onset and ≥1 mm ST-segment depression; p <0.01) were also improved [31] in the ranolazine group as compared with placebo. Furthermore, only in the subgroup of patients with previous angina, ranolazine improved health status, according to various scores [39]. In a separate analysis by gender of the MERLIN-TIMI 36 trial data, ranolazine-treated women showed a more significant reduction of recurrent ischaemia (p = 0.024), as well as fewer angina attacks (p <0.001) and reduced requests for antianginal therapy intensification (p = 0.003) [34].
In addition, treatment with ranolazine reduced the risk for primary composite endpoints (at 30 days and 1 year) in the subgroup of patients with brain natriuretic peptide (BNP) level at baseline >80 pg/ml compared with those having BNP ≤80 pg/ml [33]. Finally, a post-hoc analysis in the subgroup of diabetic patients showed that treatment with ranolazine significantly improved glycated haemaoglobin (HbA1c) as well as recurrent ischaemia [32].
The Type 2 Diabetes Evaluation of Ranolazine in Subjects with Chronic Stable Angina (TERISA) trial is the most recent study investigating the clinical efficacy of ranolazine treatment. A total of 949 patients with diabetes, coronary artery disease and SA treated with two antianginal drugs were included in the study. After a single-blind, 4-week placebo run-in, patients were randomised to 8 weeks of treatment with ranolazine SR 1000 mg twice daily or placebo. The primary outcome was the average weekly number of angina episodes recorded by an electronic diary. The ranolazine-treated arm had a significantly lower weekly angina frequency (p= 0.008) and sublingual nitroglycerin use (p = 0.003) [40] as compared with placebo.
| Table 1:Ranolazine SR efficacy in MERLIN-TIMI 36 trial and in the subsequent post-hoc analyses. | ||||||||||
| Author | Year | Number of patients | Population | Treatment groups | Primary endpoint | CV death | Recurrent ischaemia | |||
| (%pz) | HR (95% CI) | (%pz) | HR (95% CI) | (%pz) | HR (95% CI) | |||||
| Morrow et al. [30] | 2007 | 6,560 | MERLIN-TIMI 36 trial | Ranolazine vs placebo | 21.8% 23.5% | 0.92 (0.83–1.02) p = 0.98 | 4.4% 4.5% | 1.00 (0.79–1.25) p = 0.98 | 13.9% 16.1% | 0.87 (0.76–0.99) p = 0.03 |
| Wilson et al. [31] | 2009 | 3,565 | Prior chronic angina in MERLIN-TIMI 36 trial | Ranolazine vs placebo | 25.2% 29.4% | 0.86 (0.75–0.97) p = 0.017 | 11.9% 12.5% | 0.97 (0.80–1.16) p = 0.71 | 16.5% 21.1% | 0.78 (0.67–0.91) p = 0.002 |
| Morrow et al. [32] | 2009 | 2,220 | Diabetic population in MERLIN-TIMI 36 trial | Ranolazine vs placebo | – – | 1.09 (0.86–1.38) p = 0.46 | – – | 0.76 (0.41–1.39) p = 0.37 | 15.1% 19.2% | 0.75 (0.61–0.93) p = 0.008 |
| Morrow et al. [33] | 2010 | 1,935 | BNP >80 pg/ml in MERLIN-TIMI 36 trial | Ranolazine vs placebo | 23% 29% | 0.79 (0.66–0.94) p <0.05 | 7.1% 8.9% | 0.83 (0.66–1.05) p >0.05 | 14.3% 18% | 0.78 (0.62–0.98) p <0.05 |
| Mega et al. [34] | 2010 | 2,291 | Women in MERLIN-TIMI 36 trial | Women vs men | 23.9% 22.1% | 0.83 (0.10–0.99) p <0.05 | 5.3% 4% | 0.97 (0.68–1.39) p >0.05 | 15.7% 14.6% | 0.71 (0.57–0.88) p = 0.002 |
| Primary endpoint: composite endpoint of cardiovascular death, myocardial infarction or recurrent ischaemia. %pz = incidence; BNP: brain natriuretic peptide; CI = confidence interval; CV = cardiovascular; HR: hazard ratio | ||||||||||
The management of diastolic dysfunction represents one of the most serious challenges for the cardiologists. Treatment options are limited by lack of knowledge of the mechanisms underlying the prolonged relaxation of the myocardium. Among several potential mechanisms already identified, increased Ca++ current [23] and oxidative stress [19] might play a relevant role in slowing ventricular relaxation of the failing heart. Since both mechanisms are coupled with an increased INa, (which is abnormally elevated in heart failure), a pathophysiological rationale exists for the investigation of ranolazine for treating diastolic dysfunction and failure. In preclinical studies, ranolazine improved diastolic performance as monotherapy [12, 41] or in association with metoprolol [42]. Moreover, these effects were achieved without inducing a negative inotropic effect. In addition, Lovelock and coworkers recently reported that ranolazine might also have a direct effect on the contractile apparatus through the modulation of myofilament Ca++ sensitivity [43]. Twenty years ago, Hayashida and coworkers showed an improvement of diastolic function in human noninfarcted ischaemic hearts [44]. Similarly, two other studies showed the beneficial effects of ranolazine in improved left ventricular diastolic dysfunction [22] and dyssynchrony [45]. Based on these findings, the Ranolazine in Diastolic Heart Failure (RALI-DHF) trial (NCT01163734) was designed as a prospective, single-centre, randomised, double-blind, placebo-controlled proof-of-concept study, in order to investigate the effectiveness of ranolazine in improving diastolic dysfunction in patients with heart failure with preserved ejection fraction. Twenty patients were randomised to receive ranolazine or placebo (12 patients receiving ranolazine vs 8 patients treated with placebo). The treatment schedule consisted of an intravenous infusion of the study drug or placebo for 24 hours, followed by oral treatment for a total of 14 days [46]. Treatment with ranolazine improved haemodynamic measurements (left ventricular end-diastolic pressure and pulmonary capillary wedge pressure), but relaxation parameters remained unaltered as compared with the placebo group [47]. Therefore, the RALI-DHF study failed to bridge the gap between evidence from basic studies of ranolazine and human treatment.
Ranolazine appears to have potential effects on myocardial conduction activity and related metabolic pathways. The increase in late INa might directly affect myocyte electrophysiology, lengthening the action potential and hence increasing transmural dispersion of repolarisation and the QT interval [48]. At the same time, the induction of intracellular Ca++ overload can give rise to a delayed after-depolarisation [49]. These abnormalities might predispose to the onset of ventricular arrhythmias, especially “torsades de pointes”. Ranolazine might reduce the transmural and temporal dispersion of repolarisation, which are proarrhythmic triggers [50]. These effects were shown in several animal models, especially in suppressing arrhythmogenic potential in long QT3 syndrome (where a mutation in the Na+ channel induces an INa) [51–53] and also in a pilot study in human beings [54]. In the framework of the MERLIN-TIMI 36 trial, the potential antiarrhythmic effects of ranolazine were shown for the first time (although only as a safety and not as a primary endpoint). A reduced incidence of ventricular/supraventricular tachycardia (p<0.001) and, especially, atrial fibrillation (p= 0.01) were observed in the ranolazine groups as compared with placebo [55]. The therapeutic benefits of the blockade of INa peak in the setting of atrial fibrillation were widely recognised [56]. This action is largely due to a rate-dependent reduction of excitability, prolongation of the effective refractory period (secondary to the development of postrepolarization refractoriness), and blockade of conduction in a critical part of the reentrant circuit. Reduction of INa peak can also significantly decrease intracellular [Na] and, thus, Ca++ overload-mediated triggered activity. Recently, Milber and coworkers reported that ranolazine-related Na+-channel blockade also suppresses stretch-induced atrial fibrillation by increasing the interatrial conduction time and atrial postrepolarisation refractoriness [57].
Accordingly, the impact of ranolazine in prevention and treatment of atrial fibrillation was investigated as a primary endpoint in clinical trials that showed ranolazine was effective as maintainance therapy [58] as well as a “pill-in-the-pocket” strategy [59]. Ranolazine has been shown to favour successfully electrical cardioversion [60]. A clinical trial investigating this primary endpoint was recently finished and results will be available soon, potentially clarifying this issue (Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion [RAFFAELLO]) [61].
Importantly, class-III antiarrhythmic drugs were not superior to ranolazine in preventing atrial fibrillation in patients after coronary artery bypass surgery [62] or coronary revascularisation surgery [63]. Amiodarone plus ranolazine resulted in a significantly higher conversion rate of new-onset atrial fibrillation than amiodarone alone in both experimental models [64] and in humans [65]. Finally, a sponsored trial to assess a ranolazine-dronedarone combination versus dronedarone alone in paroxysmal atrial fibrillation was recently approved [66]. The results of this study will be available in the near future.
As was the case with treatments for ACS [67, 68], the development of new drugs might provide additional therapeutic options in the treatment of SA. Compared with the other drugs, ranolazine provides an anti-ischaemic effect without haemodynamic changes such as bradycardia or hypotension. This allowed the safe use of ranolazine in addition to other drug classes, improving control of anginal symptoms and representing a useful option in the presence of several comorbidities such as diabetes. Treatment with ranolazine was shown to be generally well tolerated, although it remains contraindicated in severe renal failure or moderate to severe hepatic impairment, and also has potential drug interactions through CYP450 enzymes [3]. The strength of the data reported from different clinical trials and the good pharmacological profile might suggest a potential extension of the use of ranolazine for the treatment of SA. Furthermore, it has been reported that treatment with ranolazine might be cost effective, since a better outcome brings lower costs of care [69]. On the other hand, additional data are needed potentially to recommend the use of ranolazine in the treatment of arrhythmias and heart failure. If the preliminary data discussed above are confirmed, we might have a single drug (ranolazine) that is effective on different aspects (both electrical and mechanical dysfunction) in SA, potentially improving patient quality of life and healthcare costs.
1 Fihn SD, Gardin JM, Abrams J. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
2 Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16.
3 European Medicine Agency. Ranexa (ranolazine): EU sumary of product characteristics. http://wwwemaeuropaeu/ema/indexjsp?curl=pages/medicines/human/medicines/000805/human_med_001009jsp&mid=WC0b01ac058001d124. 2012.
4 Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999;84(12):1401–6.
5 Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71(5):1231–41.
6 Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 Suppl 4(iv6–iv14.
7 Eigel BN, Hadley RW. Antisense inhibition of Na+/Ca2+ exchange during anoxia/reoxygenation in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2001;281(5):H2184–90.
8 Schafer C, Ladilov Y, Inserte J, Schafer M, Haffner S, Garcia-Dorado D, et al. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc Res. 2001;51(2):241–50.
9 Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kogler H, Moller K, et al. Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na(+)-Ca(2+) exchange. Circ Res. 2002;90(9):988–95.
10 Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther. 2007;321(1):213–20.
11 Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110(8):904–10.
12 Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17 Suppl :(S169–S77.
13 Matsumura H, Hara A, Hashizume H, Maruyama K, Abiko Y. Protective effects of ranolazine, a novel anti-ischemic drug, on the hydrogen peroxide-induced derangements in isolated, perfused rat heart: comparison with dichloroacetate. Jpn J Pharmacol. 1998;77(1):31–9.
14 Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318(1):214–22.
15 Zhang XQ, Yamada S, Barry WH. Ranolazine inhibits an oxidative stress-induced increase in myocyte sodium and calcium loading during simulated-demand ischemia. J Cardiovasc Pharmacol. 2008;51(5):443–9.
16 Maruyama K, Hara A, Hashizume H, Ushikubi F, Abiko Y. Ranolazine attenuates palmitoyl-L-carnitine-induced mechanical and metabolic derangement in the isolated, perfused rat heart. J Pharm Pharmacol. 2000;52(6):709–15.
17 Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91(4):278–80.
18 Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45(1):32–43.
19 Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44(2):192–9.
20 Fraser H, Belardinelli L, Wang L. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41(6):1031–8.
21 Shryock JC, Belardinelli L. Inhibition of late sodium current to reduce electrical and mechanical dysfunction of ischaemic myocardium. Br J Pharmacol. 2008;153(6):1128–32.
22 Figueredo VM, Pressman GS, Romero-Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther. 2011;16(2):168–72.
23 Hale SL, Leeka JA, Kloner RA. Improved left ventricular function and reduced necrosis after myocardial ischemia/reperfusion in rabbits treated with ranolazine, an inhibitor of the late sodium channel. J Pharmacol Exp Ther. 2006;318(1):418–23.
24 Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AE. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. JACC Cardiovasc Imaging. 2009;2(11):1301–9.
25 Venkataraman R, Aljaroudi W, Belardinelli L, Heo J, Iskandrian AE. The effect of ranolazine on the vasodilator-induced myocardial perfusion abnormality. J Nucl Cardiol. 2011;18(3):456–62.
26 Jain D, Dasgupta P, Hughes LO, Lahiri A, Raftery EB. Ranolazine (RS-43285): a preliminary study of a new anti-anginal agent with selective effect on ischaemic myocardium. Eur J Clin Pharmacol. 1990;38(2):111–4.
27 Thadani U, Ezekowitz M, Fenney L, Chiang YK. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Ranolazine Study Group. Circulation. 1994;90(2):726–34.
28 Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am J Cardiol. 1999;84(1):46–50.
29 Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95(3):311–6.
30 Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775–83.
31 Wilson SR, Scirica BM, Braunwald E, Murphy SA, Karwatowska-Prokopczuk E, Buros JL, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol. 2009;53(17):1510–6.
32 Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032–9.
33 Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol. 2010;55(12):1189–96.
34 Mega JL, Hochman JS, Scirica BM, Murphy SA, Sloan S, McCabe CH, et al. Clinical features and outcomes of women with unstable ischemic heart disease: observations from metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36). Circulation. 2010;121(16):1809–17.
35 Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43(8):1375–82.
36 Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309–16.
37 Sendon JL, Lee S, Cheng ML, Ben-Yehuda O. Effects of ranolazine on exercise tolerance and angina frequency in patients with severe chronic angina receiving maximally-tolerated background therapy: analysis from the Combination Assessment of Ranolazine In Stable Angina (CARISA) randomized trial. Eur J Prev Cardiol. 2012;19(5):952–9.
38 Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48(3):566–75.
39 Arnold SV, Morrow DA, Wang K, Lei Y, Mahoney EM, Scirica BM, et al. Effects of ranolazine on disease-specific health status and quality of life among patients with acute coronary syndromes: results from the MERLIN-TIMI 36 randomized trial. Circ Cardiovasc Qual Outcomes. 2008;1(2):107–15.
40 Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, et al. Evaluation of Ranolazine in Patients with Type 2 Diabetes Mellitus and Chronic Stable Angina. Results from the TERISA randomized clinical trial. J Am Coll Cardiol. 2013. doi: 10.1016/j.jacc.2013.02.011
41 Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, et al. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail. 2002;8(6):416–22.
42 Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. American journal of physiology Heart and circulatory physiology. 2008;295(5):H2149–55.
43 Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circulation research. 2012;110(6):841–50.
44 Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8(5):741–7.
45 Venkataraman R, Chen J, Garcia EV, Belardinelli L, Hage FG, Heo J, et al. Effect of ranolazine on left ventricular dyssynchrony in patients with coronary artery disease. Am J Cardiol. 2012;110(10):1440–5.
46 Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI-DHF study. Clin Cardiol. 2011;34(7):426–32.
47 Lars S. Maier MBL, MD; Ewa Karwatowska-Prokopczuk, MD, PhD; Luiz Belardinelli, MD; Stella Lee, MS; Julia Sander, MS; Christian Lang, MS; Rolf Wachter, MD; Frank Edelmann, MD; Gerd Hasenfuss, MD; Claudius Jacobshagen, MD. RAnoLazIne for the Treatment of Diastolic Heart Failure in Patients With Preserved Ejection Fraction. JACC: Heart Failure. 2013;1(2):115–22.
48 Antzelevitch C, Belardinelli L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J Cardiovasc Electrophysiol. 2006;17 Suppl 1:S79–S85.
49 Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88(4):1491–545.
50 Dhalla AK, Wang WQ, Dow J, Shryock JC, Belardinelli L, Bhandari A, et al. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2009;297(5):H1923–9.
51 Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310(2):599–605.
52 Antoons G, Oros A, Beekman JD, Engelen MA, Houtman MJ, Belardinelli L, et al. Late na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55(8):801–9.
53 Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, Owen LJ, et al. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm. 2012;9(6):953–60.
54 Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19(12):1289–93.
55 Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116(15):1647–52.
56 Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116(13):1449–57.
57 Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8(3):175–81.
58 Murdock DK, Kersten M, Kaliebe J, Larrain G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible "pill in the pocket" approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9(5):260–7.
59 Murdock DK, Kaliebe J, Larrain G. The use of ranolazine to facilitate electrical cardioversion in cardioversion-resistant patients: a case series. Pacing Clin Electrophysiol. 2012;35(3):302–7.
60 Menarini Group. Ranolazinein atrial fibrillation following an electricaL cardioversion (RAFFAELLO) [clinicaltrials.gov identifier NCT01534962]. US National Institute of Health. online http://clinicaltrialsgov. Accessed 19 Nov 2012.
61 Miles RH, Passman R, Murdock DK. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2011;108(5):673–6.
62 Tagarakis GI, Aidonidis I, Daskalopoulou SS, Simopoulos V, Liouras V, Daskalopoulos ME, et al. Effect of Ranolazine in Preventing Postoperative Atrial Fibrillation in Patients Undergoing Coronary Revascularization Surgery. Curr Vasc Pharmacol. 2012. PMID: 23140547
63 Frommeyer G, Milberg P, Uphaus T, Kaiser D, Kaese S, Breithardt G, et al. Antiarrhythmic effect of ranolazine in combination with class-III drugs in an experimental whole heart model of atrial fibrillation. Cardiovasc Ther. 2013. doi: 10.1111/1755-5922.12035.
64 Fragakis N, Koskinas KC, Katritsis DG, Pagourelias ED, Zografos T, Geleris P. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent-onset atrial fibrillation. Am J Cardiol. 2012;110(5):673–7.
65 Gilead Sciences. A study to evaluate the effect of ranolazine and dronedarone when given alone and in combination in patients with paroxysmal atrial fibrillation (HARMONY) [clinicalTrials.gov identifier NCT01522651]. US National Institute of Health, ClinicalTrials.gov (online). 2012. http://clinicaltrialsgov. Accessed 19 Nov 2012.
66 Kossovsky M, Keller PF, Mach F, Gaspoz JM. Fondaparinux versus enoxaprin in the management of acute coronary syndromes in Switzerland: A cost comparison analysis. Swiss Med Wkly. 2012;142(w13536.
67 Braunersreuther V, Mach F, Montecucco F. Statins and stent thrombosis. Swiss Med Wkly. 2012;142(w13525.
68 Phelps CE, Buysman EK, Gomez Rey G. Costs and clinical outcomes associated with use of ranolazine for treatment of angina. Clin Ther. 2012;34(6):1395–407 e4.
Funding / potential competing interests: This work was supported by Swiss National Science Foundation Grants to Dr F. Montecucco (#32003B_134963/1) and to Prof. F. Mach (#310030_118245). No other potential conflict of interest relevant to this article was reported.