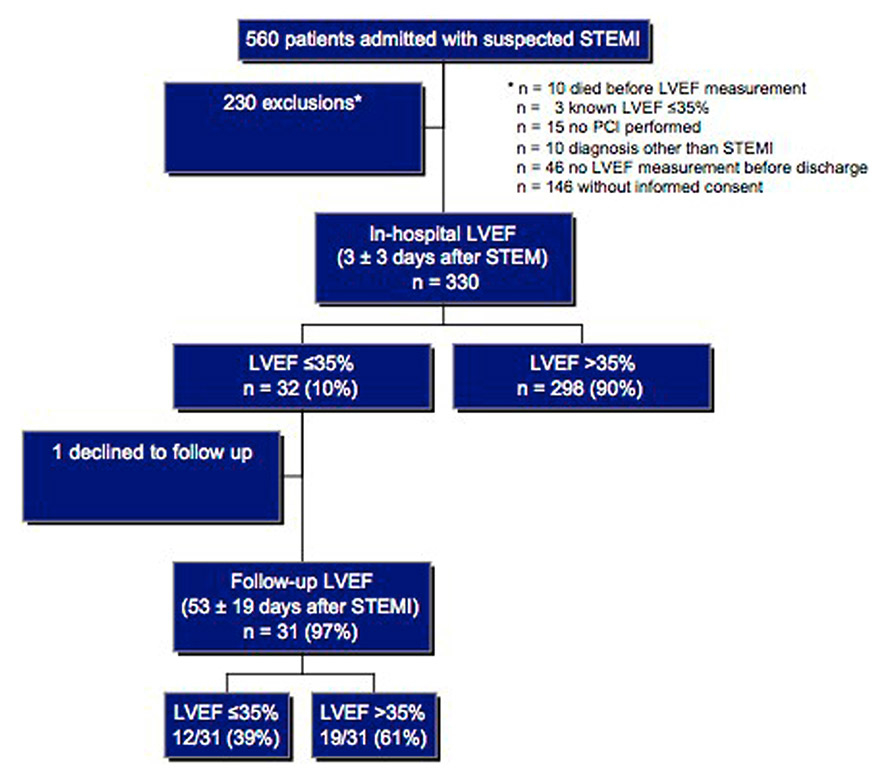
Figure 1
Study flow chart – patient recruitment and group outcome.
LVEF = left ventricular ejection fraction; STEMI = ST-segment elevation myocardial infarction
DOI: https://doi.org/10.4414/smw.2013.13869
Current guidelines for the prevention of sudden cardiac death (SCD) after myocardial infarction recommend the implantation of an implantable cardioverter defibrillator (ICD) in patients with a left ventricular ejection fraction (LVEF) ≤35% (for patients in NYHA class II or III) or ≤30% (NYHA class I) not earlier than 40 days after myocardial infarction [1]. However, the landmark studies leading to these recommendations started patient recruitment in 1990 and 1997 [2, 3]. The contemporary management of myocardial infarction with aggressive early revascularisation therapy by percutaneous coronary intervention (PCI), as well as the significant improvement of medical therapy, has markedly changed and the Multicenter Automatic Defibrillator Implantation Trial (MADIT I and II) populations do not necessarily reflect today’s post-myocardial infarction patients [4, 5]. The Defibrillator in Acute Myocardial Infarction Trial (DINAMIT) demonstrated no survival benefit for patients with a LVEF <40% receiving an ICD 6 to 40 days after an acute myocardial infarction [6]. Follow-up LVEF measurements were carried out in only 47% of patients. Hence the evolution of a depressed LVEF early after myocardial infarction in the DINAMIT population is not known. The Immediate Risk Stratification Improves Survival (IRIS) trial replicated the data, including patients with an LVEF ≤40% 5 to 31 days after an acute myocardial infarction [7]. Unfortunately, it also does not comment on the evolution of the LVEF. Stunned or hibernating myocardium may recover after revascularisation and the LVEF will improve in many patients [8]. For the clinician a common clinical problem is when to implant an ICD in a patient who suffered a myocardial infarction and has an impaired LVEF. Prospective data regarding the prevalence and time course of a severely impaired LVEF after STEMI are sparse. We conducted a prospective observational study in STEMI patients treated with primary PCI to assess the incidence of a depressed LVEF ≤35% early after a STEMI and to evaluate the evolution of the LVEF in these patients.
This study was performed as an observational study between September 2008 and March 2010 at the University Hospital Basel (UHBS) and the Kantonsspital St. Gallen (KSSG), and was approved by the local ethics committee. Patients admitted for the treatment of STEMI were screened. The data were prospectively recorded from medical charts. Patients were included if PCI was performed within 12 hours after the onset of symptoms and if the LVEF was assessed within 10 days after PCI (in-hospital LVEF). Exclusion criteria were known LVEF ≤35% of any cause before the index STEMI and inability to obtain informed consent.
The diagnosis of STEMI was based on the history of chest pain lasting for 15 minutes or more in association with ST-segment elevation in two contiguous leads (cut-off points: ≥0.2 mV in leads V2–V3 and/or ≥0.1 mV in other leads), or new or presumed new left bundle-branch block, and was confirmed by the presence of an unstable coronary lesion on angiography [9, 10]. Cardiogenic shock as a clinical state of hypoperfusion was diagnosed if systolic blood pressure was <90 mm Hg, or if the central filling pressure (wedge pressure) was >20 mm Hg, or if intravenous inotropes and/or mechanical catheter-based cardiac assist devices (UHBS: Impella, Abiomed Europe; KSSG: intra-aortic balloon pump, Sensation 7 Fr IAB Catheter, Maquet Getinge Groupe) were needed to maintain a systolic blood pressure >90 mm Hg. Periprocedural resuscitation was defined as electrical cardioversion and/or mechanical resuscitation.
LVEF assessment by echocardiography was at the discretion of the treating physician and was estimated, preferably using the Simpson biplane formula. Only LVEF measurements assessed after removal of mechanical assist devices and inotropics were recorded. Two cardiologists, blinded for the subjects study participation, reviewed all echocardiography studies. Follow-up LVEF was reassessed using echocardiography 6 to 8 weeks after the index event in patients with an in-hospital LVEF ≤35%. A telephone follow-up was performed to assess mortality at the same period in those with an in-hospital LVEF >35%. Cause of death was classified as recorded in medical records. Death, either in hospital or after hospital discharge, was assumed to be a SCD if it occurred within minutes after the onset of symptoms, resulted from a documented cardiac arrhythmia, or was witnessed and occurred unexpectedly.
Peripheral venous blood specimens were taken at the time of the PCI and during the hospital stay on a daily basis. The first available peak creatine kinase was considered to be the peak value and was used in the analysis. The serum creatinine value used was the first available, preferably from the specimen taken at the time of PCI. QRS width was measured manually on a 12-lead surface ECG performed on the day following the PCI.
PCI was performed in standard fashion. All patients received a loading dose of 600 mg clopidogrel, and aspirin-naïve patients were administered aspirin 250 mg intravenously before PCI. Glycoprotein IIb/IIIa receptor antagonists (abciximab was used at UHBS and tirofiban at KSSG) were given at the discretion of the physician performing the intervention, but was routinely administered in the presence of reduced coronary flow after successful PCI as assessed with the Thrombolysis in Myocardial Infarction (TIMI) grading system. Heparin was given as a bolus of 5,000 IU before primary PCI and all patients had either a bare metal or drug eluting stent implanted. Postprocedural antiplatelet therapy consisted of aspirin 100 mg/day and clopidogrel 75 mg/day for at least 12 months.
The initial and postprocedural blood flow in the infarct-related artery was graded by the physician performing the PCI according to the Thrombolysis in Myocardial Infarction (TIMI) grading system [11]. The diagnosis of a “no reflow phenomenon” required angiographic evidence of a patent artery after successful PCI with no evidence of residual stenosis (<50%) after intracoronary administration of nitroglycerine and exclusion of dissection, spasm, or thrombus and a TIMI flow <3, at least 10 minutes after PCI.
The primary outcome was a persistently depressed LVEF ≤35% between two time intervals, namely in-hospital (≤10 days post-PCI) and at follow-up (6–8 weeks post-PCI) in patients with an in-hospital LVEF ≤35%. Mortality during the study period was a secondary outcome.
Continuous variables are expressed as mean with standard deviation (SD) if normally distributed or as median with interquartile range (IQR) in the case of deviation from normality. An incidence of patients with a LVEF ≤35% and the 95% confidence interval (CI) is given for in-hospital and follow-up LVEF measurements. The number of patients with available in-hospital and follow-up LVEF measurements were taken as denominator. Differences between patients with and without depressed LVEF were compared using the chi-square test or Fisher exact test for categorical variables. Normally distributed numerical variables were analysed using the Student t-test and in case of deviation from normality with the Mann-Whitney U test. A two-tailed p value of <0.05 was considered to indicate statistically significant differences. Analyses were performed using Prism software package version 5.0 (Graph Pad Software for Mac OS X, Inc.)
Patient recruitment and group outcomes are summarised in figure 1.

Figure 1
Study flow chart – patient recruitment and group outcome.
LVEF = left ventricular ejection fraction; STEMI = ST-segment elevation myocardial infarction
From a total of 560 patients admitted with suspected STEMI during the study period, 230 patients were excluded for following reasons:
Ten patients in whom LVEF was not known died. Causes of death in these patients were progressive heart failure (n = 5), incessant ventricular fibrillation (n = 1), cerebral hypoxia after resuscitation (n = 3), and noncardiac (n = 1). PCI was not successful in nine, not performed in six and a diagnosis other than STEMI (e.g. Takotsubo cardiomyopathy) was made in ten patients. LVEF was known to be ≤35% before the index STEMI in three patients and forty-six patients had no LVEF measurement before hospital discharge. In 146 patients informed consent could not be obtained.
Thus, the study population consisted of 330 patients (table 1). In-hospital LVEF measured 3 ± 3 days after PCI was ≤35% in 32/330 patients (10%, 95% CI 13%–67%). LVEF was obtained using the Simpson’s biplane formula in 249/330 (76%) patients and assessed visually in the remaining. Patients with an in-hospital LVEF ≤35% presented with higher creatine kinase levels and more often in cardiogenic shock. Use of cardiac assist devices and left anterior descending coronary artery as culprit vessel or multi-vessel disease were more frequent. Length of stay at the intensive care unit and QRS duration were longer. For history of previous myocardial infarction or revascularisation (PCI or surgical), use of antithrombotic agents during PCI and symptom-to-balloon time, no difference between the groups was found. Most patients (82%) had complete revascularisation, defined as no residual coronary artery stenosis >75% after the index PCI. In the remaining patients, revascularisation was performed on average 23 ± 18 days following the index PCI (75% PCI, 25% coronary artery bypass graft [CABG]). In the group of patients with an in-hospital LVEF ≤35%, ten were revascularised 20 ± 15 days following STEMI.
Follow-up LVEF was available for 31/32 (97%) patients 53 ± 19 days following STEMI (one patient declined a follow-up visit) and improved to >35% in 19/31 patients (61%, 95% CI 42%–78%) (LVEF 42% ± 4% vs 30% ± 5%). The incidence of a LVEF ≤35% at follow-up was therefore 39% (12/31, 95% CI 22%–56%).
The LVEF recovered to >35% in seven of ten patients revascularised during the follow-up period. Patients without LVEF improvement had longer symptom-to-balloon time (290 min [194–593] vs 200 min [135–285]; table 2) when compared with those with an improved LVEF >35%. No patient died during follow-up. The demographic characteristics of the two groups are depicted in table 2. Adherence to heart failure therapy prescribed at discharge was 100% in all patients at follow-up. An aldosterone antagonist was prescribed for four patients during follow-up.
| Table 1:Baseline characteristics of the total study population and by in-hospital LVEF ≤35% and >35%. | ||||
| Total (n = 330 ) | LVEF ≤35% (n = 32) | LVEF >35% (n = 298) | p-value | |
| Age (SD), y | 63 ± 12 | 65 ± 13 | 63 ± 12 | 0.4 |
| Men, n (%) | 260 (79) | 23 (72) | 237 (80) | 0.4 |
| BMI [IQR], kg/m2 | 26 [24–29] | 27 [24–29] | 26 [24–28] | 0.8 |
| Medical history, n (%) | ||||
| Hypertension | 180 (55) | 19 (59) | 162 (54) | 0.7 |
| Hyperlipidaemia | 201 (62) | 24 (88) | 180 (60) | 0.1 |
| Smoker | 197 (60) | 19 (59) | 178 (60) | 1.0 |
| Diabetes mellitus | 42 (13) | 3 (9) | 39 (13) | 0.8 |
| Family history positive | 90 (27) | 8 (25) | 82 (28) | 0.8 |
| Previous myocardial infarction | 27 (8) | 4 (13) | 23 (8) | 0.3 |
| Previous PCI | 31 (9) | 5 (16) | 26 (9) | 0.2 |
| Previous CABG | 6 (2) | 2 (6) | 4 (1) | 0.1 |
| Procedural characteristics | ||||
| Creatinine [IQR], µg/L | 81 [70–92] | 83 [72–88] | 81 [70–92] | 1.0 |
| Peak creatine kinase [IQR], U/l | 1946 [980–3709] | 4493 [2493–6535] | 1853 [955–3224] | <0.0001 |
| Cardiogenic shock, n (%) | 15 (5) | 4 (13) | 11 (4) | 0.05 |
| Assist device, n (%) | 14 (4) | 4 (13) | 10 (3) | 0.04 |
| Culprit vessel LAD, n (%) | 164 (50) | 28 (88) | 136 (46) | <0.0001 |
| Multivessel disease, n (%) | 178(54) | 23 (72) | 155 (52) | 0.04 |
| Postprocedural TIMI flow <III, n (%) | 41 (12) | 7 (22) | 34 (11) | 0.1 |
| Mechanical ventilation, n (%) | 18 | 3 (9) | 15 (5) | 0.4 |
| GP IIb/IIIa-inhibitor use, n (%) | 248 (75) | 24 (75) | 224 (75) | 1 |
| Symptom to balloon time [IQR], minutes | 195 [145–300] | 212 [150–348] | 194 [144–300] | 0.3 |
| Duration of ICU care [IQR], d | 2 [1–2] | 2 [2–5] | 2 [1–2] | 0.0002 |
| Treatment with hypothermia, n (%) | 7 (2) | 7 (2) | n/a | |
| Echocardiographic characteristics* | ||||
| Left ventricular ejection fraction (SD), % | 49 ± 10 | 32 ± 4 | 51 ± 8 | < 0.0001 |
| LVEDD, (SD), mm | 48 ± 8 | 52 ± 8 | 48 ± 7 | 0.01 |
| LVESD, (SD), mm | 34 ± 8 | 40 ± 10 | 33 ± 7 | 0.009 |
| QRS width [IQR], ms | 96 [90–104] | 102 [91–113] | 96 [90–104] | 0.04 |
| QRS width ≥120 (SD), ms | 31 (9) | 4 (13) | 24 (8) | 0.3 |
| Left bundle branch block, n (%) | 4 (1) | 4 (1) | 0.6 | |
| Medication at hospital discharge, n (%) | ||||
| Aspirin | 329 (99) | 32 (100) | 297 (99) | 1.0 |
| Clopidogrel | 330 (100) | 32 (100) | 298 (100) | 1.0 |
| ACE inhibitor or ARB | 267 (81) | 28 (88) | 243 (82) | 0.5 |
| Beta-blocker | 307 (93) | 29 (91) | 278 (93) | 0.5 |
| Statin | 328 (99) | 32 (100) | 296 (99) | 1.0 |
| Diuretic | 37 (11) | 11 (34) | 26 (9) | 0.0002 |
| Anticoagulant | 18 (6) | 5 (16) | 13 (4) | 0.02 |
| ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blockers; BMI = body mass index; CABG = coronary artery bypass graft; GP = glycoprotein; IQR = interquartile range; LAD = left anterior descending artery; LVEDD = left ventricular end-diastolic diameter; LVEDV = left ventricular end-diastolic volume; LVESD = left ventricular end-diastolic diameter; LVESV = left ventricular end-systolic volume; PCI = percutaneous coronary intervention; SD = standard deviation; TIMI = thrombolysis in myocardial infarction. * Left ventricular diameter was available in 295/330 (89%) of subjects. | ||||
The incidence of a severely depressed LVEF ≤35% was 10% within days following revascularisation of an acute STEMI and improved to >35% within 7 weeks in 61% of these patients, resulting in an incidence of sustained depressed LVEF ≤35% of 39% (12/31, 95% CI 22%–56%).
Although overall survival was not improved by early primary preventive ICD therapy in the DINAMIT trial, death due to arrhythmia occurred more often in controls on medical therapy (hazard ratio for ICD group 0.42, p = 0.009) and deaths from nonarrhythmic causes in the ICD group were more frequent, not least due to device-related complications [6]. However, a high probability of left ventricular (LV) function improvement following revascularised acute coronary syndrome explains the missing benefit of early primary preventive ICD therapy in this population.
A recent study evaluated infarction size and the evolution of LV function by sestamibi scintigraphic imaging following reperfused STEMI [8]. Reduction of the preinterventional infarction size of 25% to 8% at 6 months follow-up was associated with improvement of LV function and long-term survival, although subjects had a preserved LVEF at baseline. The MISSION AMI trial identified 8% of patients with a severely depressed LVEF ≤35% using gated SPECT 3 months after reperfused acute coronary syndrome [12]. Of those, 4% had a LVEF ≤30% and overall 6% of patients were candidates for ICD implantation <1 year after myocardial infarction. In CARISMA an improvement of LVEF following myocardial infarction from baseline 31% ± 6% to 35% ± 10% was observed during a follow up of 6 weeks [13]. LVEF showed fewer recovery properties in the subgroup of patients with an arrhythmic event compared with patients without. In a recent series from Zwolle, 13% of 2,544 patients treated with PCI for a STEMI had a LVEF of <30% more than 30 days after infarction [14]. Although the investigators did not provide an initial LVEF, they identified multivessel disease and reinfarction within 1 year as risk factors for subsequent death, and 40% of the deaths were attributed to SCD.
Risk of persistently severe LVEF dysfunction can be explained by the magnitude of LV dysfunction and infarct size following reperfusion.
Patients with persistently severe LV dysfunction in our study showed a trend for lower LVEF early after revascularisation (see table 2). Peak creatine kinase was not different between the groups, but was higher in the group with an LVEF improvement during follow-up. This finding might be explained by more rapid coronary reperfusion as demonstrated earlier in patients with patent coronary arteries following thrombolysis–induced reperfusion compared with those with a reduced coronary flow, suggesting a washout phenomenon [15]. A lower baseline LVEF and larger infarct size have been associated with failure of LV recovery [8].
Patients with LV remodelling presented more often with a lower LVEF and larger infarct size seen on magnetic resonance imaging and as indicated by higher creatine kinase levels [16]. Peak and cumulative creatine kinase levels have been correlated to infarct size, short- and long-term impairment of LVEF, and death [17, 18]. Nienhuis et al. studied the prognostic value of creatine kinase in large-scale, prospective observational studies performed in STEMI patients treated with primary PCI. They found that patients with anterior wall myocardial infarction are at increased risk for higher creatine kinase and that the peak creatine kinase is an independent predictor of LV dysfunction and 1-year mortality [19, 20].
The median time from the onset of symptoms to the first balloon inflation was 3 hours in our study and patients with a persistently reduced LVEF ≤35% at follow-up had longer symptom-to-balloon times. Other authors reported significant longer symptom-to-reperfusion times (mean 287 vs 258 min, p = 0.03) in subjects with an in-hospital LVEF ≤40% and inducible ventricular tachycardia in an electrophysiology study performed before hospital discharge as compared with those without. In one multicentre registry, the LVEF measured before discharge was significant lower with a prolonged symptom-to-balloon time of >240 min but did not differ at 1 month [21, 22].
Although arrhythmic risk is of concern early after STEMI, those who received appropriate shocks in the DINAMIT trial had more episodes of heart failure and myocardial infarction, and nonarrhythmic deaths were more frequent, which offset the observed sudden death reduction [6, 23].
Owing to the observational character of this study, not all eligible patients were analysed because of missing data on LV function before hospital discharge. Furthermore, since the two hospitals performing PCI are tertiary centres with early transfer of the patients to the referring centres, a considerable number of patients could not provide informed consent. In addition, a fraction of the study population with an in-hospital LVEF ≤35% might have presented with a hitherto unknown LVEF ≤35%. Furthermore as LVEF measurement was at the discretion of the treating physician, some eligible patients were discharged without in-hospital LVEF assessment. The lower enzymatic infarct size in these patients as compared with patients in whom LVEF was measured during hospital stay might have influenced the physician’s decision (see table 1). Therefore, a selection bias can be assumed in the presented study population.
Cardiac troponins (cTn) are the preferred biomarker to detect myocardial necrosis and have been shown to be of predictive value in the setting of STEMI [24]. However, the enzymatic infarct size in acute STEMI patients measured as the cumulative creatine kinase release seems to correlate with peak cTnT [17]. As different cTn measurements were used in the two participating hospitals, we did not evaluate the impact of cTn.
Coronary flow following PCI was assessed angiographically using the Thrombolysis In Myocardial Infarction (TIMI) flow grade. This method is widely used clinically to describe coronary flow before and after revascularisation, but it mainly describes epicardial blood flow and neglects myocardial blood flow, and hence coronary microcirculation. However, because of the observational character of this study, quantitative assessment of coronary microcirculation (e.g. using contrast echocardiography, magnetic resonance imaging, doppler flow wires or combined pressure and temperature-tipped guidewires) was not performed [25].
As we recorded only LVEF, we are not aware of other mechanisms that might have contributed to a reduced LVEF in the in-hospital and follow-up phase (e.g. role of functional mitral regurgitation). Finally, cardiogenic shock was not necessarily due to LV dysfunction but probably due to right ventricular dysfunction in the setting of right ventricular myocardial infarction.
The low event rate of sustained reduced LVEF ≤35% might have influenced the statistical power.
| Table 2:Baseline characteristics of patients with LVEF ≤35% and >35% at follow-up. | |||
| LVEF ≤35% (n = 12) | LVEF >35% (n = 19) | p-value | |
| Age (SD), y | 66 ± 15 | 64 ± 11 | 0.2 |
| Previous myocardial infarction | 1 (8) | 3 (16) | 1.0 |
| Previous revascularisation | 2 (17) | 3 (16) | 1.0 |
| Creatinine [IQR], µg/L | 80 [72–86] | 84 [72–93] | 0.6 |
| Peak creatine kinase [IQR], U/l | 4041 [2666–5839] | 6280 [2484–6690] | 0.4 |
| Cardiogenic shock, n (%) | 1 (8) | 3 (16) | 1.0 |
| Culprit vessel LAD, n (%) | 10 (83) | 17 (90) | 0.6 |
| Multivessel disease, n (%) | 4 (33) | 5 (26) | 0.7 |
| Postprocedural TIMI flow <III, n (%) | 2 (17) | 4 (21) | 1.0 |
| Symptom to balloon time [IQR], minutes | 290 [194–593] | 200 [135–285] | 0.01 |
| Duration of ICU care [IQR], d | 2.5 [2–7] | 2 [2–2] | 0.2 |
| In-hospital LVEF, % | 30 ± 4 | 33 ± 3 | 0.09 |
| In-hospital LVEDD, (SD), mm | 54 ± 8 | 50 ± 6 | 0.1 |
| In-hospital LVESD, (SD), mm | 43 ± 10 | 37 ± 8 | 0.07 |
| QRS width [IQR], ms | 103 [86–112] | 102 [94–110] | 0.1 |
| QRS width ≥120 (SD), ms | 2 (17) | 1 (5) | 0.5 |
| Left bundle branch block, n (%) | 4 (21) | 0.1 | |
| IQR = interquartile range; LAD = left anterior descending artery; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-diastolic diameter; PCI = percutaneous coronary intervention; SD = standard deviation; TIMI = thrombolysis in myocardial infarction | |||
Our data demonstrate that the incidence of severely impaired LV function early after STEMI treated with acute PCI is 10% and that the likelihood of LVEF improvement within weeks is high, with the majority of patients having an initially severely depressed LVEF improving to >35%. These findings support an expectant strategy early after STEMI before considering primary preventive ICD implantation.
1 Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS et al. Acc/Aha/Hrs 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the Acc/Aha/Naspe 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62.
2 Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved Survival With an Implanted Defibrillator in Patients With Coronary Disease At High Risk for Ventricular Arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–40.
3 Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic Implantation of a Defibrillator in Patients With Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002;346(12):877–83.
4 Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 Focused Update of the Acc/Aha 2004 Guidelines for the Management of Patients With St-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the Canadian Cardiovascular Society Endorsed By the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With St-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117(2):296–329.
5 Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. Acc/Aha 2007 Guidelines for the Management of Patients With Unstable Angina/Non St-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non St-Elevation Myocardial Infarction): Developed in Collaboration With the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: Endorsed By the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116(7):e148–304.
6 Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic Use of an Implantable Cardioverter-Defibrillator After Acute Myocardial Infarction. N Engl J Med. 2004;351(24):2481–8.
7 Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator Implantation Early After Myocardial Infarction. N Engl J Med. 2009;361(15):1427–36.
8 Ndrepepa G, Mehilli J, Martinoff S, Schwaiger M, Schomig A, Kastrati A. Evolution of Left Ventricular Ejection Fraction and Its Relationship to Infarct Size After Acute Myocardial Infarction. J Am Coll Cardiol. 2007;50(2):149–56.
9 Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of Acute Myocardial Infarction in Patients Presenting With Persistent St-Segment Elevation: The Task Force on the Management of St-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–45.
10 Thygesen K, Alpert JS, and White HD. Universal Definition of Myocardial Infarction. Eur Heart J. 2007;28(20):2525–38.
11 Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. Timi Risk Score for St-Elevation Myocardial Infarction: A Convenient, Bedside, Clinical Score for Risk Assessment At Presentation: An Intravenous Npa for Treatment of Infarcting Myocardium Early Ii Trial Substudy. Circulation. 2000;102(17):2031–7.
12 Atary JZ, Borleffs CJ, Liem SS, Bax JJ, van der Hoeven BL, Bootsma M, et al. Structured Care for Patients After Acute Myocardial Infarction: Sudden Cardiac Death Prevention – Data From the Leiden Mission! Ami Study. Europace. 2010;12(3):378–84.
13 Huikuri HV, Raatikainen MJ, Moerch-Joergensen R, Hartikainen J, Virtanen V, Boland J, et al. Prediction of Fatal Or Near-Fatal Cardiac Arrhythmia Events in Patients With Depressed Left Ventricular Function After an Acute Myocardial Infarction. Eur Heart J. 2009;30(6):689–98.
14 Ottervanger JP, Ramdat Misier AR, Dambrink JH, de Boer MJ, Hoorntje JC, Gosselink AT, et al. Mortality in Patients With Left Ventricular Ejection Fraction </=30% After Primary Percutaneous Coronary Intervention for St-Elevation Myocardial Infarction. Am J Cardiol. 2007;100(5):793–7.
15 Zabel M, Hohnloser SH, Koster W, Prinz M, Kasper W, Just H. Analysis of Creatine Kinase, Ck-Mb, Myoglobin, and Troponin T Time-Activity Curves for Early Assessment of Coronary Artery Reperfusion After Intravenous Thrombolysis. Circulation. 1993;87(5):1542–50.
16 Lund GK, Stork A, Muellerleile K, Barmeyer AA, Bansmann MP, Knefel M, et al. Prediction of Left Ventricular Remodeling and Analysis of Infarct Resorption in Patients With Reperfused Myocardial Infarcts By Using Contrast-Enhanced Mr Imaging. Radiology. 2007;245(1):95–102.
17 Hassan AK, Bergheanu SC, Hasan-Ali H, Liem SS, van der Laarse A, Wolterbeek R, et al. Usefulness of Peak Troponin-T to Predict Infarct Size and Long-Term Outcome in Patients With First Acute Myocardial Infarction After Primary Percutaneous Coronary Intervention. Am J Cardiol. 2009;103(6):779–84.
18 Turer AT, Mahaffey KW, Gallup D, Weaver WD, Christenson RH, Every NR, et al. Enzyme Estimates of Infarct Size Correlate With Functional and Clinical Outcomes in the Setting of St-Segment Elevation Myocardial Infarction. Curr Control Trials Cardiovasc Med. 2005;6:12.
19 Nienhuis MB, Ottervanger JP, de Boer MJ, Dambrink JH, Hoorntje JC, Gosselink AT, et al. Prognostic Importance of Creatine Kinase and Creatine Kinase-Mb After Primary Percutaneous Coronary Intervention for St-Elevation Myocardial Infarction. Am Heart J. 2008;155(4):673–9.
20 Hamdan A, Kornowski R, Solodky A, Fuchs S, Battler A, Assali AR. Predictors of Left Ventricular Dysfunction in Patients With First Acute Anterior Myocardial Infarction Undergoing Primary Angioplasty. Isr Med Assoc J. 2006;8(8):532–5.
21 Zaman S, Sivagangabalan G, Narayan A, Thiagalingam A, Ross DL, Kovoor P. Outcomes of Early Risk Stratification and Targeted Implantable Cardioverter-Defibrillator Implantation After St-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Circulation. 2009;120(3):194–200.
22 Song YB, Hahn JY, Gwon HC, Kim JH, Lee SH, Jeong MH. The Impact of Initial Treatment Delay Using Primary Angioplasty on Mortality Among Patients With Acute Myocardial Infarction: From the Korea Acute Myocardial Infarction Registry. J Korean Med Sci. 2008;23(3):357–64.
23 Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck KH, Gent M, et al. Mechanisms Underlying the Lack of Effect of Implantable Cardioverter-Defibrillator Therapy on Mortality in High-Risk Patients With Recent Myocardial Infarction: Insights From the Defibrillation in Acute Myocardial Infarction Trial (Dinamit). Circulation. 2010;122(25):2645–52.
24 Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early Diagnosis of Myocardial Infarction With Sensitive Cardiac Troponin Assays. N Engl J Med. 2009;361(9):858–67.
25 Cuculi F, De Caterina AR, Kharbanda RK, Banning AP. Optimal Reperfusion in St-Elevation Myocardial Infarction – the Role of the Coronary Microcirculation. Swiss Med Wkly. 2011;141:w13313.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.