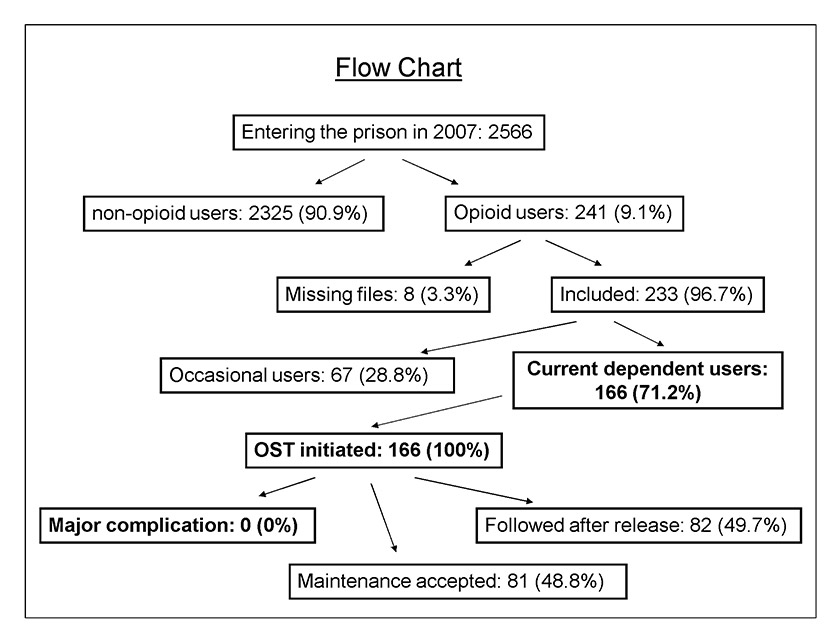
Figure 1
Flow chart of study population within the prison setting in 2007.
OST = opioid substitution treatment
DOI: https://doi.org/10.4414/smw.2013.13898
Detainees are a vulnerable population with high morbidity [1, 2]. They frequently have poor medical follow-up, given their limited previous access to healthcare as a result of educational, social and economic disadvantages [1, 3]. Prison is a significant opportunity to address the health needs of vulnerable groups. In particular, prison health services aim to reduce inequalities by providing primary care services that are similar in range and quality to those available in the community [4].
The prevalence of drug use is particularly high among detainees: in Europe, lifetime prevalence of drug use in this group ranges from 29% to 86% [5]. As many as 69% of detainees use illicit drugs regularly, and about 60% of drug users continue to use drugs in prison [5, 6]. In the US, over 50% of detainees have a history of substance abuse, actively abusing in the previous year, and about 20% of them have used intravenous (IV) drugs [7, 8]. A quarter to a third of US heroin users encounter the US justice system at some point during their lifetime [9]. In Switzerland, 20% to 50% of detainees have a history of substance use, with up to 60% of them reporting having also used drugs during incarceration [10, 11].
Infectious diseases linked to drug use are a problem in prison. Prevalence of hepatitis C infection ranges between 12% and 31% in the prison setting, and is two- to six-fold higher than in the general population [12]. Human immunodeficiency virus (HIV) prevalence among prisoners is reported to be below 5% in most European countries, and the US. However, in some countries, such as Spain, it is as high as 15% [13]. In Swiss prisons, HIV prevalence is estimated to be 1% [11].
For opioid dependence, opioid substitution treatment (OST) has proven to be a beneficial treatment among detainees, offering a 14-fold risk reduction in overdose mortality [14, 15] after release from prison. The postrelease period is a particularly vulnerable transition time for prisoners – it carries a mortality rate that is 20 times higher than that of the general population [16, 17]. Furthermore, OST decreases IV use in jail and consequently lowers the risk of infectious disease transmission [18], and the propensity towards criminal behaviour after release [19].
Given its proven use and validity, OST treatment access in detention is necessary and required in order to abide by the principles of fundamental rights [20]. However, in-prison OST is only available in 41 countries, even though treatment is available in 77 countries on a community basis (outside prison). This disparity violates the principle of equivalence of care under international recommendations, meaning that medical care in prison should be equivalent to the care provided to the general population in the same region [21, 22]. Because of the risk of opioid overdose and death at time of prisoner release (especially in cases where prisoners have undergone forced detoxification during their prison stay) [16, 17], official guidelines recommend that all opioid dependent persons have access to OST and that complete tapering be avoided during imprisonment. Follow-up after prison release should be scheduled; if OST is no longer indicated or logistically impossible, tapering of OST should be initiated in preparation for release.
OST has been available since 1990 in Switzerland’s largest remand prison, located in Geneva. Before that time, methadone was available, but only for those who were already in substitution treatment or who were in acute withdrawal. The Geneva OST programme is based on the 2007 recommendations set forth by the Swiss Society of Addiction Medicine (SSAM) and revalidated in 2010, and the World Health Organisation [23, 24].
The aims of this study were to provide a detailed description of OST in our prison in adherence to accepted recommendations, and to confirm that such treatment is safe and feasible in the pretrial term. This study contributes to the body of literature on drug treatment in prison settings, as few studies have formally described such programmes.
This retrospective cross-sectional study assessed sociodemographic characteristics, substance use diagnosis, substitution treatment and other important issues concerning drug-addicted detainees entering the facility between January 1st and December 31st in 2007. The sole inclusion criterion was to be a current opioid user. Patient cases with missing data were excluded. The research protocol was approved by the Ethics Committee of the Geneva University Hospitals (number 07-200R).
In 2007, 115 Swiss institutions housed 5,715 prisoners, of whom 29% were in pretrial detention, yielding an average of 76 prisoners per 100,000 residents – one of the lowest rates in the world [5]. Geneva, however, topped the national statistics with an average of 200 inmates per 100,000 residents. According to EMCCDA 2010, the majority of detainees in Swiss jails of this type are male (93.6%) and of foreign origin (81.4%) [5]. Of note, the canton of Geneva has the highest proportion of foreigners (38.3%) among its general resident population [25].
This study took place in Switzerland’s largest remand prison, situated in Geneva and built in 1977. Initially intended and built for 270 prisoners, the prison is now overcrowded. It had a mean occupation rate of 169% in 2007. At the time of the study, up to 20% of detainees were sentenced prisoners waiting to be transferred to another institution. The medical prison unit connected to the Geneva University Hospitals functions independently of the prison administration. All detainees admitted to the facility have a health assessment by primary healthcare nurses within the first eight hours of their admission. It is also an introduction to the facility’s explanation of access to drug treatment (detoxification, OST) and specific harm reduction measures like condom distribution, and needle and syringe exchange.
This analysis was nested within a general health study [11] of 2,566 individuals entering the detention facility during 2007; 1,510 of them (68.8%) had a primary care consultation during their stay in addition to the initial health assessment by the nurse upon entrance, whereas 685 (31.2%) only had the initial screening by the nurse. Among this population, 115 different nationalities were represented and 92.8% of patients with at least one medical consultation were of foreign origin. Morbidity of detainees was significant, with 58% of them having a somatic disorder and 32.6%having a psychiatric diagnosis. Furthermore, 18.3% needed health care for acute injury and 8% were exposed to violence at arrest.
The present observational study was composed of 241 opioid-using inmates arriving at the facility (233 complete cases). The medical screening by the nurses was used to identify current opioid users: patients reporting use in the last 30 days. OST was a consideration for patients based on this history, and prisoners were referred to the primary care physician to definitively establish if they were current dependent users, and thus eligible for OST. Inclusion into OST was based on the clinical judgment of the treating physician (from a small group of six physicians based in the prison), who had received intensive and regular training on prison medical care based on standardised tools and drug treatment curriculum in accordance with SSAM guidelines and the WHO [23, 24]. Table 1 summarises the salient points of our treatment programme, in light of these national and international recommendations.
Signs of drug withdrawal or written proof of current OST as confirmed by the physician were automatic indications for OST. Patients without symptoms or without written proof, but who reported treatment or drug use, took a urine drug screening to test for presence of opioids; if it was positive, they were eligible for OST.
The introduction dose was 30–40 mg (of methadone in the majority of cases), in accordance with the recommendation of the SSAM, depending on the severity of withdrawal signs. Those with an OST history and still taking OST at time of transfer could receive their previous dose at introduction with written proof. If for any reason a patient who was already on OST had been without treatment up until transfer to the prison setting, the last previous dose recorded was decreased by 20% per day without treatment, and used as the “new” starting dose to titrate up from.
From the nurse’s screening form, we identified other types of substance use. Cocaine use was also defined as reported in the last 30 days. Tobacco use was defined as at least one cigarette per day. The first three questions of the Alcohol Use Disorders Identification Test (AUDIT) [26] assessed for alcohol misuse, defined as excessive drinking, alcohol abuse or dependence. Screening for alcohol misuse was positive if the summed score for the first three questions was greater than or equal to four for women, and five for men. This shortened three-item AUDIT-C demonstrates good screening performance for alcohol use disorders and risky drinking, and is now considered a reliable alternative to the standard AUDIT score [27]. Regular use of cannabis or a benzodiazepine (more than once a week, without medical prescription) was recorded if the prisoner reported use during the last 30 days before admission.
Individual medical files were reviewed for data relevant to the current analysis. This information included: sociodemographic characteristics, history of opioid dependence diagnosis, route of opioid administration in the past, history and number of medical consultation visits during present prison stay (by general practitioner, psychiatrist, nurse or a psychologist), number of former stays in prison, history of opioid withdrawal signs or symptoms while in prison, history of a urine drug screen on arrival, documentation (written evidence) of current OST at arrival, whether the patient accepted OST, (substitution) medication dosing (tapering or maintenance of OST), medication used, medication dose upon prison departure (for the entire cohort, including those who stopped treatment while incarcerated), any history of skin abscess upon arrival or during current imprisonment, history of cardiotoxic medications, noted complications secondary to substitution, history of needing transfer to a hospital for higher level of care, and finally whether medical follow up was organised for the patient upon his/her prison departure.
Descriptive statistics were computed for patient characteristics and OST prescription. Statistical analyses were done with S-Plus 7.0 Enterprise Developer, and SPSS 15.0. Statistical significance was set for p-values less than or equal to 0.05.
| Table 1: Summary of the principles of opioid substitution treatment at Champ-Dollon in Geneva, following the Swiss Society of Addiction Medicine (SSAM) and the World Health Organization (WHO). | |||
| Treatment principle | SSAM | WHO | Geneva |
| Indication for OST | Opioid dependence | Same | Same |
| Preferred treatment option other than Methadone | Buprenorphine | Same | Same |
| Methadone start dose | 30 mg | 30 mg | 30–40 mg |
| Methadone dose augmentation | 5–10 mg per day | If dose is <60 mg, 5 mg every week; if >60 mg, augment by 10 mg every 3 days | 5–10 mg per day |
| Maintenance dose (mg) | 60–80 of methadone 8–12 of buprenorphine | 60–120 of methadone 8–24 of buprenorphine | Not applicable (we report mean dose upon departure) |
| Unlimited prescription duration | Promoted | Same | Same |
| Maintenance treatment until… | Stabilisation, continued abstinence | Same | Same |
| Continuity of substitution between community and prison | Promoted | Same | Same |
Among 2,566 detainees entering the prison in 2007, 241 (9.1%) were opioid users (fig. 1). Table 2 shows the baseline characteristics of the opioid users (n = 233 complete cases). Primary regions of origin were Western Europe (28.9%), North Africa and the Middle East (27.5%) and sub-Saharan Africa (20.1%). Other represented regions included Eastern Europe and South America. Among the opioid users, 95.4% were male with a mean age of 29.6 years (standard deviation [SD] 7.1), and 74.7% had a previous incarceration. Nearly 40% were IV drug users; other routes of administration included sniffing/smoking (63.1%). Almost all opioid users (94.8%) used another substance. In terms of care, they had an average of 7.7 (SD 10.4) consultations during detention. Most of these consultations were with general practitioners (4.3, SD 5.3), followed by nurses (2.0, SD 2.4) and psychiatrists (1.1, SD 4.7). Finally, 166 individuals (71.2% of 233 users) were confirmed to have current opioid dependence, and all of them received OST. Occasional users had no indication for substitution and received prevention messages, motivational intervention, and symptomatic treatment. Interestingly, 6 (6.5%) out of 93 IV drug users did not meet criteria for dependence as defined by the study. A total of 11 women (10% of the female population in prison) met criteria and received OST.

Figure 1
Flow chart of study population within the prison setting in 2007.
OST = opioid substitution treatment
Table 3 shows that withdrawal signs were used to establish most cases of dependence. The majority (80.3%) of all entering opioid users had not been receiving substitution treatment in the community. However no patients refused OST when offered by the physician in prison. Methadone was the prescribed substitution medication about 95% of patients with a mean dose of 41.7 mg (SD 29.1) on departure. Other prescribed therapies included buprenorphine (4.2%), tramadol and codeine (5.4%). Tramadol and codeine therapies were generally used for a shorter period of time, and for patients refusing methadone or buprenorphine. Among those individuals who received OST, 48.8% continued their treatment throughout the prison stay (table 3).
We present percentages of potentially harmful medication combinations among OST patients (table 3): 81.1% of these used some sort of tranquilising medication (benzodiazepines, antihistamines, chloral hydrate), 48.1% used antidepressants, and 24% took antipsychotics. Complications and serious adverse effects due to OST (including overdose), defined as need for transfer to a hospital, did not occur. There were no prisoner deaths in this institution in 2007.
Half of the patients enrolled in the OST programme received follow-up into community services after their release (49.7). Opioid users who did not receive OST (because lack of medical indication) had lower rates of follow-up (28.4%) after transition into the community.
| Table 2: Baseline characteristics of opioid users in Geneva pretrial prison setting in 2007. | |
| Characteristic | |
| Male (n = 233) | 95.3% |
| Age [years] (n = 241) | 29.6 (SD 7.1) |
| Previous incarceration (n = 233) | 74.7% |
| Origin( n = 241) | |
| Western Europe | 28.9% |
| North Africa and Middle East | 27.5% |
| Sub-Saharan Africa | 20.1% |
| Other | 23.5% |
| Length of stay less than 3 months (n = 233) | 78% |
| Opioid users with current OST (n = 233) | 19.7% |
| IV route of opiate administration (n = 230) | 39.9% |
| Another substance use( n = 233) | 94.8% |
| Cocaine | 70.8% |
| Tranquilisers | 63.5% |
| Alcohol | 55.4% |
| Cannabis | 44.2% |
| Skin abscess when arrested (n =233) | 1.7% |
| Mean consultations during detention (n = 241) | 7.7 (SD 10.4) |
| Opioid usage (n = 233) | |
| Opioid dependent | 71.2% |
| Occasional user | 21% |
| IV = intravenous; OST = opioid substitution treatment; SD = standard deviation | |
| Table 3: Information about course for opioid substitution treatment (OST) patients. | |
| OST course details | |
| Indication for OST (n = 166 ) | |
| Withdrawal signs | 51.9% |
| History and urinary drug screen | 20.2% |
| On maintenance already | 27.7% |
| Mean dosage of methadone, departure | 41.7 mg (SD 29.1) |
| Maintenance of OST until end of stay (n = 165) | 48.8% |
| Other treatment prescription (n = 166) | |
| Sedatives | 81.1% |
| Antidepressants | 48.1% |
| Antipsychotics | 24% |
| Antibiotics | 12% |
| Prokinetics | 0.4% |
| SD = standard deviation | |
This analysis identified 9.1% of detainees entering the study prison in 2007 as opioid users, of whom 71% met criteria for current dependence and thus had an indication for OST. OST programme in Geneva’s prison is a low threshold, easy-access programme without any barriers related to immigration or health insurance status. The guaranteed accessibility is confirmed by the fact that 100% of patients who were current dependent users and had an indication for OST received this treatment. There was no documentation of treatment refusal among those who met OST criteria. These findings contrast with international findings showing that lower numbers of dependent patients receive OST in prisons, even when it is available [22, 28].
A total of 29% of opioid users had no indication for OST and consequently didn’t receive OST either because they were occasional users (21.1%) and/or lacked OST criteria (7.7%) (no withdrawal signs, negative urine test or lack of proof for current OST). This finding highlights the need for healthcare professionals in prison to be trained in order to avoid inappropriate opioid prescription. Occasional users who had no indication for OST were provided with supportive and psychosocial therapy, and with interventions associated with the prevention of overdose at release [29].
This study also demonstrated that urine tests are not routinely needed to identify opioid use. In this study, only 20.2% needed a urine screen after considering (1) the clinical observation of withdrawals signs which confirmed dependence, and (2) that medical staff had systematic contacts in place with community OST centres in order to obtain written confirmation of which patients were receiving OST prior to incarceration. The majority of OST treatments were given to detainees without previous treatment, many of whom were foreigners without medical insurance or a residency permit. The fact that such excellent access to OST (without major complications) was achieved is a key message from this study.
Intravenous drug use was identified in 39.9% of all 233 opioid users – less than 5% of all 2,566 detainees entering the prison in 2007. This percentage is lower than what has been found in other studies (in Germany 13% of all detainees used IV drugs in one case study) [30]. This finding might be attributed to the high proportion of North African detainees in these prisons who rarely inject drugs. The high rates (91.7%) of concurrent substance use by current opioid drug users, mainly cocaine (70.8%), tranquilisers (63.1%) and alcohol (55.4%), correspond to study findings in other prison settings [31]. A key next step will be to investigate optimal treatment for this group of poly-drug users.
Medical care in prison must abide by the principle of equivalence of care, which means that treatment provided in prison should be equal to what is provided in the community surrounding the prison [4]. The primary OST substance prescribed in the Geneva prison was methadone (95.8%), which is also the preferred OST medication of choice in all of Switzerland (88.9%) [32]. Reasons for methadone selection are its low cost (about 0.3 Swiss francs per person per day, for 60 mg per day methadone) and its quick and simple method of delivery (oral administration). It is therefore easier to dispense in a controlled way (to avoid misuse), particularly in a jail setting. One previous study suggested that buprenorphine might be a better substance to use in prison because fewer people abandoned treatment while incarcerated, and 48% of these patients had follow-up after release [33]. However, one must note that unlike methadone, buprenorphine needs the presence of a nurse for 5 minutes to control absorption.
In our study, where mostly methadone was used, results showed that 48.8% of those who had an indication for OST desired to keep OST as a maintenance treatment, while the rest requested detoxification. From anecdotal experience, we find that the majority of those who chose to withdraw from opioid treatment did so with the intention of impressing their trial judge, who would deem detoxification as a sign of good will (a sign that the detainee wished to change his/her life). We thus conclude that alternative medical therapies would not change the long-term adherence with OST in our setting (given the noted behavioral motivations). Other possible reasons for the low rate of maintenance therapy include the patient’s fear of lifelong dependence on opioids, or the fear that OST could be a restriction to their transfer toward other desired settings. Clearly, we must do more to address patient fears and help them prioritise their health over any misperceptions of legal proceedings.
Psychoactive medications (that could potentially interact with methadone) were frequently coadministered, but this did not lead to any clinically relevant complication, simply defined “as requiring hospital transfer during the study period.” It should be noted that for the purposes of the study we did not abstract data for other adverse effects such as cognitive impairment and memory loss [34]. Thus, we did not have more detailed information about clinical sequelae secondary to medications.
When considering detainees who accepted maintenance OST, the mean dose of methadone upon departure (41.7 mg) was below the minimum international recommendations of 60 mg [21]. Based on our patient interaction experience in this setting, the lower dose may occur because inmates want to use their imprisonment as a sort of “drug rehabilitation time,” and thus request to decrease the dosage of treatment, which, as mentioned, we must continually address. Another potential reason that the dose was lower is that the short periods of stay influenced the final mean dose upon departure (less provider time with patient to increase the dose). Indeed, we did find that the dose was lower among those patients who were incarcerated for less than a week (27%).
Continuity-of-care and the organisation of follow up are important in prison as the detainee nears release [29]. Although it is encouraging that about half (49.7%) of patients with OST had a follow-up visit organised with a community centre after release, this needs improvement. Especially because, in Geneva, all ex-inmates are guaranteed access to an OST community centre, whatever their legal or health insurance status may be. However, we recognise that patients without health insurance are not able to extend this to long-term maintenance OST. The local OST community centre tapers OST over a few months postrelease if long-term OST is not financially feasible for the patient in the outpatient setting.
There are some limitations to this study. First, this was a retrospective analysis. However, data were systematically extracted from standardised files by one investigator who was a primary care physician and who had not been involved in the care of these patients. Second, the study took place in one single facility as described above and so there are limits to its generalisability and applicability to other settings and populations. Nevertheless, there are similarities between the sociodemographic profiles and substance use of detainees all over the world, which make these observations applicable. As an example, the high proportion of young males and vulnerable populations (low socioeconomic status, uninsured, immigrant status) corresponds to the demographics found in other Swiss settings and international jails [11, 35].
Our study is prone to some sources of bias: this includes the completeness of data in the detainees' individual medical files, and information bias on the patients' admission of drug use. One may question the reliability of the clinical observation of withdrawal signs when assigning patients to OST: false positive cases based on the clinical criterion and the issue of examiner-dependent differences. However, as stated, our prison providers consisted of a small group of six physicians who had all received the same standardised OST training and in a repeated fashion. If there were any doubts about a patient case, individuals in the group would consult each other to confirm diagnosis for OST need.
The strengths of the study include the large sample size, which included female inmates. Also, information was gathered on all inmates entering the detention facility over an entire year. Finally, this study was conducted in a pretrial setting, a setting that is under-described in the prison literature given the high turnover rate that characterizes the pretrial period – a barrier that not only affects the continuity of quality care for these patients, but also hinders the realisation of research projects in this important area.
Pretrial prisons are characterised by a high turnover of detainees, which complicates healthcare organisation, particularly for vulnerable patients such as those with opioid addiction. Thus, the need for adequate and unrestricted addiction treatment and harm reduction measures for addicts in prisons is clear [36]. This study describes diagnostic/treatment guidelines and patient characteristics for our OST setting. The OST programme was made possible by having well-trained general practitioners provide prescription, without restriction, to all current opioid dependent users meeting criteria, in accordance to accepted guidelines. By demonstrating that these programmes are feasible and safe, this study accelerates the development of future OST implementation in other detention centres.
1 Binswanger IA, Krueger PM, Steiner JF. Prevalence of chronic medical conditions among jail and prison inmates in the USA compared with the general population. J Epidemiol Community Health. 2009;63(11):912–9.
2 Fazel S, Baillargeon J. The health of prisoners. Lancet. 2011;377(9769):956–65.
3 Watson R, Stimpson A, Hostick T. Prison health care: a review of the literature. Int J Nurs Stud. 2004;41(2):119–28.
4 Council of Europe. Recommandation R (98) 6. 1998. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6CjZdvEkj
5 EMCDDA. Annual report on the state of the drugs problem in Europe. 2010 – 102 pp. 2010. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6CjZt2Esa
6 Bird SM, Rotily M. Les usagers de drogue dans le système judiciaire: les détenus. 2003. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6Cja4ZYzp
7 Greifinger R. Public health behind bars: from prisons to communities. New York: Springer; 2007. 576p.
8 Mumola CJ, Karberg JC. Drug Use and Dependence, State and Federal Prisoners, 2004. Washington, DC: Office of Justice Programs, Bureau of Justice Statistics. Dept of Justice publication NCJ 213530. 2006.
9 Rich JD, McKenzie M, Shield DC, Wolff FA, Key RG, et al. J. Linkage with methadone treatment upon release from incarceration: a promising opportunity. J Addict Dis. 2005,24(3):49–59.
10 Hausser D. PREVENTION DE LA TRANSMISSION DU VIH DANS LES PRISONS SUISSES. Analyse secondaire sur la base de la littérature disponible. Raisons de santé. 1999; (40). Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6Cja9WN2T
11 Wolff H, Sebo P, Haller DM, Eytan A, Niveau G, Bertrand B et al. Health problems among detainees in Switzerland: a study using the ICPC-2 classification. BMC Public Health. 2011;11:245.
12 Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48(5):1387–95.
13 Jürgens R, Ball A, Verster A. Interventions to reduce HIV transmission related to injecting drug use in prison. Lancet Infect Dis. 2009;9(1):57–66.
14 Huang YF, Kuo HS, Lew-Ting CY, Tian F, Yang CH, Tsai TI, et al. Mortality among a cohort of drug users after their release from prison: an evaluation of the effectiveness of a harm reduction program in Taiwan. Addiction. 2011;106(8):1437–45.
15 Brugal MT, Domingo-Salvany A, Puig R, Barrio G, García de Olalla P, de la Fuente L, et al. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and AIDS in a cohort of heroin users in Spain. Addiction. 2005;100(7):981–9.
16 Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, et al. Release from prison – a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–65.
17 Seaman SR, Brettle RP, Gore SM. Mortality from overdose among injecting drug users recently released from prison: database linkage study. BMJ. 1998;316(7129):426–8.
18 Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment, and post-release retention in treatment, on risk of re-incarceration. Addiction. 2012;107(2):372–80.
19 Teesson M, Ross J, Darke S, Lynskey M, Ali R, et al. One year outcomes for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). Drug Alcohol Depend. 2006;83(2):174–80.
20 Bruce RD, Schleifer RA. Ethical and Human Rights Imperatives to Ensure Medication-Assisted Treatment for Opioid Dependence in Prisons and Pre-trial Detention. Int J Drug Policy. 2008;19(1):17–23.
21 Kastelic A, Jörg P, Stöver H. Opioid Substitution Treatment in Custodial Settings A Practical Guide. WHO Europe, United Nations Office on Drugs and Crime. 2008. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6CjaR26JG http://www.webcitation.org/6CjZdvEkj
22 Harm Reduction International. The Global State of Harm Reduction 2012. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6Cjad49BY
23 Swiss Society of Addiction Medicine. Recommandations médicales pour les traitements basés sur la substitution des patients dépendants aux opioïdes. 2010.
24 Kastelic A, Pont J, Stover H. Opioid Substitution Treatment in Custodial Settings. A Practical Guide. WHO, Europe and United Nations Office on Drugs and Crimes. BIS-Verlag der Carl Ossietzky Universitat Oldenburg. 2007, 2008.
25 Office cantonal de la statistique de la République et Canton de Genève. Population résidante genevoise : + 2 278 personnes en 2007. January 2008. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6CjakRjsq
26 Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17.
27 Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29(5):844–54.
28 Larney S, Dolan K. A literature review of international implementation of opioid substitution treatment in prisons: equivalence of care? Eur Addict Res. 2009;15(2):107–12.
29 Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE, et al. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37(3):277–85.
30 Michels II, Stöver H, Gerlach R. Substitution treatment for opioid addicts in Germany. Harm Reduct J. 2007;4:5.
31 Maruschak LM. Medical problems of jail inmates. 2006. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6Cjaoq3mq
32 Der Forschungsverbund stationäre Suchttherapie act-info-FOS im Jahr 2005. Cited and archived Dec 7 2012. Available from: http://www.webcitation.org/6Cjb2FxYu
33 Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99(1-3):222–30.
34 Liebrenz M, Boesch L, Stohler R, Caflisch C. Agonist substitution – a treatment alternative for high-dose benzodiazepine-dependent patients? Addiction. 2010;105(11):1870–4.
35 Fazel S, Danesh J. Serious mental disorder in 23000 prisoners: a systematic review of 62 surveys. Lancet. 2002;359(9306):545–50.
36 Elger BS. Prison medicine, public health policy and ethics: the Geneva experience. Swiss Med Wkly. 2011;141:w13273
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.