Conversion to prolonged release tacrolimus formulation in stable kidney transplant recipients
DOI: https://doi.org/10.4414/smw.2013.13850
SHENG-WEN
WU, Hui-Ching
Tsai, Pao-Yu
Tsai, Tung-Wei
Hung, Horng-Rong
Chang, Jong-Da
Lian
Summary
PRINCIPLES: The once-daily tacrolimus formulation (Advagraf®), with the potential for improving medical adherence, has been advocated to improve long-term kidney allograft outcomes. However, experience with late conversion from the twice-daily tacrolimus formulation (Prograf®) to Advagraf in the daily care of stable kidney transplant recipients has been limited.
METHODS: The aim of this study was to observe the efficacy and safety of conversion from Prograf to Advagraf in chronic stable kidney transplant recipients in routine clinical practice. The recruited patients had postconversion follow-up at least once monthly for a total of six months, unless they had discontinued the use of Advagraf, had been lost the follow-up or had lost the graft.
RESULTS: The mean age of the 199 patients was 51.5 ± 10.4 years (60.8% male). The mean time from transplantation to the conversion to Advagraf was 8.3 ± 3.2 years and the mean tacrolimus trough level at conversion was 4.2 ± 1.4 ng/ml. After conversion, 147 patients (73.8 %) had a reduced trough level at one month and the mean change in trough level postconversion was –13.5%. The mean serum creatinine level between conversion and six months postconversion was not significantly different (1.12 ± 0.36 vs 1.10 ± 0.42 mg/dl). Thirty-four patients (17%) discontinued the treatment with Advagraf and two (1%) developed biopsy-proved acute rejection.
CONCLUSIONS: In conclusion, frequent conversion caused by a high discontinuation rate may further raise the potential risk of allograft rejection and increase unnecessary cost. In view of this, the policy of converting to Advagraf with the purpose of improving medical adherence should be individualised in routine clinical practice.
Introduction
Non-adherence to prescribed medical regimens has been proved to be associated with the risk of acute rejection and allograft survival in renal transplant recipients [1, 2]. Advagraf® (Astellas Pharma Inc., Tokyo, Japan), a new prolonged release formulation of tacrolimus, was developed to allow once daily prescription. The once daily formulation (Advagraf) is likely to improve medical adherence when compared to the traditional twice daily formulation (Prograf®).
In preliminary clinical studies, Advagraf was shown to be bioequivalent to and to have similar efficacy and comparable safety profiles to Prograf [3–5]. Since Advagraf became available on the market a few conversion studies with smaller patient populations revealed the comparable efficacy and safety of Advagraf with Prograf in stable maintenance kidney transplant patients despite the observation of reduced tacrolimus trough level after conversion Prograf to Advagraf in a certain percent of patients [6–9]. A large cohort study of stable maintenance kidney transplant patients further showed that Advagraf provided stable renal function, a low acute rejection rate, and good tolerability in the setting of routine clinical practice [10]. However, experience of stable conversion has only been reported in stable European and American kidney transplant recipients. Furthermore, the experience of late conversion in the daily care of stable kidney transplant recipients has been limited. Thus, the objective of our study is to clarify the actual efficacy and safety in a cohort of stable Chinese kidney recipients converted from twice daily Prograf to once daily Advagraf in routine clinical practice.
Materials and methods
Patients and study design
After obtaining approval from the local Institutional Research Board, we performed a retrospective cohort study of renal transplant recipients at Chung-Shan Medical University (Taichung, Taiwan) who had been receiving twice daily Prograf- based maintenance immunosuppressant for at least one year and whose clinical condition and graft function were stable. IL-2 antagonist was administered for all patients as an induction therapy. After transplantation, we keep FK-trough level around 10 to 15 ng/ml in the first three months, 7–10 ng/ml in the following one year, and 3 to 7 ng/ml thereafter. Cellcept® (1 g bid ) or Myfortic® (720 mg bid) were given in the first three months and then gradually tapered to a dose of 500 to 750 mg bid or 360 to 540 mg bid, respectively. Corticosteroid was usually discontinued one year after transplantation. The actual dose of each immunosuppressant and supplement of mammalian target of rapamycin (mTOR) was determined by the transplant team on an individual basis. The conversion from twice daily Prograf to once daily Advagraf was started in September 2010. The conversion in patients with baseline FK-trough level more than and less than 4 ug/dl was usually made on the basis of 1 to 1 and 1 to 1.1 or 1.2 of total daily dose, respectively. The recruited patients had received postconversion follow up at least once monthly for a total of six months, unless they had discontinued the use of Advagraf, had been lost to follow-up, or had lost the graft. We collected clinical condition (body weight, blood pressure, clinical events and self-reported adverse effects of drugs) and laboratory parameters (serum creatinine, alanine aminotransferase [ALT], blood sugar level, lipid profiles, urinalysis and haemoglobin) every month to every three months after conversion. Other immunosuppressive regimens, concomitant anti-hypertensive drugs, and episodes of biopsy-proved acute rejection were also recorded. The discontinuation of Advagraf could have been made either as a decision of the physician or the wish of the patient.
Statistical analyses
All data were expressed as means ± SD, unless stated otherwise. All analyses were performed using SPSS software for Windows (Version 14.0; SPSS, Chicago, IL, USA). Analyses were performed with χ2testing for categorical variables (Fisher exact test used for violations of Cochran’s assumptions) and ttest for continuous variables (Mann-Whitney test for non-normally distributed variables). A two-sided P-value of less than 0.05 was considered as statistically significant.
Results
Baseline characteristics of study population
The study included 199 stable renal transplant recipients (60.8% male) with the mean age of 54.5 years (table 1). All of them had their first transplant except one, who had a second transplant. The mean serum creatinine level and tacrolimus trough level at conversion was 1.1 ± 0.4 mg/dl and 4.2 ± 1.4 ng/ml, respectively. The mean time from transplantation to conversion was 8.3 ± 3.2 years. The conversion from twice daily Prograf to once daily Advagraf on the basis of 1 mg to 1 mg was made in 73% of patients and 1 mg to 1.1 or 1.2 mg in the remaining 27%. Within the study period 34 patients (17%) discontinued the use of Advagraf.
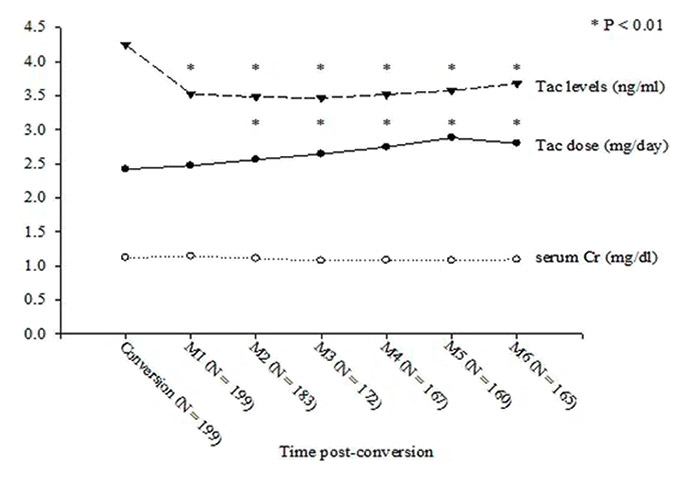
Figure 1
The evolution of serum creatinine level, tacrolimus trough level and daily dosage of Prograf or Advagraf in renal transplant patients with conversion from twice daily Prograf to once daily Advagraf. Tac level: tacrolimus trough level; Tac dose: daily dose of Prograf or Advagraf; Cr: creatinine.
The clinical efficacy and safety after conversion
After conversion to Advagraf , the mean tacrolimus trough level decreased from 4.2 ± 1.4 ng/ml at conversion to 3.5 ± 1.3 ng/ml at one month postconvervsion (p <0.01) and the average daily dose of tacrolimus increased from 2.4 ± 1.4 mg at conversion to 2.4 ± 1.5 mg at one month postconvervsion (p = 0.319) (fig. 1). Of the 199 patients studied, 147 (73.9%) had a lower postconversion tacrolimus trough level and 99 (49.7%) had a more than 20% reduction of tacrolimus trough level after conversion when compared with the level at conversion.
Within the study period, one patient (0.5%) died as an unexplained sudden death and another (0.5%) received follow up in another hospital. Advagraf was discontinued in an additional 32 patients (16.1%) because of self-reported malaise (7 patients, 3.5%), deterioration of graft function (7 patients, 3.5%), self-reported headaches (6 patients, 3.0%), persistent too low or too high tacrolimus trough level (4 patients, 2.0%), oral ulcer (3 patients, 1.5%), skin rash (2 patients, 1.0%), non-adherence to the prescription of once daily dose (1 patient, 0.5%), self-reported sexual dysfunction (1 patients, 0.5%), and neoplasia (1 patient, 0.5%). Fifty nine percent of the cases of Advagraf discontinuation in the study were determined by the patients. Among the seven patients with deterioration of graft function after conversion, five patients only had slight deterioration of graft function and refused to undergo graft biopsy. Advagraf was changed back to Prograf at their request and an additional prednisolone 5 mg per day was administered for empirical treatment. All of them had stabilised graft function in the following visits. Another two patients received graft biopsy at one and two month postconversion and both had biopsy-proved acute rejection (T cell mediated rejection, Baff 1A and 2A, respectively).
The mean serum creatinine level had been stable between the periods of conversion to six month postconversion (fig. 1). In addition, we saw no significant changes in the lipid profile, blood glucose level, liver function test and haemoglobin level.
|
Table 1:Demographic and clinical characteristics of the renal transplanted patients with conversion from twice daily Prograf to once daily Advagraf. |
| Numbers of patients |
199 |
| Age (years) |
54.5 ± 10.4 |
| Gender (Male / Female) |
121/78 |
| BMI |
24.1 ± 3.2 |
| DM (n, %) |
35 (17.6%) |
| HTN (n, %) |
138 (69.3%) |
| Hepatitis B (n, %) |
41 (20.6%) |
| Hepatitis C (n, %) |
23 (11.6%) |
| HLA- AB mismatch |
2.2 ± 0.8 |
| HLA- DR mismatch |
0.9 ± 0.5 |
| Pre-transplant PRA <10% (n, %) |
199 (100%) |
| Induction therapy with basiliximab or daclizumab (n, %) |
199 (100%) |
| Concomitant medications (n, %) |
|
| m-TOR |
29 (14.6%) |
| MMF |
170 (85.4%) |
| Corticosteroid |
41 (20.6%) |
| CCB |
29 (14.6%) |
| ACEI or ARB |
97 (48.7%) |
| Time from transplantation to conversion (years) |
8.3 ± 3.2 |
| History of acute rejection before conversion (n, %) |
20 (10.1%) |
| Serum creatinine at conversion (mg/dl) |
1.12 ± 0.36 |
| Haemoglobin at conversion (g/dl) |
13.3 ± 1.8 |
| Tacrolimus dose requirements at conversion |
|
| mg/day |
2.4 ± 1.4 |
| mg/kg/day |
0.04 ± 0.02 |
| Tacrolimus trough level at conversion (ng/dl) |
4.2 ± 1.4 |
| Numbers of Advagraf discontinuation (n, %) |
34 (17%) |
| Data were presented as n (%) or mean ± standard deviation |
| BMI: body mass index; PRA: panel reactive antibody; m-TOR: mammalian target of rapamycin; MMF: mycophenolate mofetil; CCB: calcium channel blockers; ACEI: angiotensin converting enzyme inhibitor; ARB: Angiotensin II receptor blockers |
Discussion
Although some American and European studies have confirmed the safety and efficacy of conversion from Prograf to Adavagraf in stable renal transplant recipients, some patients had actually decreased tacrolimus systemic exposure after conversion and needed a dose adjustment [7, 8, 10]. Even though conversion was already made on the basis of 1to 1.1 or 1.2 of total daily dose in 27% of our patients, a decrease in tacrolimus exposure still occurred in 147 stable kidney transplant patients (73.9%), in whom 99 (67.3%) experienced a decrease in tacrolimus trough level of more than 20%. Furthermore, two patients (1%) in this study developed biopsy-proved acute rejection, which could be attributed to low blood level of tacrolimus postconversion (approximately 2 ng/ml). We tried to determine the factors associated with the reduction of drug level after conversion, but no relative predictors were discovered (data not shown). The results of this study have called into question whether the potential benefit from improving medical adherence could overwhelm the potential risk of acute rejection from inadequate immunosuppression after conversion. In fact, some experts have questioned the assumption that Advagraf has a similar efficacy and comparable safety to Prograf in routine care of renal transplant recipients [11, 12]. They highlighted the possibility that a potential difference in the pharmacokinetics between these two drugs does actually exist and that there are discrepancies in the evolution of tacrolimus exposure from clinical trials to daily clinical practice. Therefore, conversion to Advagraf in stable renal transplant recipients still needs to be undertaken with cautious medical monitoring in routine practice.
In contrast to the discontinuation of Advagraf occurring in only a few patients in past follow up studies (seven perspective and two retrospective studies) [6, 8, 10, 13–18], 32 patients (16.1%) in our six month follow up study discontinued the use of Advagraf, in whom up to 96.9 percent of them (31 patients) went back to Prograf. The possible explanations for this discrepancy are that patients willing to participate in the prospective studies were likely to prefer once daily dose and patients with a follow up of less than six months were already excluded in one out of two retrospective studies. Moreover, we think the investigators involved in these prospective studies were probably more reluctant to respond to a patient’s request to switch back to Prograf, which could be another reason to explain the discrepancy.
It is worth noting that more than half of the Advagraf discontinuation in our study was requested by the patients, mainly because of postconversion discomfort or anxiety about the mild deterioration of renal function after conversion. This may imply that renal transplant recipients with chronic stable renal function, such as our patients (mean creatinine 1.1 mg/dl and mean post transplantation follow-up 8.3 years), were prone to have greater concern about the efficacy and safety of the new formulation. Therefore, it is possible that the postconversion discomforts in our patients were, at least in part, attributed to their insufficient reliability and confidence in the efficacy and safety of Advagraf. On the other hand, it is interesting to observe that one patient had frequently taken Advagraf twice daily because he had been used to the dosing frequency of Prograf, which could cause the excessive exposure to tacrolimus and increase risk of tacrolimus related nephrotoxicity. In fact, it is reasonable to extrapolate from the result of their long term good allograft function that most of our patients had had quite good compliance with the prescription. As such, whether or not the daily dosing could further improve treatment adherence for these patients remains undetermined.
There is no doubt that reducing dosing frequency can improve medical adherence and may also improve long term graft outcome [19–21]. However, there has been only limited data suggesting that conversion from Prograf to Advagraf could further improve treatment adherence in organ transplant recipients [10, 22]. This may not hold true for chronic stable renal transplant patients who are recognized to have good medication adherence due to their long term good allograft function. This needs to be clarified in future studies. Furthermore, there are no data available that demonstrate that conversion to Advagraf can improve the long term allograft outcome in stable renal transplant recipients. Therefore, the potential benefit and risk from conversion to Advagraf should be taken into consideration for chronic stable renal transplant recipients.
Conclusions
In summary, frequent conversion caused by the high discontinuation rate in this study may further raise the potential risk of allograft rejection and increase unnecessary cost. In view of this, the policy of conversion to Advagraf with the purpose of improving medical adherence should be individualised in routine clinical practice.
References
1 Laederach-Hofmann K, Bunzel B. Noncompliance in organ transplant recipients: a literature review. General hospital psychiatry. 2000;22(6):412–24.
2 Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(7):1305–11.
3 Hardinger KL, Park JM, Schnitzler MA, Koch MJ, Miller BW, Brennan DC. Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(4):621–5.
4 Chisholm MA, Middleton MD. Modified-release tacrolimus. The Annals of pharmacotherapy. 2006;40(2):270–5.
5 Kramer BK, Charpentier B, Backman L, Silva HT, Jr., Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(12):2632–43.
6 Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Two years postconversion from a prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007;83(12):1648–51.
7 de Jonge H, Kuypers DR, Verbeke K, Vanrenterghem Y. Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation. 2010;90(5):523–9.
8 Hougardy JM, Broeders N, Kianda M, Massart A, Madhoun P, Le Moine A, et al. Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation. 2011;91(5):566–9.
9 Tinti F, Mecule A, Poli L, Bachetoni A, Umbro I, Brunini F, et al. Improvement of graft function after conversion to once daily tacrolimus of stable kidney transplant patients. Transplantation proceedings. 2010;42(10):4047–8.
10 Guirado L, Cantarell C, Franco A, Huertas EG, Fructuoso AS, Fernandez A, et al. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(9):1965–71.
11 Hougardy JM, de Jonge H, Kuypers D, Abramowicz D. The once-daily formulation of tacrolimus: a step forward in kidney transplantation? Transplantation. 2012;93(3):241–3.
12 Barraclough KA, Isbel NM, Johnson DW, Campbell SB, Staatz CE. Once- versus twice-daily tacrolimus: are the formulations truly equivalent? Drugs. 2011;71(12):1561–77.
13 Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, et al. Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation. 2011;92(6):648–52.
14 Gallego-Valcarce E, Ortega-Cerrato A, Llamas-Fuentes F, Martinez-Fernandez G, Perez-Martinez J, Gomez-Roldan C. Conversion to tacrolimus extended-release formulation: short-term clinical results. Transplantation proceedings. 2009;41(6):2326–7.
15 Iaria G, Sforza D, Angelico R, Toti L, de Luca L, Manuelli M, et al. Switch from twice-daily tacrolimus (Prograf) to once-daily prolonged-release tacrolimus (Advagraf) in kidney transplantation. Transplantation proceedings. 2011;43(4):1028–9.
16 Diez Ojea B, Alonso Alvarez M, Aguado Fernandez S, Banos Gallardo M, Garcia Melendreras S, Gomez Huertas E. Three-month experience with tacrolimus once-daily regimen in stable renal allografts. Transplantation proceedings. 2009;41(6):2323–5.
17 Wehland M, Bauer S, Brakemeier S, Burgwinkel P, Glander P, Kreutz R, et al. Differential impact of the CYP3A5*1 and CYP3A5*3 alleles on pre-dose concentrations of two tacrolimus formulations. Pharmacogenetics and genomics. 2011;21(4):179–84.
18 Mecule A, Poli L, Nofroni I, Bachetoni A, Tinti F, Umbro I, et al. Once daily tacrolimus formulation: monitoring of plasma levels, graft function, and cardiovascular risk factors. Transplantation proceedings. 2010;42(4):1317–9.
19 Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. Journal of the American Society of Nephrology: JASN. 2005;16(6):1839–48.
20 Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–76.
21 Takemoto SK, Pinsky BW, Schnitzler MA, Lentine KL, Willoughby LM, Burroughs TE, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(12):2704–11.
22 Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transplant international: official journal of the European Society for Organ Transplantation. 2011;24(7):666–75.
