The French Military influenza surveillance system (MISS): overview of epidemiological and virological results during four influenza seasons – 2008–2012
DOI: https://doi.org/10.4414/smw.2013.13848
Sandrine
Duron, Aurélie
Mayet, Françoise
Lienhard, Rachel
Haus-Cheymol, Catherine
Verret, Serge
Védy, Patrick
Le Guen, Laurence
Berbineau, Patrick
Brisou, Philippe
Dubrous, Eric
Garnotel, Jean-Baptiste
Meynard, Xavier
Deparis, Audrey
Mérens, Christine
Bigaillon, René
Migliani
Abstract
QUESTION UNDER STUDY: Influenza is a viral infection caused by a pathogen with considerable ability for genetic mutation, which is responsible for seasonal outbreaks as well as pandemics. This article presents the results of epidemiological and virological monitoring of four successive influenza outbreaks in the French armed forces, for the period 2008 to 2012.
METHODS: The main events monitored were acute respiratory infection (ARI). Weekly incidence rates were calculated by relating cases to the number of servicepersons monitored.
RESULTS: In continental France, the incidence rates for ARI and for medical consultation attributable to influenza were highest during the pandemic and decreased to reach their lowest values in 2010–2011 and 2011–2012. In terms of virological results, the 2008–2009 outbreak was mainly due to the A(H3N2) virus, while the 2009–2010 pandemic and the following season saw the emergence of the A(H1N1) pdm09 strain. The last season 2011–2012 was characterised by a predominant circulation of A(H3N2) viruses.
CONCLUSIONS: Despite some limitations, the MISS represents a good source of information about influenza in young people. Virological results are compatible with those reported by most other influenza surveillance networks, but could be improved by a better knowledge of the other respiratory viruses in circulation in the military community.
Introduction
Influenza is a viral infection caused by a pathogen with considerable ability for genetic mutation. Owing to this characteristic, outbreaks of variable intensity occur each year. In April 2009, a novel A(H1N1) virus emerged in Mexico and rapidly spread worldwide, leading to the declaration of a pandemic situation in June 2009 [1]. The global disease burden of the A(H1N1)pdm09 pandemic was greater than during the previous seasonal outbreaks, despite differences according to the country in the Americas and in Europe [2], and it is likely that it has been under-estimated [3].
This pandemic has highlighted the importance of epidemiological and virological influenza surveillance in order to follow the influenza situation and to respond in the most appropriate way. Several complementary networks perform this surveillance worldwide under the coordination of World Health Organisation (WHO) with the Global Influenza surveillance and response System (GISRS) [4, 5]. In Europe, influenza surveillance is performed by the European influenza surveillance network (EISN) coordinated by the European Centre for Disease Prevention and Control (ECDC). In France, influenza community surveillance, coordinated by the French national watchdog Institute (Institut de Veille Sanitaire or InVS), is integrated into the EISN and involves two complementary systems: the Sentinelles network and the GROG (Groupes régionaux d’Observation de la Grippe) network. These two networks have their own specificities and the main difference between them is that the GROG network performs epidemiological and virological surveillance whereas the Sentinelles network does not perform biological sampling. As influenza can disseminate rapidly among the military due to their living conditions in confined settings, with potential detrimental consequences [6, 7], the French armed forces take part in the national and international surveillance of influenza with the Military influenza surveillance system (MISS) (SMOG in French) which is incorporated in the GROG network since 1997. The main aims of the MISS are early detection of the occurrence of influenza outbreaks and determination of circulating viral strains, to detect any variability in those strains.
The purpose of this article is to present the results of four seasons of influenza surveillance by the MISS, from 2008 to 2012. This period is particularly interesting as the 2009–2010 season saw the A(H1N1)pdm09 pandemic, and this makes it possible to evidence the evolution of the different indicators monitored by the MISS before, during and after the pandemic.
In addition, from a virological point of view, it was useful to describe which influenza viruses circulated through the period especially after the emergence of the pandemic strain.
Methods
The MISS performs seasonal epidemiological and virological surveillance of influenza on active-duty military personnel. This network relies on 30 out of 237 medical units spread across continental France in order to cover the whole territory, and a network of seven biology departments in military hospitals. MISS is activated each year from the end of September to the middle of April. During the 2009 pandemic, the activation period was longer as the surveillance was reactivated in May 2009 until April 2010 due to the emergence of the A(H1N1)pdm09 strain.
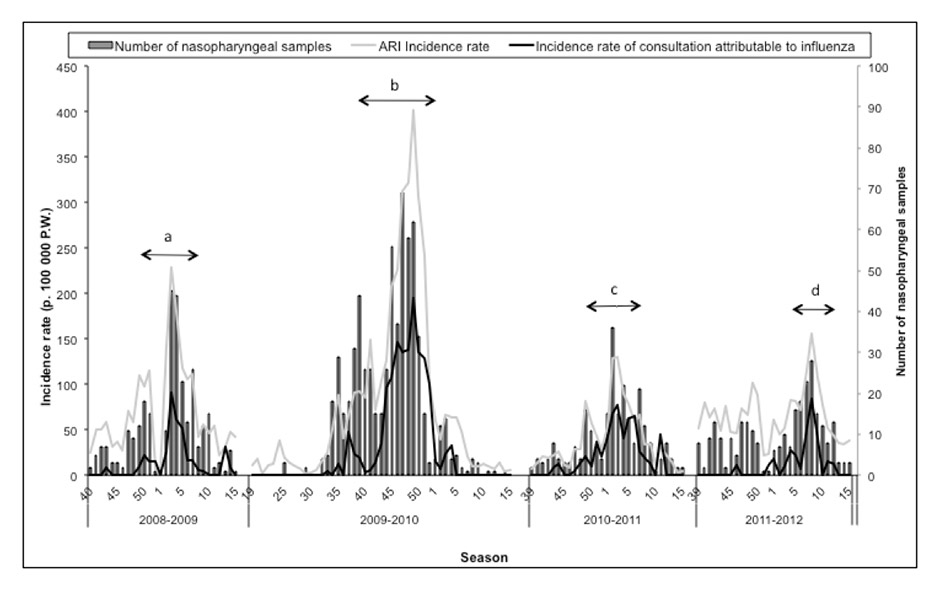
Figure 1
Incidence rate of acute respiratory infection (ARI) and medical consultation attributable to influenza, and number of nasopharyngeal samples taken , MISS network – influenza seasons 2008-2009 to 2011-2012.
a = (ARI epidemic period for 2008-2009 season) : weeks 50-2008 to 7-2009; b = (ARI epidemic period for 2009-2010 season) : weeks 38-2009 to 51-2009; c = (ARI epidemic period for 2010-2011 season) : weeks 48-2010 to 7-2011; d = (ARI epidemic period for 2011-2012 season) : weeks 4-2012 to 8-2012
MISS monitored several indicators including the two main indicators defined below:
– acute respiratory infections (ARI) defined by acute catarrh of aero-respiratory system, with oral temperature over 38.5 °C and cough;
– laboratory-confirmed influenza (immunochromatography and/or one step real-time reverse transcriptase polymerase chain reaction (rRT-PCR) and/or culture) on a nasopharyngeal swab.
Epidemiological surveillance
General practitioners (GPs) who participated in the MISS collected data, as described above, on a weekly basis. In addition, for each nasopharyngeal sample taken, a clinical form was completed and sent with the sample to one of the military hospital laboratories, depending on the localisation of the military medical unit. These forms enabled description of the characteristics of patients in terms of gender, age, medical and influenza vaccination histories, and symptoms. All these forms were collected and analysed by the Military centre for epidemiology and public health (Centre d’épidémiologie et de santé publique des armées or CESPA).
Virological surveillance
Virological surveillance relied on nasopharyngeal swabs taken by military GPs in every MISS unit. It was asked of the MISS units to take at least one swab per week. All the swabs taken were analysed by the military hospital laboratories. The A(H1N1)pdm09 pandemic represented a turning point in the virological diagnosis strategy. Indeed, before 2009 the diagnosis strategy relied on immunological tests (immunofluorescence or ELISA), followed by viral culture and one step real-time reverse transcriptase PCR (RT-PCR) for confirmation. From 2009, the biology departments taking part to the MISS used RT-PCR directly, according to WHO validated protocols or commercial validated kits. Positive samples were then sent to the National reference centres (NCR) in order to determine the viral subtype.
Statistical analysis
The ARI epidemic period was determined using the following GROG criteria (8). A week was considered as epidemic if:
– several influenza viruses were isolated in different geographical areas,
– positivity rate for nasopharyngeal swabs was at least 10%.
– an increase in two epidemiological indicators by more than 20% (as compared to the mean value for the first four weeks of the season) for two consecutive weeks.
The ARI incidence rates were obtained dividing the number of cases by the weekly number of military under surveillance (person-week [PW] rates). The incidence rate of medical consultations that could be attributed to influenza was estimated by multiplying the proportion of positive samples among all the nasopharyngeal samples by the ARI incidence rate. 95% confidence intervals of these incidence rates were estimated using Poisson regression.
Results
Epidemiological surveillance
The mean number of military under surveillance by the MISS network was 43,940, amounting to 12.9% of the military workforce in continental France (n = 339,629).
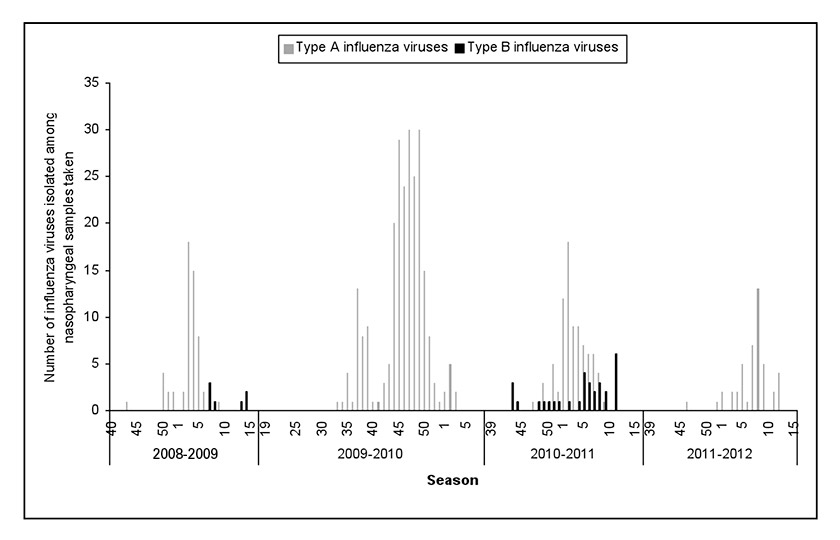
Figure 2
Number of type A and B influenza viruses isolated by MISS network – influenza seasons 2008‒2009 to 2011‒2012.
In the four influenza seasons studied, the ARI epidemic periods began mainly at the end of December and lasted 7.6 weeks on average, except for the 2009 pandemic, where the ARI epidemic period began earlier (October) and lasted longer (14 weeks) (table 1 and fig. 1).
Through the four seasons, a total of 1,472 samples were taken, among which 482 samples were positive for influenza viruses (56 [18.2%] in 2008–2009, 262 [40.9%] in 2009–2010, 119 [47.4%] in 2010–2011 and 45 [16.4%] in 2011–2012). The highest positivity rate was observed during the post-pandemic season 2010–2011 (p = 10-3) (table 2).
The highest incidence rates for ARI and medical consultations attributable to influenza were observed during the pandemic. A decrease of the incidence rates was then observed in 2010–2011 and 2011–2012, rates reached lower levels than in 2008–2009 (fig. 1 and table 1). However, while the ARI incidence rate was the lowest in 2010–2011, this season was characterised by the 2nd highest value after the pandemic season for incidence rate for medical consultation attributable to influenza, and by the highest sample positivity rate (47.4%), as compared to the other seasons (table 1).
Finally, when restricting the study to the only period that preceded the ARI epidemic period, characterised for all seasons by a preliminary moderate increase in ARI incidence rate and a relatively low sample positivity rate, we observed that the 2009–2010 and the 2010–2011 seasons were characterised by the lowest incidence rate for ARI non attributable to influenza (p = 10-4).
Virological strains
Case distribution according to the influenza virus isolated is shown in table 2 and figure 2. The influenza season 2008–2009 was characterised by a co-circulation of type A (A[H3N2] subtype isolated) viruses (87.5%) and type B viruses (12.5%) which were isolated late in the season (after week 5) (fig. 2). During the season 2009–2010, a new influenza virus named A(H1N1)pdm09 emerged and totally eclipsed the other viruses. More than 40% of the swabs were positive and nearly 100% of them enabled isolation of the pandemic virus. During the season 2010–2011, the A(H1N1)pdm09 virus was still the most frequent virus isolated (76/119 = 63.9%), but type B viruses continued to manifest themselves and were isolated (26.1%) throughout the season. Finally, during the last season 2011–2012, only type A viruses were isolated with a predominance of the A(H3N2) subtype (40/45 = 88.9%) (table 2).
Clinical description of laboratory-confirmed cases of influenza
The following clinical description was derived from 477 laboratory-confirmed cases for which clinical data were available. Whatever the season, an average of 92.7% of these cases were men (range: 90.3–95.0) and 65.7% (95% CI [61.2–69.9]) of the patients were younger than 30. However, the mean age was significantly younger (26.7 years IQR [21.4–30.2]; p = 2.10–4) and the proportion of patients aged less than 30 years was significantly higher (70.7%; p = 1.10–3) during the pandemic season 2009–2010 than during the three other seasons (table3). The onset of symptoms was sudden for 71.8% of patients but this proportion was significantly lower for 2011-2012 (p = 0.02) (table 3). The percentage of confirmed cases with measured hyperthermia (body temperature ≥38.5 °C) was significantly higher during the 2010–2011 season (p = 0.001) where 88.3% of cases suffered from a febrile form (table 3). The most frequent symptoms were cough (88.5%), asthenia (83.0), myalgia (77.8%) and chills (73.8%). No severe illness (hospitalisation, critical care admission or death) was reported.
The vaccination status of cases significantly differed according to the season (p = 0.03) (table 3). Thus, fewer confirmed cases were considered as immunised against influenza (time-lapse between immunisation and first influenza symptoms ≤1 year) during the pandemic season (2009–2010) and the post-pandemic season (2010-2011), where the proportions of immunised patients among laboratory-confirmed influenza cases were respectively 14.5% and 15.4% (table 3).
|
Table 1: Description of the main indicators monitored by the MISS and incidence rates of acute respiratory infection (ARI) and of medical consultation attributable to influenza (entire season and epidemic period) – influenza seasons 2008–2009 to 2011–2012. |
|
|
|
ARI
|
Medical consultation attributable to influenza
|
Sample positivity rate
|
|
Influenza season
|
Mean number of service personsEntire season
|
Mean incidence of ARI(extremes)
|
Incidence rate of ARI per 100,000 PWEpidemic peak
|
Incidence rate of ARI per 100,000 PW
Entire season (95% CI)
|
Incidence rate of ARI per 100,000 PW
Epidemic period
(95% CI)
|
Incidence rate of consultation attributable to influenza per 100,000 PWEpidemic peak
|
Incidence rate of consultation attributable to influenza per 100,000 PWEntire season
(95% CI)
|
Incidence rate of consultation attributable to influenza per 100,000 PWEpidemic period
(95% CI)
|
Mean sample
rate
Entire season
|
positive
%
Epidemic period
|
|
2008–2009
|
42,515 |
30.9
(0–102) |
228.3 |
72.7
(67.9–77.6) |
131.3
(119.6–143.0) |
91.3 |
13.7
(11.6–15.8) |
32.4
(26.6–38.2) |
18.2 |
24.7 |
|
2009–2010
|
42,747 |
37.3
(1–168) |
401.9 |
85.7
(81.3–90.1) |
195.2
(183.9–206.5) |
194.4 |
27.9
(25.7–30.2) |
83.2
(75.8–90.6) |
40.9 |
42.6 |
|
2010–2011
|
43,659 |
17.6
(2–61) |
129.8 |
40.3
(36.8–43.8) |
77.5
(69.5–85.6) |
77.9 |
19.1
(16.7–21.5) |
40.7
(34.8–46.5) |
47.4 |
52.4 |
|
2011–2012
|
46,838 |
31.6
(5–77) |
155.0 |
67.5
(63.1–71.9) |
101.4
(88.8–114.0) |
83.9 |
11.1
(9.3–12.8) |
37.8
(30.1–45.5) |
16.4 |
37.3 |
| PW = person-week |
|
|
|
Table 2: Description of the influenza viruses isolated by the MISS network – influenza seasons 2008–2009 to 2011–2012. |
|
Season
|
Naso-pharyngeal swabs analysed by the laboratories
|
Positive naso-pharyngeal swabs
|
Positive rate
|
Type A influenza viruses
|
Type B influenza viruses
|
| |
|
|
|
Type A total
|
A(H1N1)pdm09 virus
|
A(H3N2)
|
Non-subtyped influenza A viruses
|
|
| |
n
|
n
|
%
|
% (n)
|
% (n)
|
% (n)
|
% (n)
|
% (n)
|
|
2008–2009
|
307 |
56 |
18.2 |
87.5 (49) |
|
34.7 (17) |
63.3 (31) |
12.5 (7) |
|
2009–2010
|
640 |
262 |
40.9 |
99.6 (261) |
96.1 (251) |
|
3.8 (10) |
0.4 (1) |
|
2010–2011
|
251 |
119 |
47.4 |
73.9 (88) |
86.4 (76) |
|
13.6 (12) |
26.1 (31) |
|
2011–2012
|
274 |
45 |
16.4 |
100 (45) |
4.4 (2) |
88.9 (40) |
6.7 (3) |
|
|
Total
|
1472 |
482 |
32.7 |
91.9 (443) |
|
|
|
7.9 (39) |
| * In 2008–2009, A(H1N1) virus was isolated in one nasopharyngeal sample. |
|
Table 3: Description of clinical characteristics and vaccination status of laboratory-confirmed cases, MISS network – influenza seasons 2008–2009 to 2011–2012. |
|
|
Season
|
p-value
|
|
|
2008–2009
|
2009–2010
|
2010–2011
|
2011–2012
|
|
No of confirmed cases
|
56 |
262 |
117 |
42 |
|
|
Clinical description of the confirmed cases
|
|
|
|
|
|
| Age (mean(SD)) |
28.0 (8.9) |
26.7 (7.8) |
28.4 (8.2) |
32.8 (8.2) |
2.10–4
|
| Age <30 years-old |
64.3 (36) |
70.7 (186) |
65.0 (76) |
38.1 (16) |
1.10–3
|
| Male gender (%(n)) |
91.1 (51) |
92.7 (243) |
94.9 (111) |
90.5 (38) |
0.66 * |
| Sudden start |
73.2 (41) |
76.3 (200) |
67.5 (79) |
54.8 (23) |
0.02 |
| Body temperature ≥38.5 °C |
67.9 (38) |
46.9 (123) |
70.9 (83) |
30.9 (13)** |
1.10–3
|
|
Vaccination status
|
|
|
|
|
|
|
Confirmed cases with history of immunisation ≤1 year
|
30.3 (17) |
14.5 (38) |
15.4 (18) |
19.0 (8) |
0.03
|
| Time-lapse between immunisation and first influenza symptoms |
|
|
|
|
|
| <7 days |
23.5 (4) |
13.1 (5) |
22.2 (4) |
|
|
| 7–21 days |
5.9 (1) |
7.9 (3) |
|
|
|
| 21 days – <1 year |
70.6 (12) |
78.9 (30) |
77.7 (14) |
100 (8) |
|
|
Confirmed cases with no history of immunisation or immunisation ≥1 year
|
69.6 (39) |
85.9 (224) |
84.6 (99) |
80.9 (34) |
|
| Time-lapse between immunisation and first influenza symptoms |
|
|
|
|
|
| 1–3 years |
30.8 (12) |
6.2 (14) |
56.6 (56) |
15.0 (6) |
|
| ≥3 years |
43.6 (17) |
1.3 (3) |
15.1 (15) |
17.5 (7) |
|
| No immunisation |
25.6 (10) |
92.4 (207) |
28.3 (28) |
52.5 (21) |
|
| * Fisher’s exact test ** For 2011–2012 season, body temperature was unknown for 19 patients out of 42. |
Discussion
Epidemiological trends
In terms of epidemiological data, whether the indicator considered (ARI or medical consultations attributable to influenza), our results showed that the profile of the influenza outbreaks in the French armed forces was compatible with the trends observed in Western Europe, notably in relation to the pandemic. Indeed, according to the MISS, the ARI epidemic period and the peak occurred earlier during the pandemic than in previous seasons, this being observed by most of the influenza surveillance networks [9]. In the same manner, the characteristics of the 2011–2012 outbreak were a later epidemic period and peak and were also observed in several countries in Europe and overseas [10–14]. However, regarding the 2010–2011 outbreak, the disease burden appears to be smaller among the French armed forces compared to civilian data in France and to other European countries such as the UK or Greece [15, 16]. Indeed, in France, national data indicated that the 2010–2011 outbreak was moderate compared to the pandemic [13]. The fact that the 2010–2011 outbreak in the French armed forces appeared less marked than the national outbreak could be partly explained by a lesser investment of military general practitioners in influenza surveillance, probably because they had been called upon a lot during the pandemic to monitor several indicators on a daily basis. As it has already been described in the armed forces, it is possible that patients consulted more readily, and that military GPs reported more scrupulously any ARI during the pandemic because of the emergency context, the media coverage and the pressure of the hierarchy. Thus, it is possible that results obtained by the MISS for the pandemic were overestimated and partly attributable to report bias [17, 18]. Conversely, patients may have consulted less for ARI during the 2010–2011 season in reaction to the way pandemic was handled by health authorities, as Mytton et al. hypothesised regarding the situation in the UK in the post-pandemic season [15]. In terms of amplitude of the successive outbreaks, MISS estimations were always below the national and international estimates whatever the indicator considered. Hence, the national civilian incidence rates estimations for medical consultation attributable to influenza (1 321 P. 100 000 PW in 2009–2010 [19], 628 p. 100,000 PW in 2010-2011 and 571 p. 100,000 PW in 2011–2012 according to the GROG and Sentinelles networks [13, 14]) were much higher than those estimated by MISS. First of all, the differences observed are likely to be due to the age characteristics of the military population, the 20–49 age class accounting for more than 90% of the French military, whereas the same age class contains less than 40% of the French general population [20], keeping in mind that influenza is more likely to affect children or elderly people (older than 65) [21–23]. Standardisation (according to age) on the French general population was performed on pandemic data leading to an increase in the estimation from 194.4 p.100,000 PW to 287.8 p. 100,000 PW, still far below the national estimations [19]. Thus, factors other than age structure could play a role in the lower estimates observed. First of all, consultation of a military GP is not mandatory for the military who can rather choose to consult civilian GP, so that cases of ARI and/or influenza may have escaped the MISS network. Furthermore, the military are globally in better health than the general French population and the “healthy worker effect” can also explain the differences observed. Finally, vaccination of service members could play a role in this phenomenon as military personnel on active duty could be better vaccinated against influenza than the general population of the same age class. Indeed, vaccination against influenza is not recommended among general population for young adults in good health and with no underlying conditions such as pregnancy or obesity. In contrast, the traditional vaccine schedule for French service members includes compulsory influenza vaccination every three years (triennial scheme) and before each mission abroad [24]. For instance, 14.5% of the military on active duty were immunised with the pandemic vaccine [17], while 8% of the French general population were vaccinated [25]. However, we would need complementary data to estimate vaccine coverage for the other influenza seasons and to confirm this hypothesis.
Virological trends
Concerning the circulation of viral strains, it appears that the novel A(H1N1)pdm09 virus eclipsed the previous viral strains in circulation (A[H3N2] and B) and was the predominant virus. The following season 2010–2011 was characterised by a co-circulation of A(H1N1)pdm09 and B viruses, while during the 2011–2012 season the pandemic virus decreased, replaced by A(H3N2) viruses. This modification of the influenza viral ecology had already been observed after the previous pandemics [26]. In addition, the interaction between influenza viruses and the other respiratory viruses, notably respiratory syncytial virus (RSV) and rhinoviruses, has been discussed and it has been hypothesised that the emergence of the A(H1N1)pdm09 strain could have disturbed the circulation of the other viruses during the pandemic [27–29]. This hypothesis is in line with our results, which show significantly lower pre-epidemic incidence rates for ARI non attributable to influenza for the 2009–2010 and 2010–2011 seasons, both characterised by the predominance of the pandemic A(H1N1)pdm09 virus. The influenza outbreaks are usually preceded, during the autumn, by RSV outbreaks in France [30, 31] and our results could indicate less marked RSV outbreaks during the 2009–2010 and 2010–2011 seasons. However, this hypothesis should be explored further by screening for para-influenza viruses, RSV or rhinoviruses on swabs that are negative for influenza viruses, which is not done at the moment.
Our results are compatible with the French national data: in the 2010–2011 season, among influenza positive nasopharyngeal swabs, approximately 47% were positive for a B virus and 53% for A(H1N1)pdm09 virus [13]. In the 2011–2012 season, in 95% of the positive samples analysed by the NCRs were isolated A viruses, among which 86% were positive for the A(H3N2) viruses, and 5% were positive for B viruses [14]. The observations were globally the same at an international level, with a co-circulation of A (mostly A[H1N1]pdm09) and B viruses in 2010–2011 and a majority of A(H3N2) viruses, with B viruses but to a lesser extent in 2011–2012 [10]. For the last season, a drift was observed among A(H3N2) viruses and the increase of B/Yamagata lineage viruses led the WHO authorities to modify the vaccine composition for the northern hemisphere 2012–2013 influenza season [10, 14, 32].
The symptoms presented by laboratory-confirmed cases were classic symptoms for influenza, with no significant difference depending on the season and the predominant virus in circulation. Laboratory-confirmed cases were significantly younger during the pandemic compared to the other seasons. This age shift has been observed at the national and international level and has been widely described since 2009, because during the pandemic children and young or middle-aged adults were more likely to contract influenza, with potentially more respiratory complications, compared to elderly people [33]. The main hypothesis explaining this age shift is that people over 60 years old might have already acquired immunity from prior exposure to viruses circulating in the 1950s [2, 33]. Among armed forces, the mean age was higher and the proportion of patients aged less than 30 years-old was lower than observed among civilians as population under surveillance by the military network does not include children nor adolescents under 18. But children between 5 and 19 represented more than 46% of cases recorded in Europe [17]. However, an increase in the mean age of influenza cases was observed during 2010–2011 season whereas the predominant virus circulating was still A(H1N1)pdm09, without any clear explanation.
The other point is that none of the cases reported by MISS presented respiratory complications or severe illness, even during the pandemic. The disease burden of the pandemic was, in French armed forces as well as in France generally, less severe than predicted [34]. However, severe illnesses and deaths were reported to French civilian surveillance networks, while none was reported to the MISS, and it seems that the burden of infections due to A(H1N1)pdm09 was more important as compared to other viruses such as A(H3N2) or B [35, 36]. It is surprising that no severe cases were reported to the military network as, during the pandemic and contrary to most of seasonal influenza outbreaks, the complication and hospitalisation rates were higher among children and young adults in Europe [37]. This could be explained by the way military recruits are selected according to their medical status leading to the selection of people in a good health, without any of the chronic diseases known to be risk factors for severe influenza (e.g. non-controlled asthma, respiratory failure, morbid obesity, etc.) [19]. In addition, the population surveyed by the MISS included military personnel on active duty which implies that they were globally in good health, and our results may be partly due to the healthy worker effect [19]. Nevertheless, it remains possible that some service members could have been hospitalised in civilian hospitals for severe illnesses without having consulted their military GP. In this case, it is likely that the military GP never got the information or was informed late and did not report the case to the MISS coordination.
Finally the percentage of laboratory-confirmed cases that had been vaccinated with the seasonal influenza vaccine was significantly different according to the season (p = 0.03) and was the lowest for the pandemic season and the 2010–2011 season. Both of these influenza seasons were characterised by predominant circulation of A(H1N1)pdm09 strain which corresponded to the vaccine strain. Concerning the 2008–2009 season, despite good efficacy of the vaccine [38, 39], it appears that 1 out of 3 laboratory-confirmed cases had received the 2008–2009 seasonal vaccine, of whom 70% (12/17) presented influenza at least 3 weeks after vaccination. Concerning the 2011–2012 season, the percentage of patients contracting influenza and having received the 2011–2012 vaccine at least three weeks earlier was 19%: this could be explained by the drift of influenza viruses in circulation leading progressively to a misfit between the viral strains and the vaccine [14, 32].
Conclusion
The trends observed by MISS are globally compatible with French and European estimations, but the amplitude of the outbreaks was much smaller than that observed at national and international levels. This could be partly explained by the specific characteristics of the military population regarding demographic profile, health status or vaccine status. Despite these specificities, MISS constitutes a good observatory for influenza among young people. From a virological point of view, our results highlight the circulation of influenza viral strains across time and the changes in viral ecology from a season to another, particularly with the emergence of the A(H1N1)pdm09 strain. However, virological results could be improved by systematic screening for other respiratory viruses and PIV among negative samples.
Acknowledgment: the authors would like to thank the whole staff of the MISS units and of the biology departments who, by the quality of their work, played an active part in the MISS surveillance network.
References
1 Centers for disease control and prevention. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010. Website: http://www.cdc.gov/h1n1flu/cdcresponse.htm, Accessed on August 2nd, 2012.
2 European Centre for disease prevention and control. The 2009 A(H1N1) pandemic in Europe – A review of the experience . Website: http://www.ecdc.europa.eu/en/publications/Publications/Forms/ ECDC_DispForm.aspx?ID=575 http://www.ecdc.europa.eu/en/publications/Publications/Forms/ ECDC_DispForm.aspx?ID=575 , Accessed on August 2nd, 2012.
3 Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. Jun 26.
4 Fleming DM, Elliot AJ, Meijer A, Paget WJ. Influenza virus resistance to oseltamivir: what are the implications? Eur J Public Health. 2009;19(3):238–9.
5 Meijer A, Valette M, Manuguerra JC, Perez-Brena P, Paget J, Brown C, et al. Implementation of the community network of reference laboratories for human influenza in Europe. J Clin Virol. 2005;34(2):87–96.
6 Tarabbo M, Lapa D, Castilletti C, Tommaselli P, Guarducci R, Luca G, et al. Retrospective investigation of an influenza A/H1N1pdm outbreak in an Italian military ship cruising in the Mediterranean Sea, May–September 2009. PLoS One. 2011;6(1):e15933.
7 Jeger V, Dunki A, Germann M, Fux CA, Faas A, Exadaktylos AK, et al. H1N1 outbreak in a Swiss military boot camp – observations and suggestions. Swiss Med Wkly. 2011;141:w13307.
8 Groupes régionaux d’observation de la grippe. Website: http://www.grog.org/documents/ seuilepidemique.pdf, Accessed on September 25th, 2012.
9 Martirosyan L, Paget WJ, Jorgensen P, Brown CS, Meerhoff TJ, Pereyaslov D, et al. The community impact of the 2009 influenza pandemic in the WHO European region: a comparison with historical seasonal data from 28 countries. BMC Infect Dis.12:36.
10 Review of the 2011–2012 winter influenza season, northern hemisphere. Wkly Epidemiol Rec. Jun 15;87(24):233–40.
11 European center for disease prevention and control. Weekly Influenza Surveillance Overview (WISO) Website: http://www.ecdc.europa.eu/en/publications/Publications/Forms/ ECDC_DispForm.aspx?ID=575 http://www.ecdc.europa.eu/en/publications/Publications/Forms/ ECDC_DispForm.aspx?ID=575 , Accessed on August 2nd, 2012.
12 Sentiweb – Institut national de la santé et de la recherche médicale (INSERM). Website: http://websenti.u707.jussieu.fr/sentiweb/, Accessed on August 2nd, 2012.
13 Belchior E. Surveillance épidémiologique et virologique de la grippe en France, saison 2010–2011. Bull Epidemiol Hebd. 2011(37-38):394–8.
14 Belchior E. Epidemiological and virological influenza activity in mainland France: season 2011–2012. Bull Epidemiol Hebd. 2012(38):424–7.
15 Mytton OT, Rutter PD, Donaldson LJ. Influenza A(H1N1)pdm09 in England, 2009 to 2011: a greater burden of severe illness in the year after the pandemic than in the pandemic year. Euro Surveill.17(14).
16 Athanasiou M, Baka A, Andreopoulou A, Spala G, Karageorgou K, Kostopoulos L, et al. Influenza surveillance during the post-pandemic influenza 2010/11 season in Greece, 04 October 2010 to 22 May 2011. Euro Surveill.16(44).
17 Mayet A, Ligier C, Gache K, Manet G, Nivoix P, Dia A, et al. Adverse events following pandemic influenza vaccine Pandemrix(R) reported in the French military forces – 2009–2010. Vaccine. Mar 21;29(14):2576–81.
18 Gache K, Mayet A, Manet G, Ligier C, Piarroux M, Faure N, et al. The 2009 A(H1N1) influenza pandemic in the French Armed Forces: evaluation of three surveillance systems. Eur J Public Health. 2012 Aug 22.
19 Mayet A, Duron S, Nivoix P, Haus-Cheymol R, Ligier C, Gache K, et al. Novel influenza A(H1N1) outbreak among French armed forces in 2009: results of Military Influenza Surveillance System. Public Health. Aug;125(8):494–500.
20 Institut national de la statistique et des études économiques (INSEE). Website: http://www.insee.fr/fr/ppp/bases-de-donnees/donnees-detaillees/bilandemo/pyramide/pyramide.htm?champ=fe. Accessed on August 2nd, 2012.
21 Gasparini R, Bonanni P, Amicizia D, Bella A, Donatelli I, Cristina ML, et al. Influenza epidemiology in Italy two years after the 2009–2010 pandemic: Need to improve vaccination coverage. Hum Vaccin Immunother. 2013;9(3).
22 Lai PL, Panatto D, Ansaldi F, Canepa P, Amicizia D, Patria AG, et al. Burden of the 1999–2008 seasonal influenza epidemics in Italy: comparison with the H1N1v (A/California/07/09) pandemic. Hum Vaccin. 2011;7(Suppl):217–25.
23 Rahamat-Langendoen JC, Tutuhatunewa ED, Scholvinck EH, Hak E, Koopmans M, Niesters HG, et al. Influenza in the immediate post-pandemic era: a comparison with seasonal and pandemic influenza in hospitalized patients. J Clin Virol. Jun;54(2):135–40.
24 Haus-Cheymol R, Nicand E, Buisson Y, Berger F, Decam C, Spiegel A. Effectiveness of triennial anti-influenza vaccination in French military during the 2003–2004 influenza season. Rev Epidemiol Sante Publique. 2007;55(5):339–45.
25 Mereckiene J, Cotter S, Weber JT, Nicoll A, D’Ancona F, Lopalco PL, et al. Influenza A(H1N1)pdm09 vaccination policies and coverage in Europe. Euro Surveill. 2012;17(4).
26 Blyth CC, Kelso A, McPhie KA, Ratnamohan VM, Catton M, Druce JD, et al. The impact of the pandemic influenza A(H1N1) 2009 virus on seasonal influenza A viruses in the southern hemisphere, 2009. Euro Surveill. 15(31).
27 Linde A, Rotzen-Ostlund M, Zweygberg-Wirgart B, Rubinova S, Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14(40).
28 Casalegno JS, Bouscambert-Duchamp M, Morfin F, Lina B, Escuret V. Rhinoviruses, A(H1N1)v, RVS: the race for hivernal pandemics, France 2009–2010. Euro Surveill. 2009;14(44).
29 Casalegno JS, Ottmann M, Bouscambert-Duchamp M, Valette M, Morfin F, Lina B. Impact of the 2009 influenza A(H1N1) pandemic wave on the pattern of hibernal respiratory virus epidemics, France, 2009. Euro Surveill. 2010;15(6).
30 Respiratory syncytial virus activity – United States, July 2008–December 2009. MMWR Morb Mortal Wkly Rep. Mar 5;59(8):230–3.
31 Mullins JA, Lamonte AC, Bresee JS, Anderson LJ. Substantial variability in community respiratory syncytial virus season timing. Pediatr Infect Dis J. 2003;22(10):857–62.
32 Bonmarin I, Belchior E, Le Strat Y, Levy-Bruhl D. First estimates of influenza vaccine effectiveness among severe influenza cases, France, 2011/12. Euro Surveill.17(18).
33 Beaute J, Broberg E, Plata F, Bonmarin I, J OD, Delgado C, et al. Overrepresentation of influenza A(H1N1)pdm09 virus among severe influenza cases in the 2011/12 season in four European countries. Euro Surveill.17(9).
34 Fuhrman C, Bonmarin I, Bitar D, Cardoso T, Duport N, Herida M, et al. Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol Infect. Aug;139(8):1202–9.
35 Gubbels S, Krause TG, Bragstad K, Perner A, Molbak K, Glismann S. Burden and characteristics of influenza A and B in Danish intensive care units during the 2009/10 and 2010/11 influenza seasons. Epidemiol Infect. 2013;141(4):767–75.
36 Altmann M, Fiebig L, Buda S, von Kries R, Dehnert M, Haas W. Unchanged severity of influenza A(H1N1)pdm09 infection in children during first postpandemic season. Emerg Infect Dis. 2012;18(11):1755–62.
37 Jacks A, Ollgren J, Ziegler T, Lyytikainen O. Influenza-associated hospitalisations in Finland from 1996 to 2010: unexpected age-specific burden during the influenza A(H1N1)pdm09 pandemic from 2009 to 2010. Euro Surveill. 2012;17(38).
38 Falchi A, Amoros JP, Arena C, Arrighi J, Casabianca F, Andreoletti L, et al. Genetic structure of human A/H1N1 and A/H3N2 influenza virus on Corsica Island: phylogenetic analysis and vaccine strain match, 2006–2010. PLoS One.6(9):e24471.
39 Mossad SB. 2008–2009 Influenza update: a better vaccine match. Cleve Clin J Med. 2008;75(12):865–70.

