The importance of early treatment for the prognosis of rheumatoid arthritis
DOI: https://doi.org/10.4414/smw.2013.13865
Diego
Kyburz, Axel
Finckh
Summary
Chronic synovial inflammation in rheumatoid arthritis (RA) leads to progressive damage to articular cartilage and bone, ultimately resulting in disability. Therefore, control of the articular inflammation is of great importance to prevent joint damage. A variety of disease-modifying antirheumatic drugs (DMARDs) are available for RA patients. Conventional synthetic DMARDs, and in particular biological DMARDs, have been shown to effectively inhibit joint destruction in RA. Longitudinal assessments of radiographic changes in patients with RA in clinical trials and in large patient registries have clearly shown that delays in the initiation of DMARD therapy results in significantly increased progression of joint damage. Patients started early on DMARDs had significantly lower radiographic damage progression than patients initiating DMARD treatment later. These effects were maintained for several years, suggesting that early in the development of RA a therapeutic window of opportunity exists in which DMARD therapy decisively influences the long-term prognosis. Therefore, to improve the clinical outcome of RA, our efforts should be directed towards diagnosing RA earlier and introducing DMARD therapy immediately after the diagnosis has been made.
Key words: biologic; bone erosion; DMARD; joint damage; radiographic progression; rheumatoid arthritis; SCQM; window of opportunity
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease primarily affecting the joints. Chronic synovitis in RA leads to progressive joint damage in the great majority of patients. Inflammatory cells in the synovium produce cytokines that activate proliferation of synovial fibroblasts and macrophages. The resulting hyperplastic synovium is the source of proteases that degrade articular cartilage and of osteoclast activating factors. Activated osteoclasts lead to bone erosions, typically at the junction of bone and cartilage. Whereas erosions are usually not visible on conventional radiographs in the early stages of the disease, clinical studies using magnetic resonance imaging (MRI) have clearly demonstrated that bone erosions occur as early as 4 months after the first symptoms of joint inflammation [1]. As bone and cartilage damage is irreversible, early therapeutic intervention is of paramount importance for the prognosis of patients.
Major advances have been made in controlling joint damage with the introduction of the so-called disease-modifying antirheumatic drugs (DMARDs). Evidence from clinical trials has shown that treatment of RA with DMARDs results in less radiographically assessed damage and a better long-term prognosis [2]. In particular, it has been shown that early initiation of DMARD therapy has a long-term beneficial effect on joint destruction that extends far beyond the initial treatment period. This has led to a paradigm of RA including a therapeutic “window of opportunity” in early disease [3, 4].
In this review the mechanisms of joint damage and available outcome measures will be discussed. Then the evidence for early therapeutic intervention will be summarised with special consideration of the data from the Swiss Clinical Quality Management (SCQM) patient cohort.
Mechanisms of joint damage
Synovial hyperplasia is the origin of joint damage. Monocytes are recruited into the synovium and locally differentiate into macrophages. Proliferation of synovial fibroblasts in the synovial membrane lining is driven by cytokines produced by leukocytes in the synovium and local production by the activated synovial cells themselves. Fibroblast-like synoviocytes of patients with RA typically display an activated phenotype, characterised by resistance to apoptosis, loss of contact inhibition and the production of proinflammatory cytokines, contributing to sustained synovial hypertrophy. In addition, activated fibroblast-like synoviocytes are a source of matrix-degrading enzymes [5, 6]. Activity of matrix metalloproteinases, together with increased chondrocyte apoptosis caused by inflammatory cytokines, results in destruction of cartilage and, consequently, impairment of proper joint function.
A hallmark of RA is the formation of bone erosions, typically at the junction of cartilage and bone. Osteoclast differentiation is under the control of cytokines. Receptor activator of nuclear factor kappa-B ligand (RANKL) and granulocyte-macrophage colony-stimulating factor (GM-CSF) induce osteoclast differentiation, which is further supported by inflammatory cytokines abundantly present in the inflamed synovium, such as tumour necrosis factor-alpha (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6) [7]. Erosions can lead to significant loss of articular bone. Even in patients with sufficient control of inflammatory activity, repair of bone erosions does not occur. It is, therefore, of great importance to intervene before significant damage has occurred.
How can joint damage be measured in rheumatoid arthritis?
Imaging methods in the assessment of joint damage
Assessment of structural joint damage is an important part of disease monitoring in RA. Classically, X-ray has been used for this purpose. With conventional radiography bone erosions and periarticular osteopenia can be detected, as well as joint-space narrowing, which is the result of cartilage damage. RA typically involves the small joints of the hands and feet. Radiography allows a rapid, cost-effective and reproducible assessment of these joints. The presence of typical erosions on radiographs is highly specific for RA in patients with established, long-standing disease [8]. In the early stages of disease, the sensitivity of conventional radiography is much lower, however, with specificity remaining high [9]. Erosions are detected by conventional radiography in only 6%–40% of patients with RA at 6 months of disease duration [10]. Therefore the usefulness of conventional radiography for the early diagnosis of RA is limited. However, conventional radiography is still the method of choice for monitoring joint damage over time in patients with established disease.
Magnetic resonance imaging (MRI) has the advantage of allowing a three-dimensional assessment of the joints, including not only the bone, but also the cartilage and the soft tissues. The sensitivity of MRI for the detection of bone erosions in RA is higher than that of conventional radiography [11, 12] and is comparable to computed tomography, which is considered the gold standard for detection of erosions in RA [13].
In addition to the detection of bone erosions and cartilage degradation, MRI allows assessment of synovial inflammation and, thereby, the activity of the disease [14]. Thickening of the synovial membrane, increased synovial volume and enhancement of synovial tissue after administration of gadolinium are measures of the activity of synovitis [15]. MRI findings of synovitis have been shown to correlate well with macroscopic findings on arthroscopy and clinical disease activity [16]. Another feature seen exclusively in MRI is bone oedema. Bone oedema is nonspecific, as it can occur in traumatic and in degenerative and inflammatory bone disorders. Bone marrow oedema corresponds to cellular infiltrates in the subchondral bone [17]. Bone marrow oedema is highly prevalent in the early stages of RA and predicts erosive disease [18]. Owing to its superior sensitivity to bone damage in early RA, MRI is increasingly used in clinical studies for assessment of destructive changes.
Ultrasound has attracted a great deal of interest as a tool for diagnosis and for monitoring joint damage in RA and other rheumatic diseases. Much like MRI, it allows the detection of synovial thickening and synovial effusion. In addition, power Doppler provides a quantification of the vascularisation of the synovial tissue [19]. Ultrasonography is clearly more sensitive for the detection of bone erosions than conventional radiography in early RA [20]. Good sensitivity and specificity for erosions in metacarpophalangeal (MCP) joints has been documented for sonography in comparison with MRI [21]. However, its use is limited to joints easily accessible to examination, such as the hand joints. Assessment of cartilage thickness is possible with ultrasound. It has been shown that cartilage thickness measurements with sonography correlate with joint-space narrowing seen with conventional radiography in finger joints [22]. Ultrasound is increasingly used in clinical practice because of its availability at the bed-side and its relative inexpensiveness compared with MRI. Apart from being a powerful tool to detect synovitis in early stages of RA, power Doppler signal in the joints of patients with early RA has been shown to be predictive for radiographic progression in a longitudinal observational study [23]. Moreover, in DMARD-treated patients in clinical remission, power Doppler signal was a predictor of radiographic progression and of disease relapse [24, 25]. Thus, joint ultrasound may help to guide therapeutic decisions.
However, a variety of different scoring systems have been used in clinical studies. So far, there are no consensus definitions for the use of sonography in routine clinical practice or in clinical studies.
Imaging methods for the assessment of joint damage used in clinical studies
Several semiquantitative scoring methods have been developed to assess progressive joint damage over time on conventional radiographs. Available damage scores assess the level of damage at individual joints, which are then pooled to form a global damage score. Radiographic damage scores are considered the gold standard for assessment of disease progression in RA [26]. In fact, radiographic outcomes can be blinded for objective scoring; damage scores are not much influenced by short-term variations in disease activity, and they provide a cumulative measure of disease activity over time. Established scoring methods of radiographic joint damage include the Sharp scoring method (and various modifications of it), the Larsen scoring method, or the Ratingen scoring method, which is used by the Swiss RA cohort SCQM. These methods differ in the joints assessed or the way joint-space narrowing (measure of cartilage damage) is incorporated. Some randomised trials have added MRI scores or ultrasound scores to conventional radiographic damage scores, neither MRI scores nor ultrasound scores are currently accepted by the regulatory authorities as an outcome measure for joint damage.
|
Table 1: Conventional disease-modifying antirheumatic drugs (DMARDs) and biologics licensed in Switzerland for the treatment of rheumatoid arthritis. |
|
Conventional DMARDs
|
Biologics
|
| Methotrexate |
Tumour necrosis factor inhibitors:
Adalimumab
Certolizumab Pegol
Etanercept
Golimumab
Infliximab |
| Leflunomide |
Abatacept (T-cell costimulation blockade) |
| Sulfasalazine |
Tocilizumab (anti-interleukin-6 receptor antibody) |
| Hydroxychloroquine |
Rituximab (B-cell depleting antibody) |
| Less commonly used DMARDs:
Gold
Azathioprine
Ciclosporin |
|
Current principles of rheumatoid arthritis treatment
Once the diagnosis of RA is made, all patients should receive therapy with DMARDs. DMARDs are defined as drugs able to inhibit or delay the destructive changes occurring in joints of patients with RA. Although glucocorticosteroids also have some disease-modifying effects, they are usually not included in this group, as their side effects prevent them from being used as monotherapy for long-term control of RA. There are a variety of DMARDs with proven efficacy in RA (table 1). They are divided in conventional DMARDs, small molecules with immuno-suppressive or -modulatory function, and the so-called biological DMARDs. The latter are drugs which are biotechnologically generated and specifically target key pathways of autoimmunity and inflammation. As these drugs are usually proteins, antibodies or receptor fusion proteins, they have to be administered parenterally. The increasing number of DMARDs licensed for treatment of RA has presented rheumatologists with the difficulty of choosing the appropriate drug or combination of drugs for an individual RA patient.
A multitude of clinical studies have been performed in recent years permitting guidelines for the use of DMARDs in RA to be worked out. Recently, new guidelines by the European League against Rheumatism (EULAR) have been published [2]. To summarise these briefly, patients with RA should receive methotrexate as first-line therapy, in the absence of contraindications. If methotrexate is not tolerated or insufficiently effective, therapy can either be switched to another synthetic DMARD or another DMARD can be added to methotrexate. Biological therapy in combination with methotrexate is recommended in patients with insufficient response to methotrexate and risk factors for progressive erosive disease. Clinical trials have demonstrated that the addition of a biologic in these patients results in a significant reduction of clinical disease activity and radiographic progression [27]. Importantly, a reduction in radiographic progression on biologics was observed even in patients with residual inflammatory activity, suggesting a disconnect between synovitis and erosive processes [28]. Therefore, in patients at high risk of radiographic joint damage, biologic drugs are indicated when first-line therapy with methotrexate has failed.
Current guidelines suggest that the treatment goal should be remission in patients with early disease. To monitor the effects of therapy on disease activity, rheumatologists use disease activity measures, such as the disease activity score based on 28 joints (DAS28), which can be easily performed during a visit to the office [29]. To achieve the ambitious goal of remission, early initiation of therapy is of great importance. Although this has been generally recognised, many patients with RA are not treated with DMARDs early in their disease. In the Swiss RA cohort the delay between symptom onset and the first antirheumatic treatment was 10 months, and a recent European study showed that the median time from symptom onset to assessment by a rheumatologist was 24 weeks [30]. This study suggests that in the majority of patients, DMARD therapy is started with a significant delay.
In the following section the evidence for a benefit of early start of DMARD treatment will be discussed.
Evidence for a long-term effect of early therapy on joint damage in rheumatoid arthritis
Several studies have demonstrated that early antirheumatic intervention in RA is particularly effective in preventing structural joint damage, with large effect sizes compared with similar interventions later in the disease course. Whereas the increased effect of early intervention can easily be explained by patient selection, some studies have further suggested that early initiation of DMARD treatment may have a long-term benefit [31–33]. Based on these findings, a therapeutic window of opportunity paradigm in early RA has emerged, a long-lasting effect from a limited, initial intervention that would permanently amend the course of joint destruction towards milder disease.
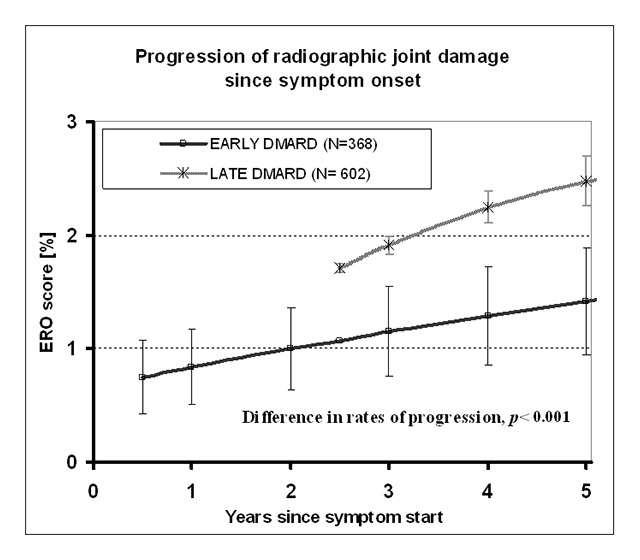
Figure 1
Progression of joint erosion score (Ratingen) over time in patients treated early vs patients treated late with disease-modifying antirheumatic drugs (DMARDs). Mean erosion (ERO) scores (± SEM) are shown as percentages of maximum damage score as a function of time since symptom onset. Regression analysis with adjustment for potential confounders of radiographic progression revealed a significant difference in the slopes of radiographic damage progression.
Reproduced from: Kyburz D, Gabay C, Michel BA, Finckh A. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: a population-based cohort study. Rheumatology (Oxford). 2011;50(6):1106–10, by permission of the British Society of Rheumatology.
A meta-analysis of 12 published studies confirmed that the long-term rates of radiographic damage are significantly lower in patients starting DMARD therapy early as compared with patients starting the same therapy later [34]. A delay of only 9 months in initiating antirheumatic treatment resulted in significantly increased subsequent joint damage over the next years. Although the benefit of early treatment has been confirmed in several other recent extension studies, a recent analysis of an early intervention study with conventional DMARDs has examined the rate of radiographic damage progression between 5 and 11 years after the initial intervention and could no longer show a difference in the rates of radiographic progression between the two treatment arms, which was present in the first 5 years [35]. Although this suggests that the benefit of early treatment may not persist for more than 5 years, the differences in absolute radiographic scores between the treatment arms reached at 5 years were maintained in the follow-up period up to 11 years. Another meta-analysis examined the prevalence of drug-free remission and found that symptom duration before DMARD treatment initiation was independently associated with remission, suggesting the existence of a “therapeutic window of opportunity” in early disease [36]. Finally, it is well established that RA has become a less severe disease over recent decades, which might be explained by more effective therapies, but this trend started long before the current biological treatments, suggesting that other factors play a role in improved disease outcomes, such as a change in the RA treatment paradigm with early aggressive therapy [37].
Considering the paucity of definitive randomised trials establishing the notion of a therapeutic window of opportunity in early RA, we studied the effects of early DMARD therapy on the long-term radiographic progression of RA using data from the Swiss Clinical Quality Management (SCQM) cohort of patients with RA [38]. This cohort offers the advantage of including longitudinal radiographic assessments.
Patients enrolled in the SCQM-RA, treated with DMARDs within the first 5 years of symptom onset and for whom serial radiographs were available were included in the analysis [39]. Patients who started a biological agent as first therapy were excluded as we considered these patients to be unrepresentative of the overall population of RA patients.
The primary outcome of the study was radiographic disease progression. The change in radiographic joint damage (Ratingen Score) compared with baseline was determined for all patients. The variable of interest was the latency between the first occurrence of symptoms and the initiation of DMARD therapy. Symptom onset rather than time of diagnosis was chosen as this is a better reflection of the period of time the patients had active disease before treatment was started, and circumvents the necessity to control for a delay in diagnosis of the disease. The patients were dichotomised into two groups according to the delay in DMARD initiation. In the “early group”, patients initiated DMARD therapy within 1 year after onset of symptoms. Patients starting DMARD therapy between 1 and 5 years after symptom onset constituted the “late group”.
A total of 970 patients were included in the analysis. Of these, 368 patients were in the early group with a median time to DMARD treatment initiation of 6 months. The 602 patients in the late group had a median time of 2.3 years to DMARD initiation. Baseline clinical criteria were not different with the exception of significantly higher disease activity in the early group. In accordance with the higher disease activity, the baseline rate of radiographic progression, calculated by dividing the baseline radiographic scores by the symptom duration, was significantly higher in the early group. More than 80% of the patients in both groups received methotrexate as a first DMARD.
The comparison of the rate of damage progression in the groups revealed that the subsequent progression of radiographic joint damage was lower in the early DMARD treatment group, although the baseline radiographic progression rate was higher in this group (fig. 1). When the result was adjusted for potential confounding factors such as clinical disease activity at baseline, cotherapy with DMARDs or glucocorticoids, or rheumatoid factor positivity, there was still significantly lower damage progression in the patients treated early with DMARD than with delayed DMARD treatment. Notably, the differences in progression rates persisted for the follow-up time of 4 years. Four years after symptom onset, mean radiographic progression was still higher in the late DMARD group than in the early group. This result was not dependent on the DMARD, as it was seen in patients treated with methotrexate as well as in patients on other conventional DMARDs. The benefit of early DMARD therapy was especially pronounced in the patients with a high radiographic progression at baseline.
Importantly, the reduced rate of radiographic progression found in the patients with early DMARD treatment translated into significantly lower absolute damage as assessed with the Ratingen score at 5 years. This result clearly shows that early initiation of DMARD treatment results in a long-lasting benefit, with reduced damage over several years. Thus, this large study of the SCQM-RA cohort strongly supports the concept of a window of opportunity in RA, suggested previously in several smaller studies.
The definition of early RA varied in the different studies. In the SCQM study, a symptom duration of less than 1 year was used to define early RA, with a median symptom duration of 6 months. Other studies have used more stringent criteria with symptom durations of 6 months or less. The majority of these studies have shown a beneficial effect of early therapy [36]. Notably, even when patients treated within 3 months were compared with patients treated at more than 3 months after symptom onset significantly lower radiographic progression could be demonstrated [32, 40, 41]. These results suggest that the therapeutic window of opportunity may be as short as a few months. The fact that a delay in therapy even of only 3 months may lead to significantly increased joint damage over the following several years is a reminder of our responsibility to initiate DMARD therapy as soon as patients are diagnosed with RA, with the aim of achieving a remission of disease.
Conclusion
Improved understanding of the pathogenesis of RA has led to important advances in the treatment of this debilitating disease. Nowadays, an increasing number of potent antirheumatic agents are available, allowing disease control in the majority of the patients. As joint damage in RA cannot be reversed with currently available treatments, antirheumatic therapy should be started as early as possible to prevent permanent structural damage and long-term functional impairment. Data from clinical trials, as well as from large patient registries such as the SCQM, have demonstrated that delays in the initiation of DMARD therapy leads to significantly increased progression of joint damage, not only in the short run, but also during at least 5 years of follow-up. Furthermore, good clinical outcomes become rarer in patients in whom effective therapy is delayed early in the disease course. These data emphasise that a “window of opportunity” exists in early RA, a period early in the course of the disease in which therapy with DMARDs can effectively influence the long-term prognosis. It is, therefore, of great importance that general practitioners and rheumatologists join in their efforts to diagnose and treat patients with RA as early as possible.
References
1 McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57(6):350–6.
2 Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–75.
3 Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4.
4 O'Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46(2):283–5.
5 Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–55.
6 Juarez M, Filer A, Buckley CD. Fibroblasts as therapeutic targets in rheumatoid arthritis and cancer. Swiss Med Wkly. 2012;142:w13529.
7 Schett G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur J Clin Invest. 2011 Dec;41(12):1361–6.
8 Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
9 Nissila M, Isomaki H, Kaarela K, Kiviniemi P, Martio J, Sarna S. Prognosis of inflammatory joint diseases. A three-year follow-up study. Scand J Rheumatol. 1983;12(1):33–8.
10 Ostergaard M, Pedersen SJ, Dohn UM. Imaging in rheumatoid arthritis--status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol. 2008;22(6):1019–44.
11 McQueen FM, Benton N, Crabbe J, Robinson E, Yeoman S, McLean L, et al. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Ann Rheum Dis. 2001;60(9):859–68.
12 Rahmani M, Chegini H, Najafizadeh SR, Azimi M, Habibollahi P, Shakiba M. Detection of bone erosion in early rheumatoid arthritis: ultrasonography and conventional radiography versus non-contrast magnetic resonance imaging. Clin Rheumatol. 2010;29(8):883–91.
13 Perry D, Stewart N, Benton N, Robinson E, Yeoman S, Crabbe J, et al. Detection of erosions in the rheumatoid hand; a comparative study of multidetector computerized tomography versus magnetic resonance scanning. J Rheumatol. 2005;32(2):256–67.
14 Cimmino MA, Barbieri F, Zampogna G, Camellino D, Paparo F, Parodi M. Imaging in arthritis: quantifying effects of therapeutic intervention using MRI and molecular imaging. Swiss Med Wkly. 2012;141:w13326.
15 Ostergaard M, Ejbjerg B. Magnetic resonance imaging of the synovium in rheumatoid arthritis. Semin Musculoskelet Radiol. 2004;8(4):287–99.
16 Ostendorf B, Peters R, Dann P, Becker A, Scherer A, Wedekind F, et al. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum. 2001;44(11):2492–502.
17 McQueen FM, Gao A, Ostergaard M, King A, Shalley G, Robinson E, et al. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis. 2007;66(12):1581–7.
18 Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67(6):794–800.
19 Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485–7.
20 Wakefield RJ, Gibbon WW, Conaghan PG, O'Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43(12):2762–70.
21 Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, Jensen KE, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8(2):R52.
22 Moller B, Bonel H, Rotzetter M, Villiger PM, Ziswiler HR. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis Rheum. 2009;61(4):435–41.
23 Funck-Brentano T, Gandjbakhch F, Etchepare F, Jousse-Joulin S, Miquel A, Cyteval C, et al. Prediction of radiographic damage in early arthritis by sonographic erosions and power Doppler signal: a longitudinal observational study. Arthritis Care Res (Hoboken). 2013;65(6):896–902.
24 Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–73.
25 Scire CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford). 2009;48(9):1092–7.
26 van der Heijde DM. Radiographic imaging: the 'gold standard' for assessment of disease progression in rheumatoid arthritis. Rheumatology (Oxford). 2000;39 Suppl 1:9–16.
27 Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69(6):976–86.
28 Dohn UM, Ejbjerg B, Boonen A, Hetland ML, Hansen MS, Knudsen LS, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis. 2011;70(2):252–8.
29 Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57, vii-viii.
30 Raza K, Stack R, Kumar K, Filer A, Detert J, Bastian H, et al. Delays in assessment of patients with rheumatoid arthritis: variations across Europe. Ann Rheum Dis. 2011;70(10):1822–5.
31 Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111(6):446–51.
32 Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43(7):906–14.
33 van Aken J, Lard LR, le Cessie S, Hazes JM, Breedveld FC, Huizinga TW. Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63(3):274–9.
34 Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55(6):864–72.
35 van Tuyl LH, Boers M, Lems WF, Landewe RB, Han H, van der Linden S, et al. Survival, comorbidities and joint damage 11 years after the COBRA combination therapy trial in early rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):807–12.
36 van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, van der Helm-van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2013.
37 Finckh A, Choi HK, Wolfe F. Progression of radiographic joint damage in different eras: trends towards milder disease in rheumatoid arthritis are attributable to improved treatment. Annals of the rheumatic diseases. 2006;65(9):1192–7.
38 Uitz E, Fransen J, Langenegger T, Stucki G. Clinical quality management in rheumatoid arthritis: putting theory into practice. Swiss Clinical Quality Management in Rheumatoid Arthritis. Rheumatology (Oxford). 2000;39(5):542–9.
39 Kyburz D, Gabay C, Michel BA, Finckh A. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: a population-based cohort study. Rheumatology (Oxford). 2011;50(6):1106–10.
40 van der Linden MP, le Cessie S, Raza K, van der Woude D, Knevel R, Huizinga TW, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62(12):3537–46.
41 Lukas C, Combe B, Ravaud P, Sibilia J, Landew R, van der Heijde D. Favorable effect of very early disease-modifying antirheumatic drug treatment on radiographic progression in early inflammatory arthritis: Data from the Etude et Suivi des polyarthrites indifferenciees recentes (study and followup of early undifferentiated polyarthritis). Arthritis Rheum. 2011;63(7):1804–11.
