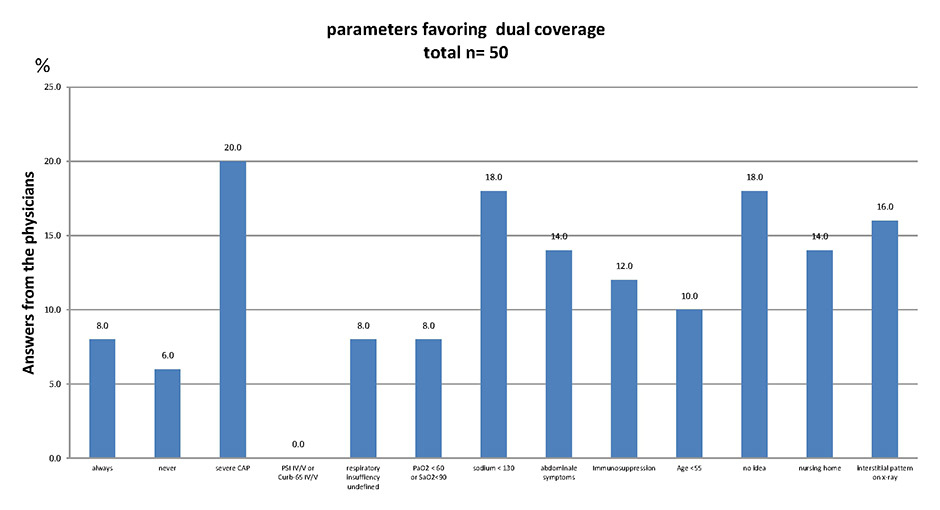
Figure 1
Answers from physicians: under what clinical circumstances should dual antibacterial therapy be prescribed for patients hospitalised with community-acquired pneumonia?
DOI: https://doi.org/10.4414/smw.2013.13870
Community-acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide. In the United States, it is estimated that 10.5% of hospitalisations are due to CAP in adults ≥65 years, and these numbers have not changed in the last 20 years [1, 2]. In Europe, the incidence of CAP varies from 1.9 to 9 cases per 1000 persons, with a hospitalisation rate of 8% to 51% [3] and a length of stay (LOS) of about 9.8 days [4]. Despite many efforts to improve the care of patients with CAP, advances in diagnostic procedures and antimicrobial chemotherapy, neither the rates of microbiological findings nor mortality rates have significantly improved in the last 30 years [5]. As the turnaround time for microbiological tests exceed the timeframe in which antibiotic treatment must be started, guidelines for empirical treatment have been developed [6, 7]. Although the beta-lactam antibiotics cover typical bacteria, macrolides are used for atypical bacteria such as Legionella, Chlamydia and Mycoplasma. Respiratory quinolones cover typical as well as atypical bacteria, but their use is debated owing to the risk of developing resistance and their (overly) broad spectrum. Contrary to the US guidelines [8], macrolide use or a respiratory quinolone are only recommended for severe CAP in European guidelines [9]. In our hospital, we also recommend empirical beta-lactam/macrolide combination therapy if the patient is immunocompromised, has an oxygen saturation below 90% or has to be admitted to the ICU. Even though some authors advocate the use of a macrolide or a respiratory quinolone in all hospitalised patients with CAP [10], in most instances the macrolide is stopped after results for Legionella antigen are negative [11]. For patients not admitted to the ICU, the value of double coverage for Streptococcus pneumoniae is much debated [12]. Furthermore, it is unlikely that one or two doses of a macrolide would be enough to treat atypical pathogens such as Mycoplasma pneumoniae or Chlamydophila pneumoniae. Therefore, in areas with a low prevalence of penicillin-resistant pneumococci, the value of the routine use of macrolides or a respiratory quinolone in the setting of hospitalised patients with nonsevere CAP is questionable [12].
As the guidelines for treatment of community-acquired pneumonia do not clearly indicate under which circumstances atypical pathogens should be covered, physicians in the emergency department may decide on the basis of gut feelings rather than upon clinical parameters. We wanted to know if the use of macrolides as a second antibiotic in empirical treatment was guided by clinical parameters.
Kantonsspital Olten is a 300 bed university-affiliated teaching hospital responsible for about 100,000 inhabitants. Patients are admitted either directly to the ward or to the interdisciplinary emergency department. First contact is normally made by one of the 24 physicians in training, but decisions about treatment are always discussed with an attending physician. All noninfectious-disease physicians were interviewed and confronted with the hypothetical situation of a patient admitted to the emergency department with community-acquired pneumonia. It was postulated that the patient was sick enough to require hospitalisation but transfer to the intensive care unit (ICU) was not necessary. The physicians were then asked under what circumstances they would include a macrolide in the treatment (e.g. a beta-lactam antibiotic plus a macrolide). We then retrospectively analysed the empirical treatment of 300 patients with documented community-acquired pneumonia consecutively admitted between November 2007 and March 2009, as we were especially interested in whether the decision to treat CAP with beta-lactam monotherapy or a beta-lactam/macrolide combination was based on clinical parameters at admission. Clarithromycin was the only macrolide used for CAP, and respiratory quinolones are not listed in our hospital. For the diagnosis of CAP, new onset of cough and one of the following was required: new focal chest signs, dyspnoea, and tachypnoea or fever for at least 4 days. We did not include procalcitonin as a diagnostic criterion. A chest X-ray was performed in all patients, and the presence of pulmonary infiltrates was required for the diagnosis of CAP.
Four parameters for the possibility of atypical pneumonia (age <55 years, abdominal symptoms, sodium <130 mmol/l, immunosuppression) and three parameters for pneumonia severity (pneumonia severity index [PSI], ICU admission, pO2<8 kPa (60 mm Hg) respective O2saturation <90%) were defined and correlated with the probability of coverage for atypical pathogens. The first measurement of oxygen saturation/pO2, before oxygen supplementation, was considered as a marker for respiratory capacity. If there was a discrepancy between the blood gas analysis and percutaneous oxygen saturation, the lower value was taken as an estimation of the true respiratory insufficiency. According to the pneumonia severity index, saturation <90% or a pO2<8 kPa (60 mm Hg) was considered to be respiratory insufficiency independent of pCO2 values. Abdominal problems (diarrhoea, vomiting, abdominal pain) were taken into account if documented. If no abdominal symptoms were documented at admission we considered these patients to have no abdominal symptoms. Immunosuppression was considered to be present in patients receiving chemotherapy, or immunosuppressive agents for rheumatological or autoimmune disorders, and those with HIV infection and CD4 + cell count ≤200/µl, or haematological malignancies.
Mortality was defined as death within 30 days of admission for CAP. If no data were available in hospital charts, the family doctor was contacted by telephone.
Descriptive statistics were performed for all patients at baseline, with continuous data expressed as mean ± standard deviation (SD) and categorical data expressed as counts. Baseline characteristics of the groups were compared by student’s t-test and X2 test. The correlations were analysed with an X2 test for a 2x2 table and odds ratio (OR). Multivariable logistic regression adjusted for severity of pneumonia (PSI), age, ICU admission, sodium <130 mmol/l, abdominal symptoms, oxygen saturation and immunosuppression; a two-sided 5% level of statistical significance was used. The outcome of the logistic regression analysis was the probability of covering atypical bacteria (e.g. beta-lactam/macrolide combination therapy) in patients with CAP in relationship to the variables listed above. Graphpad® was used for statistical analysis. Odds ratios (OR) were calculated with a 95% confidence interval (CI) and a significance level (p-value) of 0.05.
Correlation between the number of risk factors and the probability of coverage of atypical organisms was measured by correlation coefficient.
A total of 50 physicians in the medical department were asked on the basis of which clinical parameters they would favour dual coverage for hospitalised patients with community-acquired pneumonia. More than one answer was possible. For results, see figure 1.

Figure 1
Answers from physicians: under what clinical circumstances should dual antibacterial therapy be prescribed for patients hospitalised with community-acquired pneumonia?
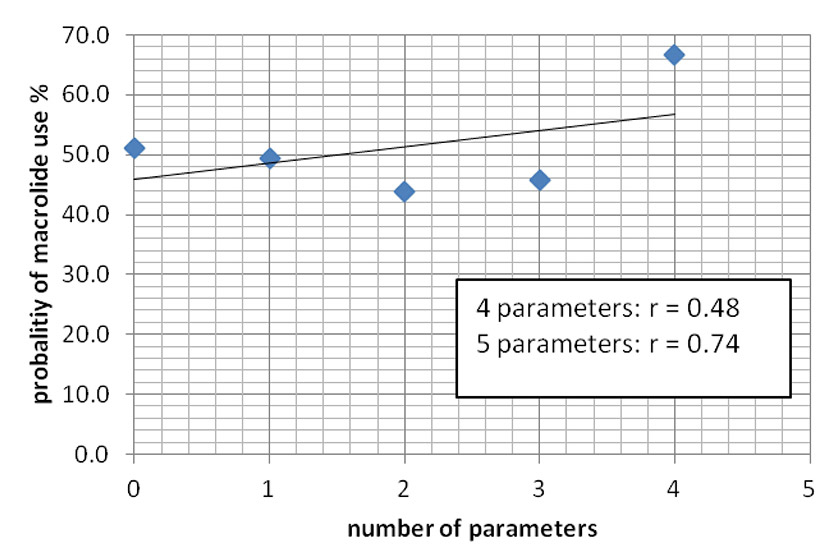
Figure 2
Correlation between the number of parameters investigated and the probability of macrolide use.
A total of 300 patients with community-acquired pneumonia were analysed, of whom 61.3% were male. Age, gender, PSI score and sodium at admission were obtained from all patients. Of the 300 patients, 13 (4.3%) were in PSI class I, and 47 (15.7%), 55 (18.3%), 110 (36.7%) and 75 (25%) in classes II, III, IV and V, respectively. In 252/300, blood gas analysis was performed at admission; in 46/48 patients in whom no blood gas analysis was performed, percutaneous oxygen saturation was measured. In only 2/300 patients was neither blood gas analysis nor oxygen saturation measurement performed. Survival data (30 days after admission) was obtained from 297/300 patients and 25/300 patients were considered to be immunocompromised.
In patients younger than 55 years, the rate of coverage of atypicals was significantly higher than in older patients (OR 2.53; 95% CI 1.25–5.14, p = 0.008). However, in 33% of the patients younger than 55 years, only a beta-lactam antibiotic was given. Among patients with a PSI score higher than III, fewer patients were treated with antibiotics that covered atypical bacteria (OR 0.77; 95% CI 0.60–0.99, p = 0.03). These differences were no longer significant upon multivariate analysis. There were no differences in empirical coverage of atypical bacteria for community-acquired pneumonia between patients with PSI >IV (OR = 1.03; 95% CI 0.61–1.74, p = 0.9).
There was no difference between patients admitted or not admitted to the ICU (OR = 1.39; 95% CI 0.87–2.47, p = 0.14). Respiratory function did not influence the prescription of antibiotics. The OR was 1.39 (95% CI; 0.87–2.21, p = 0.16) for patients with a pO2 <8 kPa compared with the group with a pO2 >8 kPa. The percentage of patients with atypical bacteria covered was also similar between patients with or without abdominal symptoms present at admission (OR 1.09; 95% CI 0.51–2.32, p = 0.82), and sodium <130 mmol/l did not influence the empirical antibiotic regime (OR 0.61; 95% CI 0.29–1.31, p = 0.2).
Also, the same number of patients with immunosuppression received antibiotic drugs with a spectrum that included atypical bacteria (OR 1.007; 95% CI 0.44–2.28, p = 1). See table 1.
There was only a weak correlation between the number of parameters used in the study and the coverage of atypical bacteria. The correlation factor r = 0.48 (p = 0.41) for zero to four factors and r = 0.74 (p = 0.07) for zero to five factors, but there were only three patients with five factors positive and these were all treated with a beta-lactam antibiotic and a macrolide (fig. 2).
The 30-day mortality rate was no different between patients treated with a combination therapy or with beta-lactam monotherapy (23/123 in the group with atypical bacteria covered vs 28/128 in the monotherapy group, p = 0.53).
| Table 1: Coverage of atypical pathogens in hospitalised patients with community-acquired pneumonia. | |||
| Atypical pathogens covered (n) | Atypical pathogens not covered (n) | ||
| Age <55 years | 27 | 13 | OR 2.53 (CI 95% 1.25‒5.14, p = 0.008) Significant, but 33% without coverage |
| Age >55 years | 117 | 143 | |
| PSI ≤III | 65 | 50 | OR 0.77 (95% CI 0.60‒0.99, p = 0.03) |
| PSI >III | 81 | 104 | |
| PSI ≤IV | 109 | 116 | OR = 1.03 (95% CI 0.61‒1.74, p = 0.9) |
| PSI >IV | 37 | 38 | |
| ICU admission | 43 | 35 | OR = 1.39 (95% CI 0.87‒2.47, p = 0.14 |
| No ICU admission | 101 | 121 | |
| p02 <8 kPa | 62 | 55 | OR 1.39 (95% CI 0.87‒2.21, p = 0.16 |
| p02 >8 kpa | 82 | 101 | |
| Abdominal symptoms | 15 | 15 | OR 1.09 (95% CI 0.51‒2.32, p = 0.82) |
| No abdominal symptoms | 129 | 141 | |
| Sodium <130 mmol/l | 12 | 20 | OR 0.61 (95% CI 0.29‒1.31, p = 0.2) |
| Sodium >130 mmol/l | 132 | 136 | |
| Immunsuppression | 12 | 13 | OR 1.007 (95% CI 0.44‒2.28, p = 1) |
| No immunosuppression | 133 | 143 | |
| CI = confidence interval; ICU = intensive care unit; OR = odds ratio; OSI = pneumonia severity index score | |||
The American Guidelines recommend a respiratory quinolone or a combination of a third generation cephalosporin and a macrolide [8]. However, the study also referred to these combinations showing a benefit of a combination therapy only in patients with a PSI of V [13] or higher risk [14]. The European Guidelines do not recommend a respiratory quinolone or a dual therapy in all patients hospitalised in a general ward [7]. They recommend individualising the strategy and leaving the decision to the treating physician, but without a clear decision tree of when to use either mono- or combination therapy.
Especially in countries with a low prevalence of penicillin-resistant S. pneumoniae (PRSP), empirical treatment with a combination therapy or a respiratory quinolone may not be necessary [15] in all patients hospitalised with CAP. Our study was retrospective and does not have the power to show differences in treatment outcome. It shows, however, that clinicians in the emergency department have difficulty in judging the severity of CAP and in guiding antibiotic treatment according to clinical parameters.
Only 2% of CAP cases are due to Legionella pneumoniae, and it is not clear if mycoplasmal and chlamydial pneumonia has to be treated with antibiotics [16]. In an older Spanish study comparing ceftriaxone with amoxicillin / clavulanic acid without a macrolide, patients with suspected atypical pathogens were excluded. However, only 18/378 (4.75%) of these patients were not included and the cure rate was almost 90% in both treatment arms, leading to the suspicion that antibiotic coverage in mild to moderate community-acquired pneumonia is not mandatory, except in cases of legionellosis [17]. Macrolide treatment is usually stopped once a negative Legionella urine antigen test is obtained. In our study, in 90% of the patients initially treated with a combination therapy, the macrolide was stopped after a negative Legionella AG (Binax) result was obtained (data not shown). That means that all CAP due to Mycoplasma or Chlamydia pneumoniae are treated for only 24 hours. The failure rate would be higher if all these patients require antibiotic treatment.
Guidelines must help physicians with diagnoses and therapeutic decision making. Hospitalisation for moderately severe pneumonia is a frequent situation [2], but the physicians in the emergency department still have difficulty in deciding under which circumstances they should use combination therapy. Despite the acceptance of the PSI- and CURB-65 Score in the literature, these tools are rarely used in daily work in the emergency department [18–20]. Moreover, the scores have shown good reliability estimating mortality, but cannot precisely estimate the severity of a pneumonia independent of a patient’s concomitant factors [21], especially in young patients [22]. It has been shown that an important fraction of patients are hospitalised despite a relatively low PSI score, and that these patients often have longer duration of hospitalisation and more complications than the PSI score would indicate [24]. In our study, 20% of patients had a PSI score of I or II. We think that physicians can make a very good decision regarding whether a patient requires hospitalisation in most instances [23, 24]. However, in judging between mono- or combination therapy, no accordance to clinical parameters could be observed. PSI or Curb-65-Score alone might not be an ideal parameter for this decision.
We are convinced that a narrow spectrum antibiotic therapy should be recommended in all possible instances. Broad spectrum antibiotics should be limited to those with severe pneumonia or at high risk of a negative outcome (e.g. immunosuppression). Although, greater focus should be put on the question about under which circumstances is monotherapy sufficient for hospitalised patients with CAP. The risk that young doctors will otherwise always use combination therapy is overt, and might also influence their choices once they practice in ambulatory care. However, in our study, physicians had difficulties in defining when broader treatment is necessary and a wide variety of answers were obtained. Interestingly, PSI or CURB-65 (as well as other scores) were never named, and respiratory insufficiency was only used by a minority of physicians. We focused on oxygenation as the most important clinical parameter for deciding if the patient needs combination therapy. We are aware that our choice of parameters: age, immunosuppression, sodium <130 mmol/l, and oxygen <8 kPa or <90% have not been validated in large clinical trials for the question of which antibiotic strategy should be applied. A recent publication suggested that hospitalisation at a threshold of 92% oxygen saturation would be safer [25]. Our study is too small to be able to show differences in mortality between patients with coverage of atypicals or not, and subgroup analysis make no sense due to the limited number of patients. We think, however, that the guidelines should be more clearly focused on the question of which patients hospitalised with CAP justify narrow spectrum antibiotic treatment.
This study has some limitations. It was a single centre study and we cannot exclude the possibility that in other hospitals better estimations of severity of pneumonia are obtained and influence antibiotic treatment. However, the interviews with the physicians were, in the majority of cases, done at the beginning of their employment in our hospital. Most of these physicians did not come directly from medical school but worked in other hospitals before. So we believe that our observation may reflect the situation in other institutions as well. The power of our study is not sufficient to observe differences in mortality, and so a subgroup analysis that might have shown differences in special groups of patients was not possible. Larger trials are needed to point out in which cases monotherapy in hospitalised patients with CAP are necessary.
1 Health, United States, 2010,with special feature on death and dying [Internet]. Hyattsville, MD: National Center for Health Statistics; 2011; cited 16.5.2011]. Available from: http://www.cdc.gov/nchs/data/hus/hus10.pdf .
2 National Center of Health Statistics. Health, United States, 2008. available at: / http://www.cdc.gov/nchs/data/hus/hus08.pdf [Internet].; 2008.
3 Woodhead M. Community-acquired pneumonia in Europe: Causative pathogens and resistance patterns. Eur Respir J Suppl. 2002;36:20s–7s.
4 Schuetz P, Albrich WC, Suter I, Hug BL, Christ-Crain M, Holler T, et al. Quality of care delivered by fee-for-service and DRG hospitals in Switzerland in patients with community-acquired pneumonia. Swiss Med Wkly. 2011;141:w13228.
5 Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90(2):223–9.
6 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
7 Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–80.
8 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
9 Management of community acquired pneumonia (CAP) in adults” [Internet].: Swiss society of Infectious disease; 2006 [updated 1.1.2006; cited 18.5.2011]. Available from: http://www.sginf.ch/ssi-home/guidelines/cap-guidelines/index.htm.
10 Weiss K, Tillotson GS. The controversy of combination vs. monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest. 2005;128(2):940–6.
11 File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, et al. Integrated analysis of FOCUS 1 and FOCUS 2: Randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis. 2010;51(12):1395–405.
12 Eliakim-Raz N, Robenshtok E, Shefet D, Gafter-Gvili A, Vidal L, Paul M, et al. Empiric antibiotic coverage of atypical pathogens for community-acquired pneumonia in hospitalized adults. Cochrane Database Syst Rev. 2012;9:CD004418.
13 Gleason PP, Meehan TP, Fine JM, Galusha DH, Fine MJ. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med. 1999;159(21):2562–72.
14 Brown RB, Iannini P, Gross P, Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: Analysis of a hospital claims-made database. Chest. 2003;123(5):1503–11.
15 Kolditz M, Halank M, Hoffken G. Monotherapy versus combination therapy in patients hospitalized with community-acquired pneumonia. Treat Respir Med. 2006;5(6):371–83.
16 Fernandez Alvarez R, Suarez Toste I, Rubinos Cuadrado G, Medina Gonzalvez A, Gullon Blanco JA, Gonzalez Martin I. Treatment and course of community-acquired pneumonia caused by atypical pathogens. Arch Bronconeumol. 2006;42(9):430–3.
17 Roson B, Carratala J, Tubau F, Dorca J, Linares J, Pallares R, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant streptococcus pneumoniae: A randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7(1):85–96.
18 Aujesky D, McCausland JB, Whittle J, Obrosky DS, Yealy DM, Fine MJ. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49(10):e100–8.
19 Barlow G, Nathwani D, Myers E, Sullivan F, Stevens N, Duffy R, et al. Identifying barriers to the rapid administration of appropriate antibiotics in community-acquired pneumonia. J Antimicrob Chemother. 2008;61(2):442–51.
20 Collini P, Beadsworth M, Anson J, Neal T, Burnham P, Deegan P, et al. Community-acquired pneumonia: Doctors do not follow national guidelines. Postgrad Med J. 2007;83(982):552–5.
21 Arnold FW, Brock GN, Peyrani P, Rodriguez EL, Diaz AA, Rossi P, et al. Predictive accuracy of the pneumonia severity index vs. CRB-65 for time to clinical stability: Results from the community-acquired pneumonia organization (CAPO) international cohort study. Respir Med. 2010;104(11):1736–43.
22 Singanayagam A, Chalmers JD, Hill AT. Severity assessment in community-acquired pneumonia: A review. QJM. 2009;102(6):379–88.
23 Seymann G, Barger K, Choo S, Sawhney S, Davis D. Clinical judgment versus the pneumonia severity index in making the admission decision. J Emerg Med. 2008;34(3):261–8.
24 Labarere J, Stone RA, Obrosky DS, Yealy DM, Meehan TP, Fine JM, et al. Comparison of outcomes for low-risk outpatients and inpatients with pneumonia: A propensity-adjusted analysis. Chest. 2007;131(2):480–8.
25 Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: A population-based cohort study. Clin Infect Dis. 2011;52(3):325–31.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.