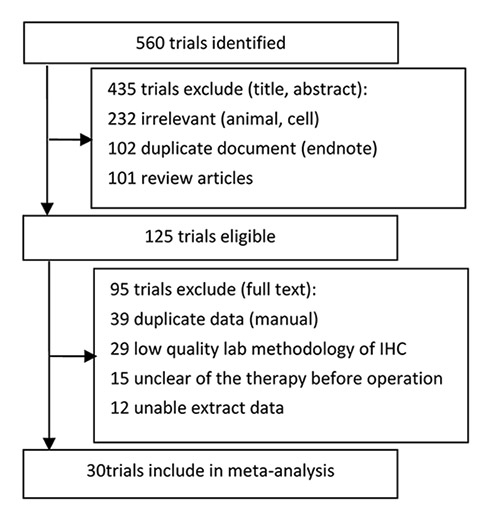
Figure 1
Flow chart of article selection in the systematic review and meta-analysis.
DOI: https://doi.org/10.4414/smw.2013.13855
Lung cancer is one of the most common malignancies worldwide and the paramount cause of cancer deaths in the world [1]. Lung cancer development is a multi-step process, driven by a series of genetic and environmental alterations. Neovascularisation and cellular adaptation to hypoxia have been recognised to be essential conditions for cancer progression. However the mechanisms of such cellular events have not yet been completely elucidated [2].
Semenza et al. [3] identified the hypoxia-inducible transcription factor (HIF-1) in 1992. HIF-1 is a heterodimer consisting of two sub-units, HIF-1α and HIF-1 β. HIF-1 β is constitutively expressed, unlike HIF-1α, which is rapidly degraded by proline hydroxylation. On the contrary, when cells are under hypoxic conditions, HIF-1α will accumulate and heterodimerise with HIF-1 β to form the transcription factor (HIF-1). Regions of hypoxia are known to exist within many tumours, and the extent of tumour hypoxia correlates with prognosis in number types [4–7]. In addition, enhanced levels of HIF-1α protein have been detected in the cytoplasm and nuclei of 40% to 80% of human carcinoma cases [8]. In recent years, hypoxia-inducible factor-1α (HIF-1α), which was used as the index of hypoxia, has been evaluated for many tumours.
Gradually, more detailed immunohistochemistry studies have indicated that HIF-1α may be involved in cell proliferation, invasion, angiogenesis and metastases. Over-expression of HIF-1α, which is common in lung cancer, may be correlated with poor prognosis, high metastatic risk, pathological types, pathological grade, tumour size, differentiation, smoking and the survival of patients. However there has been lots of controversy. Meanwhile, studies have examined the association of HIF-1α expression on disease progression, such as medium microvessel density (MVD), vasculogenic mimicry (VM) and Ki-67, and have clarified the relationship between HIF-1α and the expression of the other effectors of the hypoxia response element (HRE), such as vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGFR), cyclooxygenase-2 (COX-2), B-cell lymphoma 2 protein (bcl-2 protein) and carbonic anhydrase-9 (CA9).
However, there has not been a systematic assessment of the literature regarding the association of HIF-1α expression and clinical significance in lung cancer patients. Also, most of the individual studies had a small number of patients. We performed a meta-analysis to provide a systematic assessment of whether HIF-1 α expression is associated with clinical significance in lung cancer patients. It could help diagnosis and optimise selection for targeted therapy in lung cancer patients.
Firstly, a computer literature search was conducted in Cochrane library, PubMed and EMbase which are English Databases, and CNKI, CBM, VIP and Wan Fang which are Chinese Databases from inception to May 2012. The keywords “hypoxia-inducible factor” OR “HIF-1α” OR “HIF-1”, “lung cancer” OR “lung neoplasm” and “immunohistochemistry” OR “immunocytochemistry” were used. No language restrictions were applied. Various combinations of the keywords were applied.

Figure 1
Flow chart of article selection in the systematic review and meta-analysis.
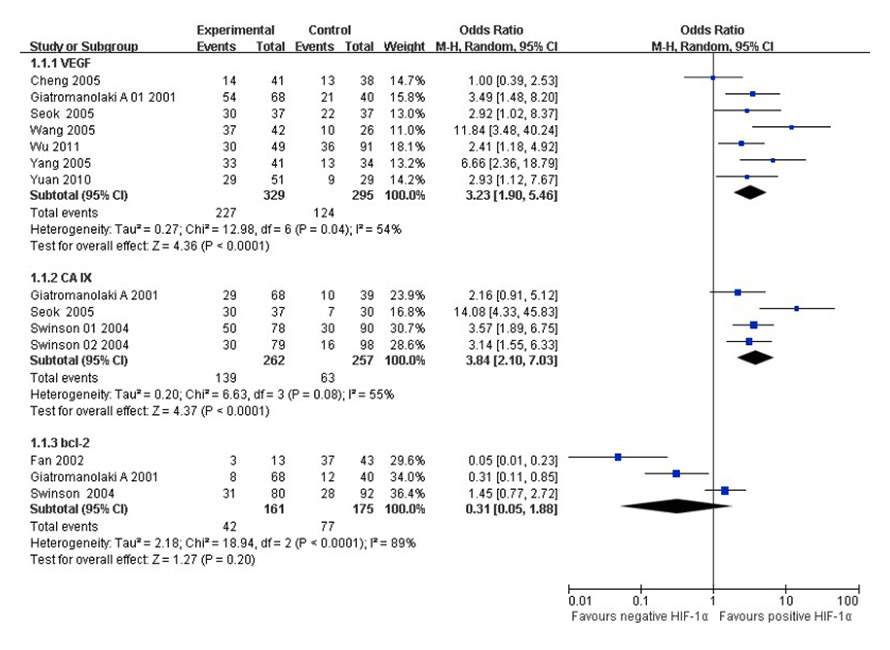
Figure 2
Forest plot of VEGF, CA IX and bcl-2 positive expression in positive and negative HIF-1α tumour tissues, respectively (Giatromanolaki A 01 2001: Giatromanolaki A 2001 [14], Giatromanolaki A 2001: Giatromanolaki A 2001 [15], Swinson 01 2004: pCA IX, Swinson 02 2004: mCA IX).
Secondly, all abstracts were read by two independent reviewers (Ren WW and Li Z), and then the full-texts were independently read and checked carefully. Finally, disagreements were resolved through consensus with a third reviewer (Mi DH). For studies using the same sample in different publications, only the most complete information was included following careful and exhaustive examination. Consultation with experts in the field was performed to further identify additional published and unpublished studies.
As the study design of articles which were included in the meta-analysis were not single cohort studies or case-control studies, we could not use the Newcastle-Ottawa Quality Assessment Scale [9]. To assess laboratory methodology, two reviewers (Ren WW and Mi DH) read each of the acceptable studies in duplicate independently, and performed selection criteria according to Steele’s method [10–12]. Discrepancies between the two reviewers were resolved by discussion and consensus with a third reviewer (Yang KH). The final results were reviewed by all investigators to avoid bias.
Inclusion criteria for primary studies were as follows: (1) primary lung cancer patients should be pathologically proven ; and (2) HIF-1α expression should be detected with immunohistochemistry (IHC); and (3) the association between clinicopathologic variables and positive HIF-1α expression; or (4) the association between HIF-1α and the expression of the other effectors; or (5) provides information on survival data; and (6) laboratory methodology of IHC: (6.1) clear and detailed description of protein (nuclear, cytoplasm or extracted from cellular components) and antibodies (type of tissue or liquid sampled); and (6.2) tissue sample conservation(fixation in formalin, alcohol or paraffin); and (6.3) description of the revelation test procedure of the biological factor with the first antibody type and clone identification, second antibody type, reaction characteristics, colouration method , epitope unmasking method; and (6.4) description of the negative and positive control; and (6.5) test reproducibility control; and (6.6) definition of the level of positivity of the test; or (6.7) the pathologist evaluating the IHC outcome was double-blind (or random) to patient clinicopathologic data and outcome. When studies were retrospective, the pathologist blinding was simple-blind, and the studies were defined as medium quality.
Exclusion criteria for primary studies were as follows: (1) review, abstract, animal studies and cell line, or a case report; or (2) not possible to extract the exact data; or (3) patients received chemotherapy, radiotherapy, targeted therapy before operation; and (4) laboratory methodology of IHC: (4.1) the study design was not defined; or (4.2) was unclear and no detailed description of standard laboratory methodology about IHC; or (4.3) the pathologist blinding was unblinded.
Studies were considered to be of high and medium quality in this meta-analysis if they met each of inclusion criteria well, and low quality studies were excluded from further analysis.
To reduce the bias and to improve the reliability, two reviewers (Ren WW and Li Z) checked all relevant studies independently. Data on the following characteristics were also extracted: (1) the first author, year of publication; (2) the number of cancer cases and controls for positive HIF-1α (HIF-1α high expression , score≥++: semi-quantitatively assessing the percentage of tumour cells expressing HIF-1α, intensity of cell staining and extent of staining were included in the scoring system); (3) the number of test cases (≥60 years, male, smoking, lymph nodes metastasis) and control cases (<60 years old, female, no smoking, no lymph nodes metastasis) for positive HIF-1α; (4) the number of test cases (moderate or high differentiation) and control cases (poor differentiation); (5) the number of test cases (squamous cell carcinoma) and control cases (adenocarcinoma) for positive HIF-1α; (6) the number of test cases (non small cell lung cancer) and control cases (small cell lung cancer) for positive HIF-1α; (7) the number of test cases (I‒II stage) and control cases (III‒IV stage) for positive HIF-1α; (8) the hazard ratio of overall survival, 5-year survival; (9) the association of other protein positive expression (VEGF, COX-2, CA IX, bcl-2 and so on) in HIF-1α positive expression and negative expression tumour tissues (the data of dual staining of them in the same tissue section or in consecutive sections of same tumour tissue).
We estimated the odds ratio (OR) or relative risk (RR) for test cases and control cases. Statistical heterogeneity assumption among studies was checked using the X2-based Q-test [13]. When I2was no more than 50%, pooled odds ratios, relative risk and 95% confidence intervals (CIs) were calculated using Mantel-Haenszel method with fixed-effect models. Whereas significant heterogeneity (p <0.1, I2>50%) among the studies was detected, a random-effect model (Der Simonian and Laird method) was adopted. If necessary, a sensitive analysis was also performed to evaluate the influence of individual studies on the final effect. All p-values were two-sided. A p-value <0.05 was considered significant. All the statistical analyses were performed using RevMan 5.1 software (The Cochrane Collaboration, Oxford, United Kingdom, 2011).
| Table 1: Characteristics of the 30 selected studies. | |||||
| Author (year, reference) | Country | Stage | Histology | HIF-1α positive (negative) | Outcome(s) |
| Giatromanolaki A 2001 [14] | UK | I‒II | Squamous and adenocarcinomas | 68 (40) | K |
| Giatromanolaki A 2001 [15] | UK and Greece | I‒II | Squamous and adenocarcinomas | 68 (40) | LM |
| Wu XH 2011 [16] | China | I‒III | Squamous and adenocarcinomas | 49 (91) | BCFHIJKNOP |
| Chen 2009 [17] | China | I‒III | Squamous and adenocarcinomas | 70 (50) | ABHIJ |
| Seok JK 2005 [18] | USA and Korea | I‒II | Squamous, adenocarcinomas and large cell carcinoma | 37 (37) | FJKL |
| Sigve A2011 [19] | Norway | I‒III | Squamous, adenocarcinomas and large cell carcinoma | 25 (303) | OP |
| Hirami YJ 2004 [20] | Japan | I‒II | Squamous, adenocarcinomas and large cell carcinoma | 34 (46) | BFIJP |
| Park SH 2011 [21] | Korea | I‒IV | NSCLC (squamous, adenocarcinomas and other) | 79 (74) | BFHJP |
| Swinson DE 2004 [22] | UK | I‒IV | NSCLC (squamous, adenocarcinomas and other) | 80 (92) | JLMP |
| Li 2011 [23] | China | I‒IV | Squamous and adenocarcinomas | 43 (28) | ABCFHIK |
| Xiang 2008 [24] | China | I‒III | Squamous, adenocarcinomas and SCLC | 33 (42) | BDGHJO |
| Yang 2005 [25] | China | I‒III | Squamous, adenocarcinomas and SCLC | 33 (42) | K |
| Zhu 2007 [26] | China | I‒IV | Squamous and adenocarcinomas | 19 (26) | AFH |
| Wang 2011 [27] | China | I‒III | NSCLC (squamous, adenocarcinomas and other) | 28 (32) | ABCDEFHIJNO |
| Wang 2009 [28] | China | I‒IV | Squamous and adenocarcinomas | 24 (21) | ABDEFHJ |
| Wu 2011 [29] | China | I‒IV | Squamous and adenocarcinomas | 78 (82) | BFHP |
| Qin 2008 [30] | China | I‒III | Adenocarcinomas | 27 (18) | ABCEHIJN |
| Liu 2011 [31] | China | I‒III | Squamous, adenocarcinomas and large cell carcinoma | 108 (18) | BCFHIJ |
| Jiang 2011 [32] | China | I‒III | Squamous and adenocarcinomas | 29 (21) | ACFHIJ |
| Ding 2009 [33] | China | I‒IV | Squamous and adenocarcinomas | 38 (20) | ABCFHIJ |
| Deng 2010 [34] | China | I‒IV | Squamous and adenocarcinomas | 14 (15) | ABFHI |
| Lu 2009 [35] | China | unclear | Squamous and adenocarcinomas | 29 (31) | ABCEFIJ |
| Cheng 2005 [36] | China | I‒IV | Squamous and adenocarcinomas | 12 (36) | BCFIJK |
| Han 2008 [37] | China | I‒IV | NSCLC and SCLC | 37 (27) | ABCGHIJ |
| Zuo 2008 [38] | China | I‒III | Squamous and adenocarcinomas | 34 (14) | ABCFHIJ |
| Fan 2002 [39] | China | I‒III | NSCLC and SCLC | 17 (43) | GHIJM |
| Huo 2010 [40] | China | I‒IV | Squamous and adenocarcinomas | 53 (30) | ADFHJ |
| Zhao 2005 [41] | China | I‒IV | NSCLC and SCLC | 40 (36) | ABCDGHIJ |
| Wang 2005 [42] | China | I‒IV | Squamous, adenocarcinomas and large cell carcinoma | 29 (39) | ABDFHIJK |
| Yuan 2010 [43] | China | I‒IV | Adenocarcinomas | 51 (29) | ABDFHIJKP* |
| A: the control of benign tissues, B: gender, C: age (≥60 years or <60 years old), D: tumour diameters (≥5 cm or <5 cm), E: smoking (yes or no), F: histology (adenocarcinomas vs. squamous cell carcinoma), G: histology (NSCLC vs. SCLC), H: stage, I: differentiation, J: lymph node metastasis, K: VEGF, L: CA IX, M: Bcl-2, N: COX-2, O: 5-year’s survival rates, P: overall survival (HR), P*: overall survival (RR). | |||||
The original search identified 560 articles in PubMed, EMbase, CNKI, CBM, VIP, and Wan Fang Databases. Searching through the Cochrane database did not identify any articles. We excluded 435 studies after review of the title and abstract, because they contained duplicate documents, or were irrelevant studies and review articles. Afterwards, 125 articles were read in full, independently by two investigators, to assess their accordance with the predefined inclusion criteria. Finally, 30 articles were considered eligible for inclusion in the meta-analysis. A total of 9 articles [14–22] were published in English, and 21 articles [23–43] were published in Chinese. The study flow diagram is shown in figure 1, and the characteristics of eligible studies are summarised in table 1.
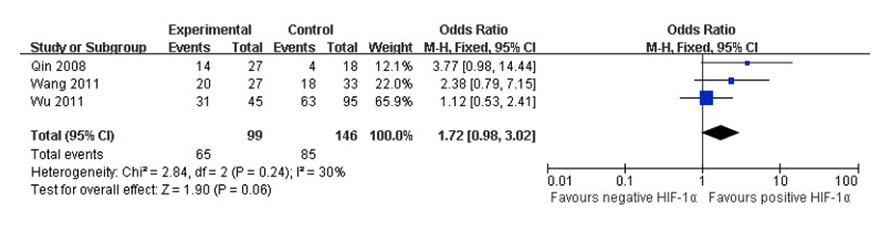
Figure 3
Forest plot of COX-2 positive expression in positive and negative HIF-1α tumour tissues.
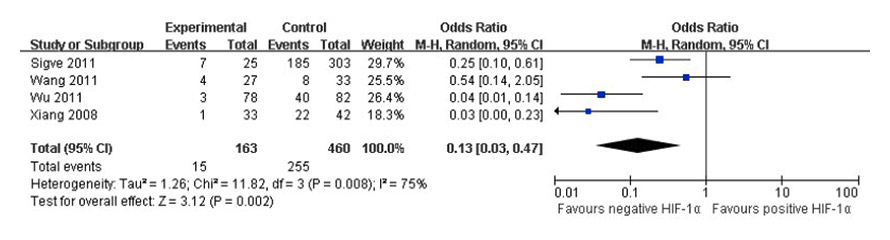
Figure 4
Forest plot of association between HIF-1α expression and 5-year survival rates.
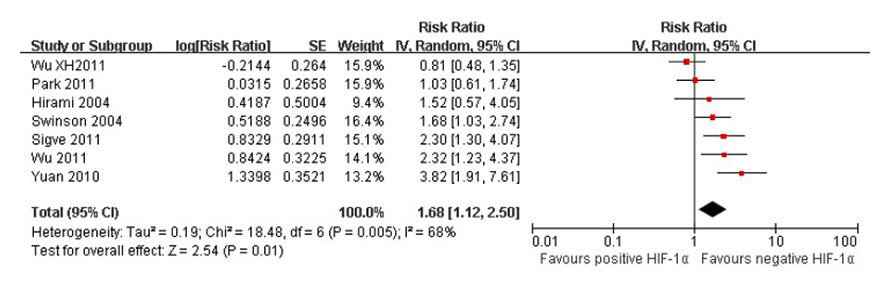
Figure 5
Forest plot of association between HIF-1α expression and overall survival.
Table 2 showed the results of meta-analysis. Overall, there was no association between genders, age, or smoking and HIF-1α positive expression (p >0.05). The OR (95% CI) was 1.00 (0.80, 1.26) for male versus female, 1.14 (0.85, 1.52) for age (≥60 years vs. <60 years), 2.16 (0.77, 6.05) for smoking versus no smoking, respectively. In accordance with the p value of 0.05, we could not draw a firm conclusion regarding whether there was no association between tumour diameters and HIF-1α positive expression (p = 0.05). However, the positive HIF-1α expression was associated with malignant tissues, tumour stage, lymph node metastasis, degrees of differentiation or histology (NSCLC vs. SCLC, adenocarcinomas vs. squamous cell carcinoma) in lung cancer patients (p <0.05). The OR (95% CI) was 19.00 (12.12, 29.78) for malignant tissues versus benign tissues, 3.31 (2.02, 5.44) for lymph node metastasis (yes vs. no), 0.23 (0.14, 0.36) for stage (I‒II VS. III‒IV), 0.47 (0.31, 0.70) for differentiation (well vs. moderately or poorly), 0.24 (0.07, 0.77) for NSCLC versus SCLC, 0.78 (0.63, 0.98) for adenocarcinomas versus squamous cell carcinoma, respectively. Interestingly, the expressions of HIF-1α was significantly higher than those in normal lung tissue; and III‒IV stage, lymph node metastasis, poorly differentiation, SCLC and squamous cell carcinoma were significantly higher than those in I‒II stage, no lymph node metastasis, well differentiation of lung tissues, NSCLC and adenocarcinomas, respectively.
Two studies [15, 27] assessed double staining in the same tissue section, one study [16] assessed the tissue micro-array, eight studies [18, 22, 25, 30, 36, 39, 42–43] assessed consecutive (serial) sections of the same tumour tissue, one study [23] did not assess the type of sections (data not extracted), significant heterogeneity existed in seven [15–16, 18, 25, 36, 42–43] studies when VEGF positive expression was compared in positive and negative HIF-1α tumour tissues, and there were three [15, 18, 22] studies for CA IX, and three [15, 22, 39] studies for Bcl-2 as well (I2 = 54%, I2 = 55%, I2 = 89%). The random effects model was used to pool the result (fig. 2). No significant heterogeneity existed in 3 [16, 27, 30] studies when COX-2 positive expression was compared in positive and negative HIF-1α tumour tissues (I2 = 30%). The fixed effects model was used to pool the result (fig. 3). There was an association between VEGF or CA IX positive expression and HIF-1α positive tumour tissues (p <0.05). The OR (95% CI) was 3.23 (1.90, 5.46), 3.84 (2.10, 7.03) for positive versus negative HIF-1α tumour tissues, respectively. There was no association between Bcl-2 or COX-2 positive expression and HIF-1α positive tumour tissues (p >0.05). The OR (95% CI) was 0.31 (0.05, 1.88), and 1.72 (0.98, 3.02) for positive versus negative HIF-1α tumour tissues, respectively. Thus, VEGF or CA IX positive expression was significantly higher in HIF-1α positive tumour tissues than those in HIF-1α negative tumour tissues, respectively.
Significant heterogeneity existed in 4 [19, 24, 27, 29] studies when 5-year survival rates were compared in positive and negative HIF-1α tumour tissues and 7 [16, 19–22, 29, 43] studies for overall survival, too (I2 = 75%, I2 = 68%). The random effects model was used to pool the result (fig. 4, fig. 5). There was an association between positive and negative HIF-1α tumour tissues for 5-year survival rates (OR = 0.13, 95% CI: 0.03–0.47, p = 0.002) and overall survival (RR = 1.68, 95% CI: 1.12–2.50, p = 0.01) in lung cancer patients. Thus, a survival difference was observed in positive and negative HIF-1α tumour tissues of lung cancer patients. The patients of negative HIF-1α tumour tissues had higher 5-year survival rates and overall survival than positive HIF-1α.
In order to prove robust results of high heterogeneity outcomes (diameter, smoking, NSCLC vs. SCLC, stage, lymph node metastasis, differentiation, VEGF, CA IX, bcl-2, 5-year survival rates and overall survival), sensitivity analyses were conducted as mentioned above. The influence of outcome on the overall meta-analysis estimate was investigated by omitting some obviously different studies at the time (table 3). When no significant heterogeneity (p >0.1, I2 <50%) among the studies was detected, the heterogeneity did not appear to impact significantly on the main outcomes of our analyses (NSCLC VS SCLC, stage, lymph node metastasis, differentiation, VEGF, CA IX, 5-year survival rates and overall survival), indicating that our results were statistically reliable. However sensitivity analyses showed that exclusion of a single study [30] considerably altered the main outcomes of our analyses (smoking), with a range from 2.16 (95% CI: 0.77–6.05, p = 0.14) to 3.52 (95% CI: 1.81–6.87, p = 0.0002). Exclusion of two studies [27, 43] considerably altered the main outcomes of our analyses (diameter), with a range from 1.84 (95% CI: 1.00–3.39, p = 0.05) to 1.30(95% CI: 0.84–2.01, p = 0.23). Exclusion of a single study [22] significantly altered the main outcomes of our analyses (bcl-2), with a range from 0.31 (95% CI: 0.05–1.88, p = 0.20) to 0.41 (95% CI: 0.02–0.83, p = 0.03), but did still not alter the significant heterogeneity (p = 0.05, I2 = 74%).
| Table 2: Meta-analysis of association between clinicopathologic variables and positive HIF-1α expression. | ||||||||||
| Clinicopathologic variables | Included studies | Test cases | Control cases | Heterogeneity | Meta-analysis model | Outcome(s) | ||||
| n | N | n | N | I2 | p | OR(95%CI) | p | |||
| Tumor vs. benign tissues | 16 [17, 23, 26–28, 30, 32–35, 37–38, 40–43] | 579 | 1085 | 21 | 385 | 0% | 0.91 | Fixed | 19.00 (12.12, 29.78) | 0.00001 |
| Male vs. female | 20 [16–17, 20–21, 23–24, 27–31, 33–38, 41–43] | 621 | 1173 | 236 | 438 | 0% | 0.50 | Fixed | 1.00 (0.80, 1.26) | 0.99 |
| Age (≥60 years vs. <60 years) | 12 [23, 27, 29–33, 35–38, 41] | 253 | 428 | 249 | 438 | 0% | 0.91 | Fixed | 1.14 (0.85, 1.52) | 0.38 |
| Diameter (≥5 cm vs. <5 cm) | 7 [24, 27–28, 40–43] | 125 | 205 | 132 | 282 | 57% | 0.03 | Random | 1.84 (1.00, 3.39) | 0.05 |
| Smoking vs. no smoking | 4 [27–28, 30, 35] | 66 | 111 | 41 | 99 | 68% | 0.03 | Random | 2.16 (0.77, 6.05) | 0.14 |
| AD vs. SCC | 18 [16, 18, 20–21, 23, 26–29, 32–36, 38, 40, 42–43] | 309 | 611 | 440 | 795 | 20% | 0.22 | Fixed | 0.78 (0.63, 0.98) | 0.03 |
| NSCLC vs. SCLC | 4 [24, 37, 39, 41] | 91 | 233 | 30 | 42 | 54% | 0.09 | Random | 0.24 (0.07, 0.77) | 0.02 |
| Stage (Ⅰ-Ⅱ vs. Ⅲ-Ⅳ) | 21 [16–17, 21, 23–24, 26–34, 37–43] | 412 | 991 | 466 | 655 | 67% | 0.00001 | Random | 0.23 (0.14, 0.36) | 0.00001 |
| Lymph node metastasis (yes vs. no) | 22 [17–18, 20–22, 24, 27–33, 35–43] | 601 | 901 | 374 | 886 | 72% | 0.00001 | Random | 3.72 (2.38, 5.80) | 0.00001 |
| Differentiation (well vs. poorly) | 18 [17, 20, 23, 27, 29–39, 41–43] | 352 | 757 | 321 | 524 | 54% | 0.003 | Random | 0.47 (0.31, 0.70) | 0.0002 |
| AD: adenocarcinomas, SCC:squamous cell carcinoma, NSCLC: non small cell lung cancer, SCLC: small cell lung cancer. | ||||||||||
| Table 3: Sensitivity analyses of high heterogeneity outcomes in meta-analysis. | ||||||||||
| Heterogeneity outcomes | Omitted(excluded) studies | Test cases | Control cases | Heterogeneity | Meta-analysis model | Outcome(s) | ||||
| n | N | n | N | I2 | p | OR(95%CI) | p | |||
| Diameter (≥5 cm vs. <5 cm) | 2 [27, 43] | 90 | 160 | 89 | 187 | 0% | 0.37 | Fixed | 1.30 (0.84, 2.01) | 0.23 |
| Smoking vs. no smoking | 1 [30] | 56 | 91 | 24 | 74 | 0% | 0.56 | Fixed | 3.52 (1.81, 6.84) | 0.0002 |
| NSCLC vs. SCLC | 1 [37] | 62 | 180 | 24 | 31 | 0% | 0.75 | Fixed | 0.13 (0.05, 0.34) | 0.0001 |
| Stage (Ⅰ–Ⅱ vs. Ⅲ–Ⅳ) | 2 [16, 29] | 366 | 806 | 385 | 540 | 0% | 0.63 | Fixed | 0.26 (0.20, 0.34) | 0.00001 |
| Lymph node metastasis (Yes vs. No) | 6 [22, 24, 29, 31, 33, 42] | 369 | 567 | 240 | 571 | 0% | 0.48 | Fixed | 3.27 (2.50, 4.27) | 0.00001 |
| Differentiation (well vs. moderately or poorly) | 6 [20, 29, 31, 34, 38, 39] | 220 | 504 | 199 | 296 | 0% | 0.59 | Fixed | 0.32 (0.24, 0.45) | 0.00001 |
| VEGF | 2 [36, 42] | 176 | 246 | 101 | 231 | 0% | 0.65 | Fixed | 3.24 (2.17, 4.82) | 0.00001 |
| CA IX | 1 [18] | 109 | 225 | 56 | 227 | 0% | 0.65 | Fixed | 3.03 (2.00, 4.59) | 0.00001 |
| bcl-2 | 1 [22] | 11 | 81 | 49 | 83 | 74% | 0.05 | Random | 0.14 (0.02, 0.83) | 0.03 |
| 5-year survival rates | 2 [19, 27] | 4 | 111 | 62 | 124 | 0% | 0.75 | Fixed | 0.04 (0.01, 0.11) | 0.00001 |
| 5-year survival rates | 2 [29, 24] | 11 | 52 | 193 | 336 | 0% | 0.34 | Fixed | 0.32 (0.15, 0.66) | 0.002 |
| Overall survival | 2 [16, 21] | – | – | – | – | 0% | 0.75 | Fixed | 2.19 (1.65, 2.89)* | 0.00001 |
| NSCLC: non small cell lung cancer, SCLC: small cell lung cancer, VEGF: vascular endothelial growth factor, CA IX: carbonic anhydrase-9, bcl-2: B-cell lymphoma 2 protein, *: relative risk (RR). | ||||||||||
Tissue hypoxia is an essential characteristic of solid tumours and promotes biologic processes involved in tumour progression [44]. It is well known that hypoxia inducible factor-1α (HIF-1α) is the unique sub-unit that determines the HIF system activity and is a member of the basic helixloop-helix-PAS protein family [45], is usually increased under hypoxic conditions, and can activate transcription of many genes that are critical for cellular function under hypoxic conditions.
The prognostic significance of HIF-1α expression has now been evaluated in a number of solid tumours. Increased HIF-1α expression has certainly been reported to be a negative expression in benign tissues, so our results of the meta-analysis (lung cancer tissues vs. benign tissues) showed a strong association with lung cancer risk (OR = 19.00, 95% CI = 12.12–29.78). However different studies showed a different trend of HIF-1α expression in different clinicopathologic variables of tumour. Meanwhile, conflicting results had been reported in lung cancer patients. Sample size may contribute to conflicting results among original studies, and a small sample size in a single study may be under statistical power and incapable to draw a reliable conclusion [46]. Meta-analysis as an important statistical method for medical research can extremely improve statistical power by enlarging sample size, and afterwards a more reliable conclusion can be drawn [47]. The results of the meta-analyses showed there was association between positive HIF-1α expression and tumour stage, lymph node metastasis, histology (NSCLC vs. SCLC, adenocarcinomas vs. squamous cell carcinoma) or degrees of differentiation in lung cancer patients. Thus the process of HIF-1α expression may be controlled by different mechanisms between histology [48], but the precise mechanism in the hypoxia-sensitive pathway of different histology is still not clear. In those studies [16, 20, 22, 24, 29, 31, 33–34, 37–39, 42], the choice of the cutoff value may be the main reason of heterogeneity. When there were no significant heterogeneity (p >0.1, I2 = 0%) by omitting those obviously different studies, the heterogeneity did not appear to impact significantly on the main outcomes of our analyses. Based on a meta-analysis of data obtained from 7 [24, 27–28, 40–43] studies, this review showed no evidence of difference in HIF-1α expression for tumour diameter (p = 0.05). As we know, the size of tumour does not necessarily predict a benign or malignant tumour, meanwhile sensitivity analyses showed that there was no evidence of difference in HIF-1α expression for tumour diameter (p = 0.23). Therefore, we are drawn to the conclusion that there was no relationship between the size of tumour and HIF-1α expression, but the measurement method of tumour diameter still has influence on the heterogeneity of the main outcomes of our analyses. Sensitivity analyses showed that exclusion of a single study considerably altered the main outcomes of our analyses (smoking). The detailed histories of smoking were not assessed in these studies [27–28, 30, 35]; therefore, smoking exposure variables (quantity and time) may have significant influence on the heterogeneity of the main outcomes of our analyses (smoking). In a word, these conclusions are needed to develop the further verification.
It is well known that hypoxia inducible factor-1α (HIF-1α) can regulate more than 40 downstream genes, which are involved in adaptive responses to hypoxia, and regulate many biological behaviours of cells, such as tumour metabolism, growth and angiogenesis [49]. The results of the meta-analyses showed there was association between VEGF or CA IX positive expression and HIF-1α positive tumour tissues. Given that these studies [18, 22, 25, 30, 36, 39, 42–43] have analysed the markers separately but on sequential tissue sections from one tumour, and used different methods of the immunohistochemistry (SP, PV9000, MaxVisionTM, EnVisionTM, ABC, the catalysed signal amplification kit), these may be the main reason of heterogeneity. When there were no significant heterogeneity (p >0.1, I2 = 0%) by omitting those obviously different studies [18, 39, 42] (Seok JK 2005 for CA IX; Cheng 2005 and Wang 2005 for VEGF), the heterogeneity did not appear to impact significantly on the main outcomes of our analyses. Sensitivity analyses showed that exclusion of a single study considerably altered the main outcomes of our analyses (bcl-2). Two studies [14, 39] have shown that a significant inverse association of the HIF1α with bcl-2(cytoplasm) expression, but no association was found between HIF-1α and Bcl-2 (unclear) expression [22]; therefore, the difference of protein accumulation region (cytoplasm, nuclear) may have significant influence on the heterogeneity of the main outcomes of our analyses (bcl-2).However maybe due to including a small sample size, there was no association between positive and negative HIF-1α tumour tissues for Bcl-2 or COX-2 positive expression. Meanwhile, owing to including few (less than three) studies about other proteins and variables, such as p53 protein, BNIP3, survivin protein, VEGFR, MMP-9, MVD, VM and Ki-67, we could not perform a meta-analysis of those variables. Moreover, some studies [50–51] reported the relationship between other proteins and HIF-1α expression, but there was limited data of HIF-1α to perform a meta-analysis and therefore they could not be included in our meta-analysis. Furthermore, from a clinical point of view it would be interesting to know something about the role of HIF-1α in molecular (KRAS wt/mut, EGFR wt/mut, ALK rearranged) sub-types of NSCLC, but there were the absences of actual data of molecular subtypes in our included studies. All of these will be needed to provide sufficient data to calculate the effect sizes in future research.
All of the survival data was confounded by variable use of postoperative adjuvant therapy, such as adjuvant chemotherapy and adjuvant radiotherapy, or no treatment after surgery. This may be the main reason of heterogeneity, and undoubtedly affected patient’s survival. Only one study [19] reported postoperative adjuvant radiotherapy, three studies [24, 27, 29] did not report postoperative therapy (5-year survival rates); three studies [16, 29, 43] did not report postoperative therapy, and four studies [19–22] reported postoperative adjuvant radiotherapy, chemotherapy or radiotherapy with chemotherapy (overall survival). When there were no significant heterogeneity (p >0.1, I2 = 0%) by omitting those obviously different studies, the heterogeneity did not appear to impact significantly on the main outcomes of our analyses. Adjuvant treatments after surgery are unavoidable, taking into account the issues of ethics and patient interests. So combined with the results of our study about association between positive HIF-1α expression and clinicopathologic variables, we could draw the firm conclusion that HIF-1α expression has an influence on the survival of lung cancer patients.
Some limitations of this systematic review and meta-analysis are as follows: First, this meta-analysis had to address heterogeneity issues. We found significant heterogeneity among these studies. The heterogeneity could be explained by immunohistochemistry techniques used to detect protein expression (including antigen retrieval methods, choice of antibody, tumour specimens and the choice of the cutoff value), methods of the immunohistochemistry (SP, PV9000, MaxVisionTM, EnVisionTM, ABC and the catalysed signal amplification kit) and postoperative adjuvant treatment have been mentioned above. Specifically, there were 15 studies [14–15, 17, 23, 26–27, 31, 34–41] which used SP, 5 studies [24–25, 30, 32–33] which used PV9000, 4 studies [16, 20, 29, 43] used EnVisionTM, 3 studies [18, 19, 42] used ABC, 2 studies [21–22] used the catalysed signal amplification kit, and 1 study [28] used the MaxVisionTM method of the immunohistochemistry, but the sensitivity and specificity of these methods are differences in the immunohistochemistry. However, average immunohistochemistry measurements are more reproducible, stable, and less affected by bias [52]. Nonetheless, the potential limitations of this study should be considered. We also conducted sensitivity analyses to assess the accuracy and reliability of our results by the removal of obviously different studies. When no significant heterogeneity (p >0.1, I2 = 0%) among the studies was detected, the heterogeneity did not appear to impact significantly on the main outcomes of our analyses (NSCLC vs. SCLC, stage, lymph node metastasis, differentiation, VEGF, CA IX, 5-year survival rates and overall survival) (table 3). Secondly, publication bias is a wide phenomenon for all forms of meta-analysis, such as positive results easily published by journals, including double articles published in Chinese. Therefore, the test for publication bias was not performed and possible bias still could not be ruled out. Third, some studies which were included in our study used continuous variables and r value of statistical methods, but due to the limitation of quantity (less than three studies) they could not be used to develop a meta-analysis, too. These could affect the comprehensive and integrity of our research data.
In conclusion, despite the limitations of this meta-analysis, our study confirmed that HIF-1α is associated with differentiation degree of lung cancer cell, lymph node metastasis, postoperative survival time and histology (NSCLC vs. SCLC, adenocarcinomas vs. squamous cell carcinoma). HIF-1α which combined other proteins, such as VEGF or CA IX, might serve as important parameters in evaluating biological behaviour and prognosis of lung cancer; it will be benefit to clinical treatment and prognostic evaluation.
1 Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–80. doi: 10.1056/NEJMra0802714.
2 Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5.
3 Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–54.
4 Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60(17):4693–6.
5 Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. Hypoxia-inducible factor-1- independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64(5):1765–72.
6 Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1 alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573–81.
7 Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, et al. Hypoxia-inducible factor-1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26(1):56–64.
8 Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5.
9 GA Wells, B Shea, D O’Connell, J Peterson, V Welch, M Losos, et al. The Newcastle – Ottawa Scale (NOS) for assessing the quality of non randomized studies in meta-analyses. 2012. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
10 Steels E, Paesmans M, Berghmans T, Branle F, Lemaitre F, Mascaux C, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18(4):705–19.
11 Gould Rothberg BE, Bracken MB. E-cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res Treat. 2006;100(2):139–48.
12 Wu Y, Liu HB, Ding M, Liu JN, Zhan P, Fu XS, et al. The impact of E-cadherin expression on non-small cell lung cancer survival: a meta-analysis. Mol Biol Rep. 2012;39(10):9621–8.
13 Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11):1539–58.
14 Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85(6):881–90.
15 Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61(21):7992–8.
16 Wu X, Qian C, Yuan K. Correlation of hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression with angiogenesis factors expression and prognosis in non-small cell Iung cancer. Chin Med J (Engl). 2011;124(1):11–8.
17 Chen Y, Zhao C, Li W. Effect of hypoxia-inducible factor-1α on transcription of survivin in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28(1):29.
18 Kim SJ, Rabbani ZN, Dewhirst MW, Vujaskovic Z, Vollmer RT, Schreiber EG, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer. 2005;49(3):325–35.
19 Andersen S, Eilertsen M, Donnem T, Al-Shibli K, Al-Saad S, Busund LT, et al. Diverging prognostic impacts of hypoxic markers according to NSCLC histology. Lung Cancer. 2011; 72(3):294–302.
20 Hirami Y, Aoe M, Tsukuda K, Hara F, Otani Y, Koshimune R, et al. Relation of epidermal growth factor receptor, phosphorylated-Akt, and hypoxia-inducible factor-1alpha in non-small cell lung cancers. Cancer Letters. 2004;214(2):157–64.
21 Park S, Ha SY, Cho HY, Chung DH, Kim NR, Hong J, et al. Prognostic implications of hypoxia-inducible factor-1α in epidermal growth factor receptor-negative non-small cell lung cancer. Lung Cancer. 2011;72(1):100–7.
22 Swinson DE, Jones JL, Cox G, Richardson D, Harris AL, O’Byrne KJ. Hypoxia-inducible factor-1 alpha in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer. 2004;111(1):43–50.
23 Li H, Li J, Wang J, Zhang H, Huang J, Wang H. Expression of HIF-1α, VEGF in non-small cell lung cancer. Journal of Hebei Medical University. 2011;32(4):455–8 [Article in Chinese].
24 Xiang F, Wu C, Yang D, Niu Z, Shen Y. Prognostic significance of HIF-1α in pulmonary carcinoma and its relationship to proliferative activity of cancer cells. China Journal of Modern Medicine. 2008;18(1):28–32 [Article in Chinese].
25 Yang D, Xiang F. Expression of vascular endothelial growth factor in patients with pulmonary carcinoma and relationship with biological behavior of hypoxia and tumor and prognosis. Chinese Journal of Clinical Rehabilitation. 2005;9(10):115–8 [Article in Chinese].
26 Zhu L, Chen P, Jiang Y. Expression of COX-2 and HIF-1 in non-small cell lung cancer and the relationship between them and tumor angiogenesis. Journal of Chinese Physician. 2007;9(10):1340–2 [Article in Chinese].
27 Wang X, Yin L. Expression of HIF-lα, P-gp and COX-2 in non-small cell lung cancer. Ning xia Med J. 2011;33(5):411–4 [Article in Chinese].
28 Wang Q. Expression of hypoxia-inducible factor-1α and BNIP3 in human non-small cell lung cancer and their correlation. China: Nan Chang University Press. 2009;1–48 [Article in Chinese].
29 Wu S, Cheng Z, Yu L, Song W, Tao Y. Expression of CD28/KAI1 and HIF-1α in non-small cell lung cancer and their relationship to vasculogenic mimicry. Chin J Lung Cancer. 2011;14(12):918–25 [Article in Chinese].
30 Qin J. Study on the effect and mechanism of HIF-1α on the expressions of COX-2 and E-cadherin in lung adenocarcinoma cell during hypoxia. China: Tian Jin Medical University Press. 2008; 1–90 [Article in Chinese].
31 Liu J, Zhang N, Xu C, Li Z, Liu J, Sun Y. Co-expression of hypoxia-inducible factor-1α and hepatocyte growth factor in non-small-cell lung cancer and their association with lymphangiogenesis. Journal of Shan Dong University (Health Sciences). 2011;49(11):112–6 [Article in Chinese].
32 Jiang X, Dai P, Song D, Wu J, Li S. Expressions of HIF-1α、VEGF and VEGFR2 in non-small cell lung cancer and their clincical significance. Journal of Clinical Pulmonary Medicine. 2011;16(3):386–8 [Article in Chinese].
33 Ding Y, Da C, Li Y, Wu J. Expression of HIF-1α and VEGF-C and the relationship of lymphatic metastasis in non small cell lung cancer. Journal of Qing Hai Medical College. 2009;30(2):93–8 [Article in Chinese].
34 Deng S, Nie W, Wang C. The Expressions of HIF-1α and COX-2 in non-small cell lung carcinoma and its clinical significance. Journal of Basic and Clinical Oncology. 2010;23(5):369–71 [Article in Chinese].
35 Lu P, Zhang D, Cai C, Chang J. Expression and significance of HIF-1αand P300/CBP in squamous cell carcinoma and adenocarcinoma of lung. Modem Oncology. 2009;17(1):55–7 [Article in Chinese].
36 Cheng C, Pan T, Chen T, Xu Q, Gao S. Relationship of hypoxia-inducible factor-1 alpha (HIF-1α) expression with vascular endothelial growth factor (VEGF) and microvessel density (MVD) in human non-small cell lung cancer. China Journal of Modern Medicine. 2005;15(15):2285–8 [Article in Chinese].
37 Han Y, Wang J, Wang G, Ao Q. Vasculogenic mimicry and HIF-1α expression in lung cancer and their significance. J Clin Exp Pathol. 2008;24(3):269–72 [Article in Chinese].
38 Zuo S, Wang J, Guo J. Expressions and correlation of HIF-1α and VEGF-C in non-small cell lung cancer. The Journal of Practical Medicine. 2009;25(1):59–61 [Article in Chinese].
39 Fan L, Diao L, Chen D, Liu X, Zhu L, Li H, et al. Expression of HIF-1α and its relationship to apoptosis and proliferation in lung cancer. Chinese Journal of Cancer. 2002;21(3):254–8 [Article in Chinese].
40 Huo R, Teng L, Zhang X, Liu Q. Expression and significance of HIF-lα and MMP-9 in non-small cell lung cancer. Modern Medicine &Health. 2010;26(6):802–3 [Article in Chinese].
41 Zhao J, Yang J, Li Y. Expressions and clinical significance of PTEN and HIF-1α proteins in human lung carcinoma using tissue microarray. Medical Journal of Wu han University. 2005;26(4):399–442 [Article in Chinese].
42 Wang X, Wan Y, Zhang Q. Expression and Significance of HIF-1α and VEGF in non-small cell lung cancer. Cancer Research on Prevention and Treatment. 2005;32(8):487–9 [Article in Chinese].
43 Yuan Y, Zhong H, Mei J, Zhang P, Bao C, Li B, et al. Expression and clinical significance of HIF-1αin non-small cell lung cancer. Progress in Modern Biomedicine. 2010;10(15):2814–7 [Article in Chinese].
44 Harris AL. Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47.
45 Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423(6940):593–5.
46 Attia J, Thakkinstian A, D’Este C. Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol. 2003;56(4):297–303.
47 Nordmann AJ, Kasenda B, Briel M. Meta-analyses: what they can and cannot do. Swiss Med Wkly. 2012;142:w13518. doi: 10.4414/smw.2012.13518.
48 Lee CH, Lee MK, Kang CD, Kim YD, Park DY, Kim JY, et al. Differential expression of hypoxia inducible factor-1 alpha and tumor cell proliferation between squamous cell carcinomas and adenocarcinomas among operable non-small cell lung carcinomas. J Korean Med Sci. 2003;18(2):196–203.
49 Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
50 Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63(17):5376–80.
51 Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, et al. BNIP3 expression Is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10(16):5566–71.
52 VanDiest PJ, vanDam P, Henzen-Logmans SC, Berns E, vanderBurg ME, Green J, et al. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European organization for research and treatment of cancer-gynaecological cancer cooperative group. J Clin Pathol. 1997;50(10):801–4.
Funding / potential competing interests: This study was supported by the Research Programme for health of Gansu Province of China (No. GSWST09-06), the Natural Science Foundation of Gansu Province of China (No. 1010RJZA162) and the Postgraduate Business Programme of Evidence-Based Medicine Center of Lanzhou University (No. 2010LDEBM-A). None of the authors has any conflict of interest.