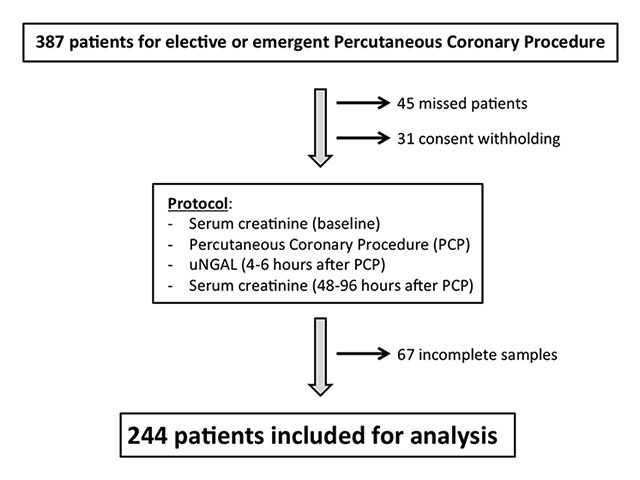
Figure 1
Study flowchart. uNGAL = urinary neutrophil gelatinase-associated lipocalin.
DOI: https://doi.org/10.4414/smw.2013.13853
Contrast-induced acute kidney injury (CI-AKI) is a decrease in renal function following the injection of radio-contrast media (CM). In inpatient settings, CI-AKI is one of the most common iatrogenic causes of acute renal failure [1]. In current practice, the diagnosis of CI-AKI still relies on serum creatinine (SCr) kinetic monitoring. Unfortunately, SCr elevation is a late indicator of acute changes in renal function, because of a 24–48 hour delay between renal insult and significant SCr elevation [2]. As CI-AKI is associated with an increased short- and long-term morbi-mortality [3–6] and as the number of cardiac procedures is constantly increasing, the early detection of CI-AKI is of utmost clinical relevance in invasive cardiology. This point is in accordance with the recent statement from a multidisciplinary panel of experts that gave the highest research priority in nephrology for the discovery of new biomarkers and for the translation of scientific laboratory advances into clinical practice [7].
Several biomarkers have recently been under investigation in search of a rapid and accurate indicator of CI-AKI [8]. Among those increasing rapidly after a renal insult, neutrophil gelatinase-associated lipocalin (NGAL or Lipocalin-2 – LCN2) is of particular interest, and has even been considered as a “renal troponin” [9]. NGAL is a small (25 kDa) protein of the lipocalin superfamily that was first isolated in 1993 from the supernatant of activated human neutrophils [10, 11]. It is massively and rapidly upregulated in the ascending loop of Henle and in collecting duct epithelia following an ischaemic or nephrotoxic injury, and is thought to participate in limiting renal parenchymal damage [12, 13]. Several studies have shown encouraging results for uNGAL for early detection of AKI in different clinical settings. In patients developing AKI following cardio-pulmonary bypass, Benett and colleagues found that NGAL is significantly elevated in urine (uNGAL) already two hours following the intervention, with a peak at four to six hours [14]. Similarly, Haase et al. calculated in their meta-analysis, that when uNGAL is measured within six hours after CM exposure during percutaneous coronary procedures (PCP), the sensitivity, specificity and AUC for the detection of CI-AKI using a cut-off for uNGAL of ≥100 ng/ml are respectively 0.78, 0.96, and 0.89 [15].
Most of the previous studies addressing the clinical use of uNGAL were based on “custom-made” ELISA assays. More recently, an automated immunoassay for fast (36 minutes) uNGAL measurement was developed and validated by Abbott for their clinical laboratory platform ARCHITECT [16, 17]. Therefore, the purposes of this prospective observational study were: (1) to investigate the clinical usefulness of uNGAL measured with this standardised assay for the early detection of CI-AKI in unselected adult patients undergoing PCP, and (2) to assess the correlation between the post-procedural uNGAL levels and the amount of CM used.
Between November 2011 and February 2012, all patients aged ≥18 years who underwent PCP (angiography and/or angioplasty) in the department of Cardiology at Fribourg University & Hospital, Switzerland, were eligible for the present study. The pre-defined exclusion criteria were dialysis-dependent chronic kidney disease and lack of written consent. This study complies with the Declaration of Helsinki and was approved by the local ethics committee at Fribourg University & Hospital, Switzerland (013–REP-CER-FR; NCT01612117). All patients were informed and gave written consent for inclusion in the study.
According to the good-practice recommendations that aims to reduce the risk of CI-AKI in patients undergoing PCP, we optimised the hydration status, interrupted concomitant nephrotoxic medications, avoided iterative expositions to CM and used a minimal volume of low-osmolar non-ionic CM in the majority of the study patients [8, 18]. Hydration was performed whenever possible based on time limits and cardiac function before and after PCP with normal saline [18–20] in nondiabetic patients, or glucosaline in diabetic patients. The standard hydration protocol for daycare patients was l ml/kg/h of NaCl 0.9% starting the morning of procedure and continuing for a minimum of five hours thereafter. In hospitalised patients the perfusion was started the evening before PCP and kept until the following morning. Use of N-acetylcystein was left to the discretion of the interventional cardiologist, but was only rarely used. Coronary angiograms were acquired using a dedicated monoplane digital flat-panel detector with 3D acquisition (Philips Allura XPer FD10), low-osmolar non-ionic contrast medium Iomprolum (Iomeron 350®; 350 mg/ml of Iodine) and an automated contrast injector (CVi Acist medical). As a general rule, the CM amount was kept to a minimum, especially in patients with baseline SCr ≥130 µmol/l.
Two definitions were used: (1) The classical definition – used in most available literature in the field –that considers CI-AKI as a relative (≥25%) or an absolute (≥44.2 µmol/l) increase in SCr from baseline [21]. (2) The Acute Kidney Injury Network (AKIN) definition that considers CI-AKI as a relative (≥50%) or an absolute (≥26.5 µmol/l) increase in SCr from baseline, or a reduction in urine output (documented oliguria of less than 0.5 ml/kg per hour for more than six hours) [22]. Although infrequently used in clinical trials in the field, this latter definition is supported by the current KDIGO guidelines [21].
Blood samples for SCr determination were harvested from a peripheral vein during the 24 hour period before and between 48 and 96 hours after PCP (heparinised sampling tube). SCr was measured as routine, using a kinetic colorimetric assay based on the Jaffé method (Cobas 6000, Roche Diagnostics).
Mid-stream urine specimens were taken in all patients four to six hours after PCP for urinary analysis and peak uNGAL determinations [15]. Urinary samples for creatinine determination were processed rapidly according to our standard laboratory proceeding and were measured by a kinetic colorimetric assay (Cobas 6000, Roche Diagnostics).
Urinary samples for peak uNGAL determinations were stored at –80° immediately after drawing and up to measurement. Urinary NGAL was measured with the automated immunoassay developed and validated by Abbott (ARCHITECT, Abbott Dx, Abbott Park, IL) with respect to the manufacturer’s protocol. Briefly, urine samples were thawed to room temperature, hand-homogenised and centrifugated (3,000 rpm, 8 minutes, 20 °C). The next steps were completely automated in the Abbot ARCHITECT system; sample and wash buffer (saline solution containing phosphate) are combined to create a 1:10 dilution sample. A mixture of the pre-diluted sample, wash buffer, and paramagnetic microparticles coated with anti-NGAL antibodies (Abbott Diagnostics Division, Abbott Laboratories, Abbott Park, Illinois) is performed to allow the NGAL of the sample to bind to the microparticles coated with anti-NGAL antibodies. After a wash-out cycle, acridinium-labelled anti-NGAL antibodies (Abbott Diagnostics Division, Abbott Laboratories, Abbott Park, Illinois) are added. Following another wash-out cycle, pre-trigger (hydrogen peroxide) and trigger (sodium hydroxide) solutions are added, and the resulting chemiluminescent reaction is measured as relative light units. Concentration of uNGAL is finally retrieved from the manufacturer’s correlation curve [23]. Normal upper limit for uNGAL in healthy volunteers is 132 ng/ml (95 th percentile) with this assay [23]. Coefficient of variation for this automated assay has been reported to be ≤5% and sensitivity (uNGAL concentration corresponding to a coefficient of variation of 20%) was found to be <2 ng/ml [17].
Estimated glomerular filtration rate (eGFR) was calculated with the Cockcroft-Gault formula [24]. The predicted risk of CI-AKI development was calculated according to the “simple risk score” by Mehran et al.[25]. We defined urgent PCP as a PCP performed within twelve hours of emergent presentation. Diabetes mellitus was defined according to the WHO [26]. Hypercholesterolaemia was defined according to the Adult Treatment Panel III [27], and arterial hypertension according to the 7 th report of the Joint National Committee (JNC-VII) [28]. Heart failure was classified as being either present or absent according to the clinical definition of the New York Heart Association [29]. Obesity was considered a body-mass index of ≥30 kg/m2[30].
Continuous variables are expressed as a median with interquartile range [IQR] and analysed with the Mann-Whitney test. Categorical variables are expressed as counts with percentage (%) and differences assessed by Pearson’s chi-square test. Analyses were performed with SPSS 18.0 software (SPSS Inc, Chicago, IL, USA). No adjustments are made for multiple comparisons. All tests are two-sided and differences are considered statistically significant at p-value ≤0.05.
| Table 1: Baseline clinical and biological characteristics in patients undergoing PCP. Data are presented as median [interquartile range] or number of patients (%). BMI = body mass index; CI-AKI = contrast-induced acute kidney injury; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; SCr = serum creatinine. | |
| Patients(n = 244) | |
| Age (years) Age >75 years | 66.6 [59.5–74.7] 59 (24%) |
| Female | 73 (30%) |
| Body mass index (kg/m2) Obesity (BMI ≥30 kg/m2) | 28.3 [25.1–30.9] 86 (35%) |
| Arterial hypertension | 179 (73%) |
| Diabetes mellitus | 50 (21%) |
| Hypercholesterolemia | 140 (57%) |
| Family history of CVD | 115 (47%) |
| Smoking, past & current | 132 (54%) |
| Known coronary heart disease | 111 (46%) |
| Heart failure | 68 (28%) |
| Left ventricular ejection fraction (%) ≤50% ≤35% | 62 [50–70] 63 (26%) 22 (10%) |
| Baseline SCr (µmol/l) Baseline SCr ≥130 µmol/l (max. 197 µmol/l) | 79.6 [70.7–97.2] 9 (4%) |
| Baseline eGFR (ml/min) ≤30 ml/min >30–60 ml/min >60–120 ml/min >120 ml/min | 86.5 [66.1–114.6] 1 (0.4%) 39 (16%) 155 (64%) 49 (20%) |
| Mehran’s CI-AKI risk score [25] Low risk (≤5 points) Intermediate risk (6–10 points) High risk (≥11 points) | 4.0 [2.0–7.0] 155 (64%) 63 (26%) 26 (11%) |
Figure 1 depicts the flow chart of the study. During the inclusion period, 387 patients underwent PCP at our institution. Of these, 45 patients were lost due to off-hour procedures or urgent transfer for surgery, 31 patients refused participation in the study, and 67 initially included patients were excluded from analysis due to missing urine or blood samples (principally secondary to missed follow-up appointments). The remaining 244 patients constitute the study population.

Figure 1
Study flowchart. uNGAL = urinary neutrophil gelatinase-associated lipocalin.
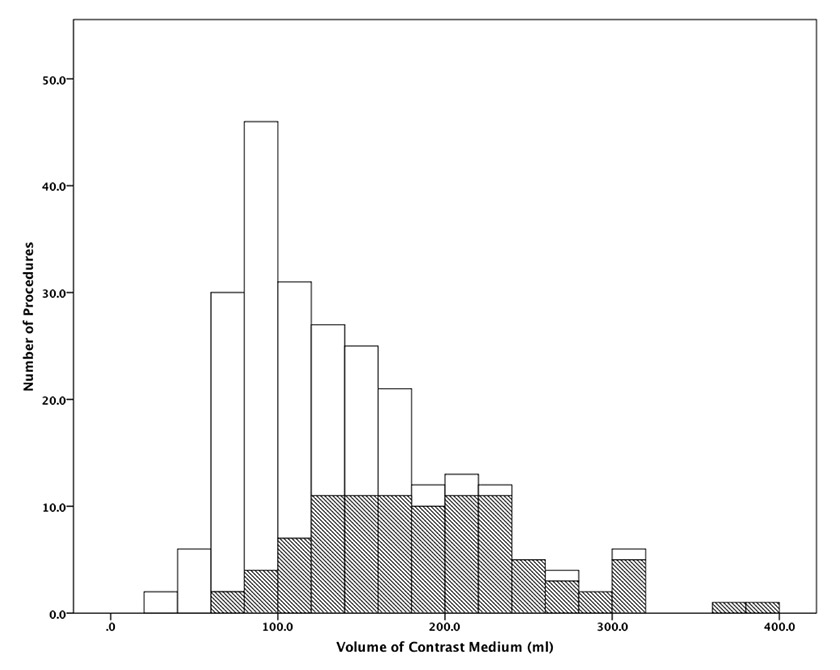
Figure 2
Distribution of the volume of contrast medium used for diagnostic (white areas) and for therapeutic (dashed areas) percutaneous coronary procedures.
Relevant baseline characteristics of the 244 patients are summarised in table 1. Median age was 66.6 [59.5–74.7] years and 171 (70%) patients were male. The majority of our patients (n = 155, 64%) were at low risk for CI-AKI, with an average Mehran risk score of four [2–7] points. The median baseline eGFR was 86.5 [66.1–114.6] ml/min, with 40 (16.4%) patients having an eGFR ≤60 ml/min (table 1). The median baseline SCr level was 79.6 [70.7–97.2] µmol/l, with nine (4%) patients having baseline SCr ranging from 130 to a maximum of 197 µmol/l.
Procedural characteristics are summarised in table 2. In addition to coronary angiography, 204 (84%) patients underwent levography and 10 (4%) had other additional angiographic diagnostic procedures. Ninety-five (39%) patients underwent a percutaneous coronary intervention, with a median of 2 [1–2] stents implanted per procedure. The median volume of CM used per procedure was 122 [88–168] ml – corresponding on average to 43 [31–59] grams of iodine – and 40 patients (16.4%) received >200 ml of CM. Almost twice the CM volume was used in therapeutic compared to diagnostic procedures (180 [139–220] ml vs 95 [80–130] ml; p <0.001). The exact distribution of CM volume used in diagnostic and therapeutic procedures is depicted in figure 2. As expected, due to our preventive measures, a lower CM volume per procedure was used in patients with baseline SCr ≥130 µmol/l compared to those with SCr <130 µmol/l (95 [73–124] ml vs 130 [88–170] ml, respectively; p = 0.05).
Twenty-five (10%) patients developed CI-AKI according to the classical diagnostic criteria (≥25% increase in SCr from baseline), whereas only eight (3.3%) patients fulfilled the AKIN definition classification. No patient in our cohort needed renal replacement therapy.
The median post-PCP uNGAL level was 7.7 [4.0–14.5] ng/ml and was significantly higher in patients with baseline eGFR ≤60 ml/min when compared to the other patients (12.1 [5.1–41.0] vs 7.3 [3.8–13.3] ng/ml; p = 0.003). As illustrated in figure 3, three patients (1.2%) had uNGAL levels higher than the normal range of the used assay (>132 ng/ml), none of them developed CI-AKI.
When considering the classical definition of CI-AKI, the uNGAL levels did not differ significantly between the 25 patients with CI-AKI and those without (6.4 [3.4–10.4] vs 8.2 [4.1–15.1] p = 0.2). A subgroup analysis with stratification according to Mehran risk score or to baseline renal function (eGFR ≤60 or >60 ml/min) did not show any significant difference either. Similarly, when considering AKIN criteria for CI-AKI definition, there was no difference in uNGAL levels between the eight patients developing CI-AKI and those who did not (8.6 [6.7–11.3] vs 7.5 [4.0–14.6] ng/ml, p = 0.70).
It should be emphasised that the data presented are absolute uNGAL values. However, using uNGAL-to-creatininuria ratios instead had no significant impact on the results, with no difference in the ratios between patients with and without CI-AKI (classical criteria: 1.5 [0.7–4.5] vs 1.8 [0.9–3.4], p = 0.6; AKIN stage 1: 1.8 [0.9–3.4] vs 3.0 [0.9–5.8], p = 0.4).
As shown in table 2, the median volume of CM used per procedure was 122 [88–168] ml. The overall median CM amount-to-baseline eGFR ratio was 1.5 [1.0–2.2] and, as expected, was significantly higher in patients with baseline eGFR ≤60 ml/min compared to the others (2.6 [1.9–3.1] vs 1.4 [0.9–1.9]; p <0.001).
As illustrated in figure 3, there was no significant correlation between the amount of CM used during the procedure and the uNGAL levels (Spearman correlation –0.11). Similarly, there was also no relationship between CM amount-to-baseline eGFR ratios and uNGAL levels (Spearman correlation 0.07). The absences of any significant correlation remained even after stratification according to the Mehran risk-score.
| Table 2: Procedural characteristics in patients undergoing PCP. Data are presented as median [interquartile range] or number of patients (%). CM = contrast medium; eGFR = estimated glomerular filtration rate; PCP = percutaneous coronary procedure. | |
| Patients(n = 244) | |
| Urgent PCP | 44 (18%) |
| PCP with intervention Number of stents/intervention | 95 (39%) 2 [1–2] |
| Levography | 204 (84%) |
| CM volume (ml) ≤50 ml >50–100 ml >100–200 ml >200 ml | 122 [88–168] 8 (3.3%) 86 (35.2%) 110 (45.1%) 40 (16.4%) |
| CM volume – to – eGFR ratio – in patients with eGFR ≤60 ml/min. | 1.5 [1.0–2.2] 2.6 [1.9–3.1] |
During the last decades, the number of radiologic and angiographic exams using CM has steadily increased. As most exams are performed on an outpatient basis, follow-up SCr measurements are only occasionally performed. Therefore, most episodes of CI-AKI might be missed. Since CI-AKI worsens renal but also overall prognosis [3, 4, 6, 31], even in patients with normal baseline renal function [3–5], new biomarkers allowing for early detection of CI-AKI even in ambulatory patients are eagerly awaited. We investigated whether the most promising biomarker, namely uNGAL, might answer this need in the daily cathlab practice, in which most exams are done in outpatients at low-risk for CI-AKI.
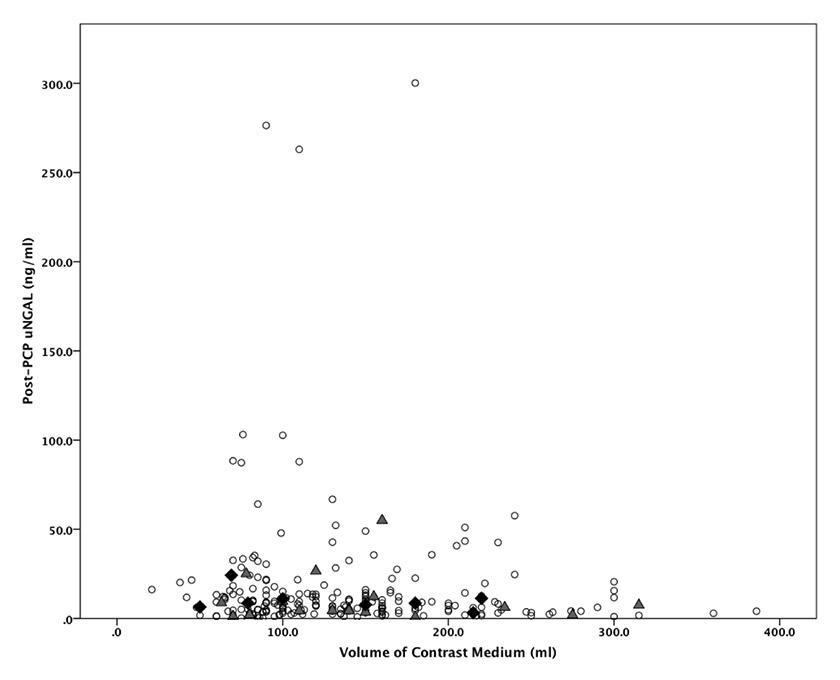
Figure 3
Relationship between the volume of contrast medium used and the post-PCP uNGAL levels in patients without CI-AKI (white disk), and with CI-AKI defined by the classical criteria (grey triangle) or by the AKIN criteria (black diamond) (normal uNGAL range ≤132 ng/ml). CI-AKI = contrast-induced acute kidney injury; PCP = percutaneous coronary procedure; uNGAL = urinary neutrophil gelatinase-associated lipocalin.
The main findings of our trial are that: (1) CI-AKI occurred in 3% to 10% of patients undergoing PCP depending on the definition used. uNGAL levels measured four to six hours after coronary angiography did not predict its occurrence, even in the subanalysis focusing on patients with higher Mehran’s risk scores, or lower baseline eGFR (≤60 ml/min). (2) there was no correlation between uNGAL levels and the amount of CM.
Our results clearly show that the incidence of CI-AKI largely depends on the definition considered. It outlines that the AKIN criteria are less sensitive to detect CI-AKI than the classical definition used in the literature. According to this latter definition, a 10% incidence of CI-AKI in our unselected cohort is in accordance to comparable previous trials [8]. The relatively low renal toxicity following PCP is reflected by the fact that only 8 of 244 patients (3%) fulfilled the more restrictive AKIN definition and none needed renal replacement therapy. This good outcome might be due to the systematic use of preventive measures already implemented at our institution [8]. In that view, it should be noted that modern angiographic equipment allows the performance of PCP with a lower CM-amount than previously. Accordingly, the median CM-amount used in the present study (122 ml) is much lower than the amounts reported in previously published works in the years 2003–2008 (180 ml) [6], and in 1994–2000 (256 ml) [5].
The available literature addressing uNGAL in invasive cardiology brings confusing conclusions about its predictive power for the early diagnosis of CI-AKI following PCP. To date, six studies using various ELISA assays focused on the importance of uNGAL after PCP in adults [10, 32–36]. Most trials included a limited number of patients (ranging from 25 to 60) with normal SCr values [10, 32–35], and none reported any subanalysis focusing on patients with decreased eGFR (≤60 ml/min). Five studies were performed using a dedicated ELISA kit (Antibodyshop, Gentofte, Denmark) at the Medical University of Bialystok, Poland, and reported the kinetic of uNGAL levels before and 2, 4, 8 (or 12), 24 and 48 hours after PCP [10, 32–34, 36]. The results of these five studies showed that uNGAL level significantly increases at four hours compared to baseline and peaks between four and eight hours post-PCP. Interestingly, SCr remained stable in three of their five studies (86 cumulated patients) with no patient fulfilling the usual diagnostic criteria for CI-AKI [10, 32, 33], while the baseline uNGAL levels (8–12 ng/ml) increased on average 2–3 times at four hours after PCP (up to 16–25 ng/ml). This latter suggests that increases in uNGAL do not necessarily parallel SCr or the occurrence of CI-AKI. Consistently, and based on the two largest studies from this research group (200 patients included) [34, 36] and on the results of Ling et al., [35] a relative increase in uNGAL was associated with a rather low sensitivity and specificity to predict CI-AKI in adult patients. Contrary to this, the only trial published so far, focusing on this issue in children, reports a very good diagnostic accuracy for uNGAL: Hirsch and colleagues used uNGAL (measured with a self-generated ELISA assay) to detect CI-AKI in 91 children with congenital heart disease undergoing cardiac angiography. In children without CI-AKI, uNGAL levels remained unchanged up to six hours after PCP (10–18 ng/ml). Contrasted with the children developing CI-AKI, uNGAL levels increased rapidly and significantly from 17.7 ± 6.8 ng/ml before angiography to 135 ± 32 ng/ml at two hours and even 172 ± 30 ng/ml at six hours after PCP (p <0.001). Using a cut-off for uNGAL of ≥100 ng/ml, when measured at six hours after PCP, the ROC analysis yielded good sensitivity (90%) and specificity (99%) for predicting CI-AKI (AUC 0.97) [37].
Taken together, the above mentioned results suggest that the kinetic of uNGAL in cases of CI-AKI differ greatly between children and adults, and may indicate that the kidneys react differently in children compared to adults in cases of CM-mediated toxicity. In this view, the results from our relatively large cohort of adult patients show that a single measurement of uNGAL is unfortunately not useful for an early detection of CI-AKI in unselected adults undergoing coronary angiography.
Since the amount of CM correlates with tubular toxicity and uNGAL is a marker of acute tubular injury, we hypothesised that these two parameters may correlate. However and in accordance to two previous studies [32, 33], our data clearly indicate that uNGAL is not related to the amount of CM. This was the case for the overall population, as in patients developing CI-AKI. It is important to note that uNGAL levels were low. Only three patients had uNGAL levels greater then the normal upper limit of the assay (>132 ng/ml). Interestingly, from these patients, none developed CI-AKI. In light of this, it has been suggested that an isolated elevation in uNGAL without concurrent changes in SCr might indicate the occurrence of a kidney damage not severe enough to affect glomerular filtration [16], and Haase et al. have already demonstrated an increased risk of adverse outcomes in patients with “subclinical” CI-AKI [2]. Clearly, upcoming studies should continue to address the prognostic value of renal tubular biomarker.
The strengths of our study are its prospective design and its relatively large size (n = 244) in comparison to previous trials addressing the role of uNGAL following PCP, the largest of which matched 70 diabetic patients with 70 sex- and age-matched non-diabetic patients [36]. Conversely, one must consider several limitations when interpreting the results of this study. First, it is a single-centre study. Second, our patients had no uNGAL level measurements before PCP. However, as all patients with CI-AKI already had very low post-PCP uNGAL levels, pre-procedural uNGAL measurements would most likely have failed to provide further relevant information. Third, the majority of patients in our cohort had a low-risk profile for CI-AKI. Therefore, our results may not apply to patients with increased risk factors for development of contrast-induced nephropathy. Accordingly, we feel that further studies concerning the early detection of CI-AKI should focus on selected groups of patients at higher risk for renal toxicity following PCP, as well as on the use of biomarker panels including uNGAL together with other promising early renal biomarkers such as kidney injury molecule 1 (KIM-1), interleukin 18 (IL-18) or Cystatin-C.
Although uNGAL may be a sensitive biomarker for AKI in the emergency department [38], after cardiac surgery [39, 40], as well as in pediatric patients undergoing PCP [37], the results of the present study do not support the use of post-PCP urinary NGAL measurement for the early detection of contrast-induced acute kidney injury in unselected adult patients undergoing coronary angiography.
Acknowledgement:The authors thank the study nurses of the clinical trial unit for the excellent data gathering, the intensive care unit team and hospital nurses for their participation in urine collecting, as well as the laboratory staff for the conscientious measuring of samples. We also thank especially Mrs. Bernadette Mermillod for her precious advice concerning statistics.
1 Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–8.
2 Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61.
3 James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–16.
4 James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–9.
5 James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–9.
6 Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–107.
7 Parikh CR, Garg AX. Testing new biomarkers for acute kidney injury: association, prediction, and intervention. Am J Kidney Dis. 2009;54:987–9.
8 Perrin T, Descombes E, Cook S. Contrast-induced nephropathy in invasive cardiology. Swiss Med Wkly. 2012;142:w13608.
9 Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology. 2010;15:419–28.
10 Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S. NGAL (neutrophil gelatinase-associated lipocalin) and L-FABP after percutaneous coronary interventions due to unstable angina in patients with normal serum creatinine. Adv Med Sci. 2009;54:221–4.
11 Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89–94.
12 Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22:101–8.
13 Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13.
14 Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73.
15 Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24.
16 Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis. 2008;52:395–9.
17 Grenier FC, Ali S, Syed H, Workman R, Martens F, Liao M, et al. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem. 2010;43:615–20.
18 Mautone A, Brown JR. Contrast-Induced Nephropathy in Patients Undergoing Elective and Urgent Procedures. J Interv Cardiol. 2010.
19 Brar SS, Shen AY, Jorgensen MB, Kotlewski A, Aharonian VJ, Desai N, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. J Am Med Assoc. 2008;300:1038–46.
20 Klima T, Christ A, Marana I, Kalbermatter S, Uthoff H, Burri E, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012;33:2071–9.
21 Kidney Disease Improving Global Outcome. Chapter 4.1: Contrast-induced AKI. In: KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:69–88.
22 Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
23 Abbott Diagnostics Division Baar. Architect Urin NGAL. Package insert 1P37, G2–2964/R04. 2011.
24 Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
25 Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9.
26 World Health Organisation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. 2006. Available at: whqlibdoc.who.int/publications/2006/9241594934_eng.pdf.
27 Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143.
28 Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al.; The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. J Am Med Assoc. 2003;289:2560–72.
29 The Criteria Committee of the American Heart Association NYCA. Diseases of the heart and blood vessels, nomenclature and criteria for diagnosis. 9th revision. Boston, Mass: Little, Brown & Co; 1994:253–6.
30 World Health Organization. Obesity and overweight. Fact Sheet No 311. 2013. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/.
31 Ricci Z, Ronco C. New insights in acute kidney failure in the critically ill. Swiss Med Wkly. 2012;142:w13662.
32 Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26:287–92.
33 Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant. 2007;22:295–6.
34 Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Poniatowski B, Pawlak K, et al. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol. 2008;127:290–1.
35 Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–81.
36 Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31:910–9.
37 Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–95.
38 Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9.
39 Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–57.
40 Seitz S, Rauh M, Gloeckler M, Cesnjevar R, Dittrich S, Koch AM. Cystatin C and neutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgery. Swiss Med Wkly. 2013;143:w13744.
Funding / potential competing interests: This work was supported by the “Schweizerische Herzstiftung” (Bern, Switzerland), the “Fondation Lausannoise pour la Recherche en Hypertension” (Lausanne, Switzerland) and the “Fonds Scientifique Cardiovasculaire” (Fribourg, Switzerland). There was no industry involvement in the design, conduct, or analysis of the study.