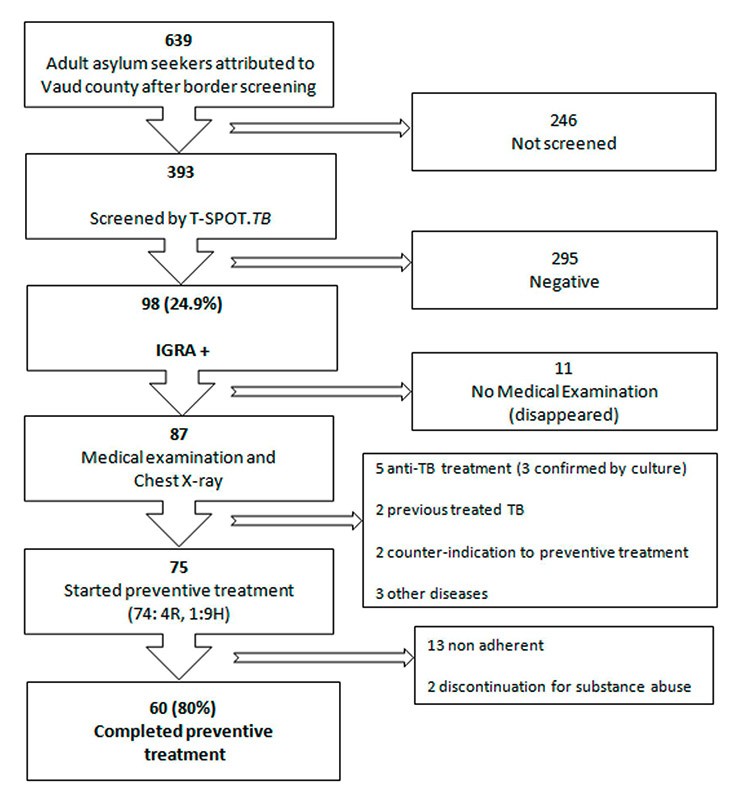
Figure 1
Preventive treatment completion flowchart.
* IGRA = Interferon Gamma Release Assay
DOI: https://doi.org/10.4414/smw.2013.13860
Latent tuberculosis infection (LTBI) is a widespread condition among asylum seekers born in or who have lived in countries with a high prevalence of tuberculosis [1]. Persons with LTBI have a known risk of developing active tuberculosis at a later stage [2] and can be screened by tuberculin skin testing or by one of the new Interferon Gamma Release Assays (IGRA), which offer a greater specificity [3]. Among the persons with positive IGRA, those most at risk of developing an active infection are children, immunosuppressed persons and those with recently acquired infection. The highest risk for the development of tuberculosis among infected persons is during the first few years following infection. The risk of reactivation, defined as the progression from latent infection to active disease can be decreased by preventive treatment [4].
Asylum seekers (AS) from high incidence countries entering in low incidence countries have a higher rate of LTBI than the local population, therefore some of them may develop TB after entering the country, by reactivation from remote infection. In some low incidence countries AS or migrants are screened for active TB and LTBI upon entry. In Switzerland AS are screened at the border for active TB only. Cases with LTBI are not detected and not offered a preventive treatment so that the possible progression from infection to disease after entry is not prevented. The treatment of latent tuberculosis infection (LTBI) is generally considered costeffective and a component of the strategy towards elimination of tuberculosis in low-incidence countries [5–7]. The key features of a cost effective LTBI screening strategy are proper screening, selection of persons at highest risk of progression and the completion of preventive treatment by eligible persons with LTBI who are expected to follow a long treatment in spite of being asymptomatic [8].
The low adherence to LTBI treatment is the main challenge in the preventive strategy. According to various studies the completion rate of preventive treatment varies from 10% to 86% [9, 10]. The duration and side effects of preventive treatment along with the lack of awareness on active tuberculosis disease by people with LTBI, and the high social and economical vulnerability of some population groups like asylum seekers render adherence to treatment a highly challenging issue for both the physician and the patient. No factor seems to reliably predict the adherence to preventive treatment [11]. Our study aims to assess the rate and conditions of adherence to preventive treatment in a population of asylum seekers with latent tuberculosis infection recently arrived in Canton Vaud. All aspects regarding screening results, demography and treatment decision were described in a separate paper [12].
We conducted a prospective cohort study to assess the feasibility and adherence of preventive treatment in a population of asylum seekers (AS) with LTBI newly arrived in the Swiss Canton Vaud, who had recently passed a border screening for the detection of active TB. The AS resided in two migrant centres at Sainte – Croix and Crissier. AS aged over 16 years were offered a free IGRA screening for LTBI by T-SPOT.TB (Oxford Immunotec, Abingdon, UK). All asylum seekers received written information about tuberculosis and the aims of the study were translated into five major languages (English, French, German, Russian and Arabic). All participants signed a consent form. A translator was used if needed. The study was approved by the ethics commission of the University of Lausanne.
The results of IGRAs were categorised as positive, indeterminate or negative. An additional SPOT.TB was offered to AS with an indeterminate result.
AS with a positive test result were referred for further examination to a physician for the exclusion of active TB and a decision on preventive treatment. The physician obtained a detailed history of TB exposure or treatment, and performed a clinical examination. A chest X-ray was taken and human immunodeficiency virus (HIV) screening was offered to the IGRA positive participants. AS with suspect clinical manifestations or abnormal radiological findings had further investigations including sputum examination. AS with documented or possible active tuberculosis were treated according to the current Swiss guidelines [13].
AS with LTBI without evidence of active disease were offered a preventive treatment according to the current Swiss recommendation with four months of rifampicin (first choice) or nine months of isoniazid. The rifampicin scheme was preferred over the isoniazid because of its simplicity of prescription (no need for vitamin substitution, short treatment time) and follow up (better tolerance, no need for routine liver function test assessment, visible urine colouration) advantages that could enhance compliance with treatment [6]. We did not use incentives. Adherence to treatment was evaluated during the monthly visit to the physician where clinical examination, side effects and symptom assessment was performed. If normal, the preventive treatment prescription was renewed. In cases of abnormality, blood tests were requested. The treatment was self-administered but the nurse in charge of the centre met regularly (if possible daily) with the treated AS and reinforced the treatment instructions. If the physician was not assured of the adherence to the treatment directly observed treatment (DOT) for a month was prescribed.
The adherence to treatment was considered as satisfactory if the AS were compliant with all scheduled visits and asked for a repeat prescription. No tablet count was performed. Individuals who did not attend two of the scheduled appointments were considered as non-adherent. The treatment was suspended in individuals with poor or problematic adherence.
The screening assay with T-SPOT.TB among 393 adult AS living in two asylum seeker centres in Canton Vaud revealed positive IGRA results in 98 (24.9%), who were referred for further medical examination. The mean age of the AS with LTBI was 27.63 years. 74% were men, 66% were Africans, and 17% were Asians. Balkanic and former Soviet Union nationals were both 8% and 8% of the collective. No AS with LTBI had a known immunosuppressive treatment or condition, notably HIV infection.

Figure 1
Preventive treatment completion flowchart.
* IGRA = Interferon Gamma Release Assay
Of them, 11 did not attend the first scheduled medical evaluation. After examination, documented or possible tuberculosis or another pulmonary disease was discovered in 8/87 AS (of whom 5 were prescribed an antituberculosis treatment). Two other patients had a history of prior treatment for active tuberculosis. Of the 77 IGRA positive asymptomatic individuals with normal chest X-ray, 2 had counter indications (severe liver disorder and pregnancy) for preventive treatment (fig. 1).
An LTBI treatment with daily rifampicin for four months was offered to 74 AS, one received isoniazid for 9 months due to counter indication to rifampicin. During the follow up three participants defaulted on the first follow-up visit, three on the second and seven on the third (total 13 cases). In two individuals preventive treatment was suspended because of poor compliance and substance abuse. During the follow-up, no serious adverse event was observed and no treatment was interrupted for medical reasons. The final rate of completion of preventive treatment in our cohort was 80% (60/75) (fig. 2).
In this prospective study, the rate of adherence to LTBI preventive treatment in AS was high with 80%.
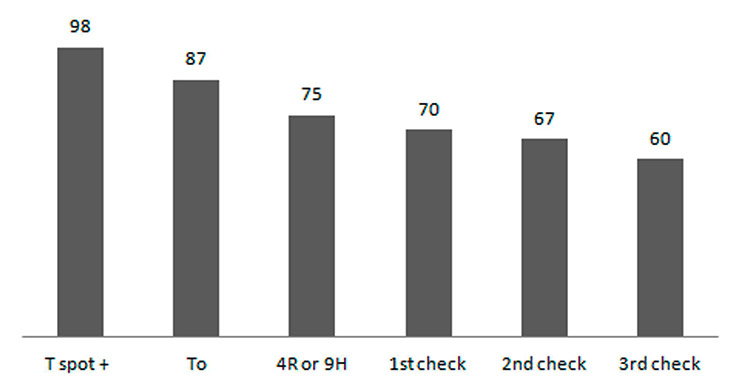
Figure 2
Monthly assessment and drop outs.
T spot+: Positive T-SPOT.TB, To: initial examination of the participants, 4R or 9H: Preventive treatment start either four months of rifampicin or nine months of isoniazid.
One particular feature of the migrant population is the young mean age, allowing the assumption that many persons with LTBI must have been infected recently and are therefore still in a period with a high risk of reactivation. One argument for this is the fact that migrants arriving in Switzerland by land or sea/ land travel had a higher rate of LTBI than migrants travelling by air, indicating that travel conditions were a risk factor for TB exposure and recent infection (data in a separate publication) [12]. An indication for this is the fact that about 75% of all TB observed in AS in Switzerland occurs more than three months after the border screening, probably by progression from a recent infection in young adults.
Variable rates of completion are reported with partially contradictory conclusions regarding the risk factors for non-adherence. A recent study by Horsburgh et al. reported that 93.7% of contacts of patients with contagious TB accept the treatment, whereas only 48.3% of health-care workers with LTBI detected by routine screening accept it [9]. A study conducted in Arkansas, US, among patients treated with isoniazid for LTBI found no correlation between adherence and age, diagnosis, mode of administration and duration of treatment, but demonstrated that education at the first visit could improve the adherence rate for further treatment [11]. An analysis conducted by Hirsch-Moverman et al. in the US and Canada reported rates of completion from 35% to 64% among contacts with TB, 32% to 61% in prisoners and jail inmates, 22% to 90% among foreign-born persons, with better rates among recent migrants [10]. Reduction of treatment duration from six or nine months isoniazid to four months rifampicin was followed by an increase in the completion rate up to 91%. Another study by the same group reported a progressive increase of the completion rate from 37% in 1996–1999 to 56.1% in 2002–2005 with tighter follow-up of patients [14]. Trajman et al. confirmed that the overall completion rate was satisfactory (73%) but that the reduction of the treatment duration to four months improved the rate of completion [15]. Similar conclusions were made in a Swiss study comparing the tolerance and rate of completion in contacts with LTBI treated with isoniazid for six months or rifampicin for four months, where the rate of treatment interruption because of liver toxicity decreased from 6.1% to 2% and the rate of treatment completion increased from 74% to 83% [16].
On the contrary, a prospective study conducted in Boston in 2006 reported a rate of completion of 28.6% (or 33.3% in recent contacts) [17]. In one study in South Africa, only 20% of children less than five years exposed to parents with TB completed the preventive treatment [18].
In Switzerland, the results varied. In a study of asylum seekers screened at the border with tuberculin skin test, where the subjects with a positive test result were supposed to have a medical examination and a prescription of preventive treatment, a large proportion were not prescribed a treatment or did not complete it [19]. Other reports were more favourable. A study conducted at the TB Dispensary in Lausanne reported a global completion rate of 76% among persons with a positive tuberculin skin test prescribed a preventive treatment with isoniazid for six months (70% among foreign-born persons, 93% among foreign workers and 90% in the local population) [20].
Several studies have reported a higher adherence and a lower rate of adverse events in subjects with LTBI who were prescribed a short treatment (four months of rifampicin) than among those who had to take a long treatment (six to nine months of isoniazid) [21, 22]. A report from the Centre Antituberculeux in Geneva reported an acceptance rate of 83% and a completion rate of 67% among the patients (mostly contacts of TB cases) who started the treatment with four months of rifampicin [23]. In underserved population groups, the results are less favourable. In a study of undocumented migrants in Lausanne, 10/22 persons with a positive IGRA test accepted the preventive treatment but only 5 completed it [24].
Not prescribing the preventive treatment in infected persons with a high risk of progression to active tuberculosis can be considered as a missed opportunity of reducing the pool of future cases of disease and the transmission of mycobacteria to contacts [25]. In a recent survey in the Netherlands, the risk of progression to TB among migrants with a positive IGRA was between 386 and 590/100,000, depending on the incidence rate in the country of origin [26].
Our study has some limitations. The screening and acceptance of preventive treatment for LTBI was voluntary. 39% of the AS were not screened and some of them may have been infected and at risk for progression to active tuberculosis disease [26]. Considering the fact that the screening was offered to all participants but that this population was extremely mobile, some staying for only few days in the centre, we consider that this pilot study was representative of the target population. Furthermore, AS were clearly informed that acceptance or refusal of the proposed screening and preventive treatment would have no impact on the final decision regarding granting refugee status or not. Also, although the efficacy of rifampicin is less extensively documented than isoniazid, because of the enhanced adherence to this preventive treatment scheme this option is a recommended alternative to the classical isoniazid scheme [16, 27].
Future uncertainties, difficulties of integration and in communication associated with a divergent representation of health and disease compared with locals are some of the problems AS need to overcome. The reasons for the high rate of completion of LTBI treatment in this population of migrants in spite of social, economical and legal problems are probably related to the selection of a well-tolerated short treatment schedule ( four instead of six or nine months), a stable environment (accommodation in a shelter with dedicated social workers and nurses caring for inmates), regular assessment of the drug intake by the nurses, close coordination with the medical staff of the referral hospitals and repeated information about the importance of treatment). This study demonstrates that a policy of screening for LTBI and preventive treatment can be implemented in a migrant population with a high rate of adherence if all obstacles are carefully considered in advance.
Acknowledgements: The authors thank the medical and nursing staff of the shelters in Sainte-Croix and Crissier and the staff of the TB Dispensary at the Department of ambulatory care and community medicine, Faculty of biology and medicine, Lausanne, for their support and contribution to the care of the patients and to Oxford-Immunotec for donating their analytical kit.
1 Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, White PJ, Abubakar I, Lalvani A. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011.
2 Horsburgh CR, Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350(20):2060–7.
3 Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, et al. Interferon-{gamma} release assays for the diagnosis of latent M. tuberculosis infection: A systematic review and meta-analysis. Eur Respir J. 2010.
4 Leung CC, Rieder HL, Lange C, Yew WW. Treatment of latent infection with m. tuberculosis: update 2010. Eur Respir J. 2010.
5 Horsburgh CR, Jr., Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364(15):1441–8.
6 Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179(11):1055–60.
7 Bennett DE, Courval JM, Onorato I, Agerton T, Gibson JD, Lambert L, et al. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care Med. 2008;177(3):348–55.
8 Leung CC, Rieder HL, Lange C, Yew WW. Treatment of latent infection with Mycobacterium tuberculosis: update 2010. Eur Respir J. 2011;37(3):690–711.
9 Horsburgh CR Jr., Goldberg S, Bethel J, Chen S, Colson PW, Hirsch-Moverman Y, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–9.
10 Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis. 2008;12(11):1235–54.
11 Eidlitz-Markus T, Zeharia A, Baum G, Mimouni M, Amir J. Use of the urine color test to monitor compliance with isoniazid treatment of latent tuberculosis infection. Chest. 2003;123(3):736–9.
12 Sarivalasis A, Zellweger J, Faouzi M, Daher O, Deslarzes C, Bodenmann P. Factors associated with latent tuberculosis among asylum seekers in Switzerland: a cross-sectional study in Vaud County. BMC Infectious Diseases. 2012 ;12:285.
13 Ligue Pulmonaire S. Manuel de la tuberculose (2e edit). Ligue Pulmonaire Suisse, http://www.tbinfo.ch , Berne, 2007.
14 Hirsch-Moverman Y, Bethel J, Colson PW, Franks J, El-Sadr W. Predictors of latent tuberculosis infection treatment completion in the United States: an inner city experience. Int J Tuberc Lung Dis. 2010;14(9):1104–11.
15 Trajman A, Long R, Zylberberg D, Dion MJ, Al-Otaibi B, Menzies D. Factors associated with treatment adherence in a randomised trial of latent tuberculosis infection treatment. Int J Tuberc Lung Dis. 2010;14(5):551–9.
16 Fresard I, Bridevaux PP, Rochat T, Janssens JP. Adverse effects and adherence to treatment of rifampicine 4 months vs isoniazid 6 months for latent tuberculosis: a retrospective analysis. Swiss Med Wkly. 2011;141: w13240.
17 Shieh FK, Snyder G, Horsburgh CR, Bernardo J, Murphy C, Saukkonen JJ. Predicting non-completion of treatment for latent tuberculous infection: a prospective survey. Am J Respir Crit Care Med. 2006;174(6):717–21.
18 Marais BJ, van ZS, Schaaf HS, van AM, Gie RP, Beyers N. Adherence to isoniazid preventive chemotherapy: a prospective community based study. Arch Dis Child. 2006;91(9):762–5.
19 Breuss E, Helbling P, Altpeter E, Zellweger JP. Screening and treatment for latent tuberculosis infection among asylum seekers entering Switzerland. Swiss Med Wkly. 2002;132(15-16):197–200.
20 Racine-Perreaud E, Zellweger JP. Chimioth‚rapie antituberculeuse preventive chez 250 patients du Dispensaire antituberculeux de Lausanne. Schweiz Med Wschrift. 1994;124:705–11.
21 Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170(4):445–9.
22 Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149(10):689–97.
23 Langenskiold E, Herrmann FR, Luong BL, Rochat T, Janssens JP. Contact tracing for tuberculosis and treatment for latent infection in a low incidence country. Swiss Med Wkly. 2008;138(5-6):78–84.
24 Bodenmann P, Vaucher P, Wolff H, Favrat B, de Tribolet F, Masserey E, et al. Screening for latent tuberculosis infection among undocumented immigrants in Swiss healthcare centres; a descriptive exploratory study. BMC Infectious Diseases. 2009;9(1):34.
25 MacIntyre CR, Plant AJ, Yung A, Streeton JA. Missed opportunities for prevention of tuberculosis in Victoria, Australia. Int J Tuberc Lung Dis. 1997;1:135–41.
26 Mulder C, van Deutekom H, Huisman EM, Toumanian S, Koster BF, Meijer-Veldman W, et al. Role of the QuantiFERON(R)-TB Gold In-Tube assay in screening new immigrants for tuberculosis infection. Eur Respir J. 2012;40(6):1443–9.
27 Landry J, Menzies D. Preventive chemotherapy. Where has it got us? Where to go next? Int J Tuberc Lung Dis. 2008;12(12):1352–64.
Funding / potential competing interests: The study was supported by a grant from the Department of public health of Canton Vaud and from the local section of the Swiss lung association. The kits for the T-SPOT.TBwere donated by Oxford Immunotec. No authors declared any competing interest in the performance of this study.
Author’s contribution: AS performed medical assessment, preventive therapy prescription and treatment follow-up, acquired the study data and helped to the results interpretation, drafted the manuscript and revised it. PB helped in the study coordination; contributed to the interpretation of results and revised the manuscript. EL and CL-F performed medical assessment, preventive therapy prescription and treatment follow-up of the study subjects. OD participated in the study coordination. JPZ conceived and designed the study, helped in the study coordination, interpretation of the results and revised the study manuscript. All authors read and approved the final manuscript.