Zurich University Hospital lung transplantation programme: update 2012
DOI: https://doi.org/10.4414/smw.2013.13836
Ilhan
Inci, Mace
Schuurmans, Annette
Boehler, Walter
Weder
Summary
Lung transplantation is an established therapeutic option for end-stage lung disease in selected patients. During the last 30 years more than 34,000 transplantations have been performed worldwide. Emphysema, pulmonary fibrosis, cystic fibrosis and primary pulmonary hypertension are the most common indications. This type of surgical treatment is increasingly successful, with better early and late survival rates. However, lung transplantation is still hampered by persisting problems such as donor organ shortage, primary graft dysfunction, late graft dysfunction and morbidity related to long-term immunosuppression. The first lung transplantation in Switzerland was performed the 10th November 1992 at Zurich University Hospital. Since then the lung transplant programme has progressively increased its yearly transplant volume. Since the beginning of our lung transplantation programme, overall patient survival has increased steadily and has been at benchmark levels since the year 2000. The most important factors influencing this result are presumably good teamwork among all involved specialists, improved surgical techniques, and close and long-term patient follow-up by the transplant pulmonologists. In this paper we present our programme structure, managing strategies for some specific problems and outcome after lung transplantation. The results presented here are from recipients who underwent lung transplantation up to the end of 2011.
Programme structure
A potential lung transplant candidate is seen at least three times as an outpatient by the pulmonologists in order to assess urgency of evaluation and possible contraindications. Information concerning evaluation and follow-up after lung transplantation is provided. Pretransplant work-up is done during a hospitalisation on the pulmonary ward. During this evaluation period, members of the multidisciplinary team see the patient and document their decision in writing before attending the interdisciplinary colloquium which evaluates the listing of the patient. The evaluation team includes dedicated lung transplant specialists from thoracic (cardiac) surgery, pulmonary medicine, psychiatry, endocrinology, cardiology, anaesthesia, physiotherapy, nutrition, infectious diseases and surgical intensive care, and transplant coordinators. The core transplant care team, consisting of thoracic surgeons and pulmonologists, meets every week to discuss the recently transplanted patients who are on the ward, and the current condition of the patients on the waiting list.
In the early postoperative phase, patients are managed by the surgical intensive care members on the intensive care unit (ICU), with the main contribution coming from thoracic surgery and pulmonary medicine specialists. After transfer to the ward, recipients are followed up by the thoracic surgery team, with daily visits from a pulmonologist. After discharge, the transplanted patients are followed up closely for life by transplant pulmonologists. In the event of hospital readmission for any reason, the patient is admitted at our university hospital to the pulmonary ward or other wards under the supervision of pulmonologists.
Patient evaluation and selection criteria
After a referral for lung transplantation, we provide a rapid and efficient work-up procedure for the potential candidate. This work-up includes, but is not limited to, standard laboratory tests, chest X-ray, high resolution computed axial tomography scanning, abdominal ultrasound, bone densitometry, mammography, pulmonary function tests, arterial blood gas analysis, 6-minute walking test, electrocardiogram, echocardiography, left and sometimes right heart catheterisation, lung perfusion scan, and expert evaluation by specialists in thoracic surgery, pulmonology, gastroenterology, otorhinolaryngology, cardiology, ophthalmology, endocrinology, angiology, dermatology, psychiatry, anaesthesia and nutrition.
For patient selection our programme follows the widely accepted criteria established by the International Society for Heart and Lung Transplantation (ISHLT) [1]. The list of absolute contraindications is given in box 1. Owing to the increased experience at our centre, single relative contraindications are seldom considered prohibitive, especially if they are amenable to treatment before or after transplantation. An accumulation of relative contraindications is generally prohibitive for transplantation. Single organ failure (respiratory insufficiency) with extracorporeal membrane oxygenation (ECMO) or mechanical ventilation with or without tracheostomy are no longer prohibitive relative contraindications for our programme (box 2).
|
Box 1: Absolute contraindications to transplantation. |
| Malignancy in the last 2 years. In general, a 5-year disease-free interval is prudent.
Untreatable advanced dysfunction of another major organ system (e.g. heart, liver or kidney).
Incurable chronic extrapulmonary infection including chronic active viral hepatitis B, hepatitis C and human immunodeficiency virus.
Significant chest wall / spinal deformity.
Documented nonadherence or inability to follow through with medical therapy
Untreatable psychiatric or psychological condition associated with the inability to cooperate or comply with medical therapy.
Substance addiction (e.g. alcohol, tobacco or narcotics) that is either active or within the last 6 months.Adapted from Orens et al. (2006) [1] |
|
Box 2:Relative contraindications to transplantation. |
| Age older than 65 years.
Critical or unstable clinical condition (e.g. shock, mechanical ventilation or extracorporeal membrane oxygenation).
Severely limited functional status with poor rehabilitation potential.
Colonisation with highly resistant or highly virulent bacteria, fungi or mycobacteria.
Body mass index (BMI) exceeding 30 kg/m2
Severe or symptomatic osteoporosis.Adapted from Orens et al. (2006) [1] |
Donor selection criteria
As for all solid organ transplants, the demand for suitable donor lungs exceeds the supply. The lower utilisation rates of lungs in comparison with other solid organs are, generally, due to the fact that the lungs are more fragile and sensitive than other solid organs. Therefore, direct trauma to the chest, resuscitation manoeuvres, neurogenic oedema due to brain death, and aspiration and ventilator associated pneumonia lead to lower donor lung acceptance rates.
Currently accepted ideal donor criteria are given in box 3 [2]. In order to overcome the problem of donor organ shortage, some centres use less ideal donors, so called “extended” or “marginal” donors, with similar outcomes to those of ideal donors [3–5]. In our experience utilisation of extended donors does not compromise the outcome after lung transplantation [6].
|
Box 3. Ideal donor criteria |
| Age <55 years
Blood group compatibility
Normal chest X-Ray
PaO2 >300 mm Hg on oxygen challenge
Smoking history <20 pack years
Normal findings on bronchoscopy
Adapted from Orens et al. (2003) [2] |
Operative technique
Until the year 2000 organs were preserved with Euro-Collins solution; since then we use Perfadex® (Vitrolife, Gothenburg, Sweden). Before an antegrade flush, 500 µg prostaglandin E1 (Prostin VR, Upjohn, Puurs, Belgium) is injected into the pulmonary artery in all cases. Harvesting of the donor lungs is undertaken en bloc after perfusion. Since 2000, we also use a retrograde flush with Perfadex® at the time of the back-table preparation. The decision to perform size-reduced lung transplantation is made in the operating theatre during implantation. Peripheral segmental wedge resections are undertaken with a commercially available stapler device. For lobar transplants, lobectomy is done on the back-table. For bilateral sequential lung transplants, a bilateral trans-sternal anterior thoracotomy (clamshell incision) or two separate anterolateral thoracotomies (since 2000) are performed. First, the bronchial anastomosis is done followed by venous (atrial cuff) and pulmonary-artery anastomoses. The recipient’s main bronchus is divided one ring proximal to the upper-lobe bronchus branch. Bronchial arteries are ligated of the peribronchial tissue without electrocoagulation. All dissection on the bronchus is performed using “minimal” or “no-touch” techniques in order to keep the peribronchial tissue intact. The donor bronchus is cut back as close as possible to the origin of the upper lobe bronchus, with special attention to the peribronchial tissue. Absorbable suture material polydioxanone (PDS, Ethicon Inc, NJ, USA) is used. A continuous suture to the membranous wall (PDS, 4/0) and end-to-end anastomosis with interrupted single stitches (PDS, 3/0) to the cartilaginous part is performed [7, 8].
Postoperative management
According to our standard protocol, patients receive induction therapy (antithymocyte globulin or basiliximab) and triple immunosuppressive therapy including ciclosporin, azathioprine or mycophenolate mofetil (since 1997), and prednisone. Anti-infective prophylaxis is used in accordance with our centre’s protocol, which is described elsewhere [9, 10]. Post-transplant management at our centre includes routine surveillance bronchoscopies with transbronchial biopsies and bronchoalveolar lavage during the first 6 months after transplantation, serial laboratory lung function tests, and regular outpatient clinic follow-up visits.
Postoperative complications
Complications following lung transplantation can occur immediately after surgery, or days or weeks later. Clinical suspicion and close follow-up of these patients are strategies to reduce the morbidity and mortality in those who survive the procedure. The main complications after lung transplantation can be divided into three major groups: (1) surgical complications; (2) immunological reactions; and (3) side effects of immunosuppressive drugs. Surgical complications can be classified as: (1) technical complications; (2) pleural space complications; (3) primary graft dysfunction; and (4) others (such as, lung hernia, inguinal lymphocele, phrenic nerve injury, etc.). In our series, the overall complication rate was 38%. Tracheotomy was performed for 19% of the recipients. We observed pleural space complications in 24.5% of the cases. Haemothorax requiring surgical intervention occurred in 11% of patients. The rate of early abdominal complications requiring surgical intervention was 6%.
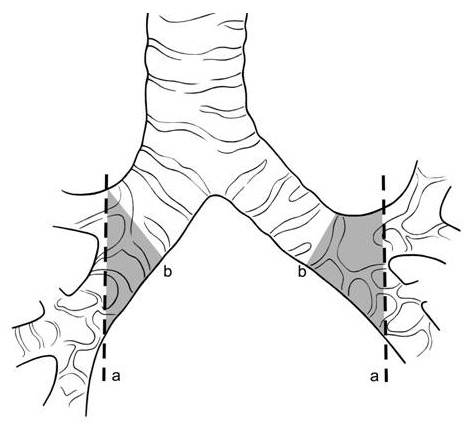
Figure 1
Cut points on the donor bronchus. (a) The donor bronchus should be cut back as close to the upper lobe bronchus origin as possible in an oblique plane with special attention to keep peribronchial tissues undisturbed (b) if the donor bronchus is cut at this level there will be an risk zone for bronchial ischaemia (gray zone) (from Weder et al. [7]).
Airway complications after lung transplantation
Refinements in lung preservation and surgical technique, and improvements in patient selection, postoperative care and immunosuppression have reduced the incidence of airway complications [11]. Reflecting these changes, the contemporary rate of anastomotic lesions after lung transplantation has dropped from 80% before 1983 [12] to below 3%; but a wide range of 2.6%–23.8% remains [11, 13–20].
Bronchial ischaemia is reported to be a significant risk factor for the development of airway complications [15]. The viability of the donor bronchus is initially dependent upon retrograde low-pressure collaterals derived from the pulmonary artery, since bronchial arterial circulation is lost during the harvest of the donor lungs [21].
The very low incidence of airway complications encountered in our programme is strong evidence in favour of our rigorous and specific technique, which we have used for years [7, 8]. The surgical approach for performing the anastomosis may vary among transplant centres. Telescoping, end-to-end anastomosis with a running suture for the membranous part and interrupted sutures for the cartilaginous part, and end-to-end anastomosis with a single running suture are most often used [11, 15, 18, 22].
Furthermore, we are convinced that resection of the donor bronchus down to the lobar carina in an oblique plane (fig. 1), in conjunction with keeping the peribronchial tissue intact, is a critical step when performing the bronchial anastomosis. The often cited disadvantages of a single-stitch technique compared with a running suture – more time required, a higher load of foreign tissue due to the need to tie multiple knots and an overall increased tissue irritation – are not reflected in our experience [7].
Currently, in over 650 anastomoses performed at our institution, no significant dehiscence was observed. An airway complication is defined as any bronchial anastomotic complication that becomes symptomatic owing to airflow limitation, bleeding or a fistula, and that may require an intervention, either endobronchially or as an open revision. In one patient, a small fistula was detected and closed surgically on postoperative day five. In a previous analysis, in only 4.9% (10/206) of recipients was luminal narrowing found at the first surveillance bronchoscopy in a consecutive series of 391 bronchial anastomoses (narrowing was found in 18/391 [4.6%] anastomoses). This rate decreased to 2.3% (9/391) after 6 months. None of these patients required any intervention, and there was no death related to bronchial anastomotic complications [7]. We know that bronchial anastomotic complications can be avoided by use of a standardised surgical technique that respects the fact that the donor bronchus is poorly vascularised. Prevention of fungal infections with aggressive antifungal treatment may play an important additional role [7].
Outcomes
From November 1992 to December 2011, a total of 342 lung transplantations were performed at the Zurich University Hospital. Bilateral lung transplants were performed in 308 patients, single lung transplants in 33 and a heart-lung transplant in 1. The number of transplantations performed in each calendar year are shown in figure 2. The number of retransplantations was 14. The censor date for all survival data is 15th November 2012. Retransplantations (n = 14) and heart-lung transplantation (n = 1) were excluded from the survival analyses. Overall survival rates at 1, 3 and 5 years were 84%, 71% and 66.5%, respectively (fig. 3). The 29th annual report of the ISHLT (2012) stated that 39,835 adult lung transplantations were performed up to June 2011 [23]. In this report the survival rate was 79% at 1 year, 64 % at 3 years and 53% at 5 years. The Japanese Lung transplantation report of 2012 recorded 86% survival at 1 year, 79% at 3 years and 73% at 5 years [24]. Kreisel et al from Washington University, St Louis, reported 84% 1-year survival and 56% at 5 years in 1,000 patients who underwent lung transplantation [25].
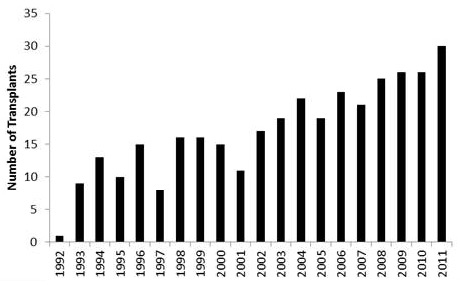
Figure 2
The number of lung transplantations performed each year.
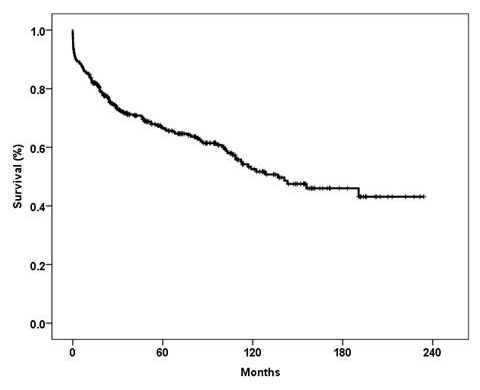
Figure 3
Overall survival of patients who underwent lung transplantation at the Zurich University Hospital. Censor date for all survival data is 15 November 2012. Retransplantation (n = 14) and heart-lung transplantation (n = 1) were excluded.
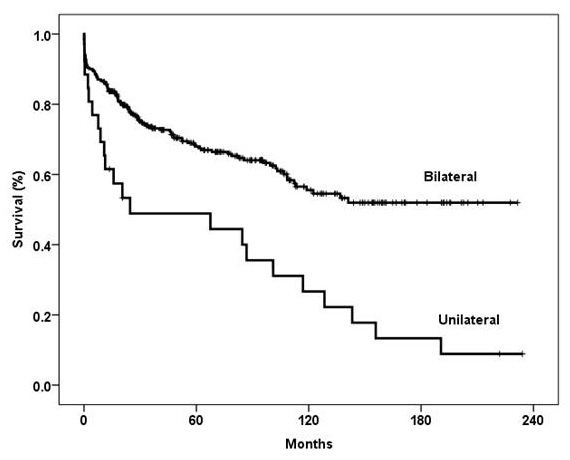
Figure 4
Kaplan-Meier survival curve showing a significantly better outcome in patients who underwent bilateral versus unilateral lung transplantation (p = 0.0001, log-rank test).
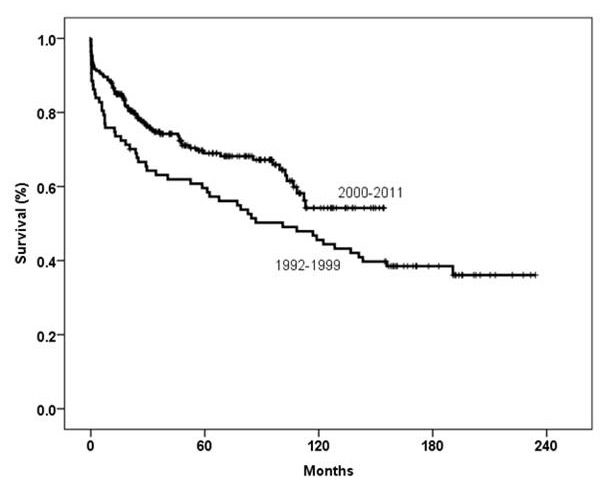
Figure 5
Kaplan-Meier survival curve comparing two different eras (p = 0.02, log-rank test).
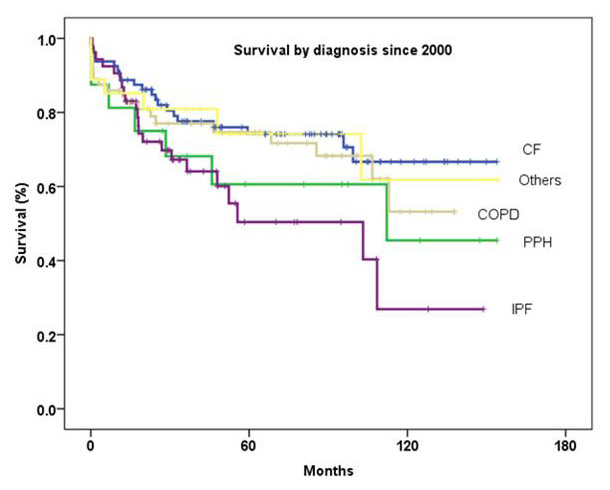
Figure 6
Survival by diagnosis since 2000 (p = 0.1, log-rank test).
Type of the procedure: unilateral versus bilateral lung transplant
Overall survival rates at 1, 3 and 5 years for recipients undergoing bilateral lung transplantation were 86%, 74% and 68%, respectively, and for unilateral lung transplantation were 61.5%, 49% and 49%, respectively (p = 0.0001, log-rank test; fig. 4). In our programme, the number of unilateral lung transplantations has significantly decreased over the years. Between 1992 and 1997, 36% of the transplants were unilateral; this decreased to 3% in the period between 2006 and 2011.
Era effect
We performed 87 lung transplants (1 retransplant was excluded) between 1992 and 1999 with 1-, 3- and 5-year survival rates of 76%, 63% and 60%, respectively. Between the year 2000 and the end of 2011, 240 transplants were performed with survival rates of 87%, 75% and 69%, respectively (p = 0.02, log-rank test; fig. 5) (14 retransplants and 1 heart-lung transplant were excluded). In the second era we increased the number of transplants performed in our centre. The improved survival rates are due to better lung preservation, and improvements in intensive care management, immunosuppressive treatment, antibiotic and anti-fungal treatments, as well as continued long-term follow-up by transplant pulmonologists.
Effect of underlying diagnosis on survival
Cystic fibrosis
In our transplant programme, cystic fibrosis (CF) was the most common indication, accounting for 35% of all lung transplants performed. According to the recent ISHLT registry data, CF constitutes the third most common indication for lung transplantation at a global level [23]. Ultimately, it is the only treatment option that can allow patients with advanced CF lung disease to obtain normal respiratory health [26]. Good post-transplant outcome for CF has been reported from transplant centres worldwide [26–28]. Despite transplant-related and medical comorbidities, prolonged survival and good quality of life have been reported for CF recipients [28–32].
Although single-centre reports indicate increased mortality rates in patients with Burkholderia cepaciacomplex, especially Burkholderia cepeciagenomovar III, in Zurich we do not systematically exclude these patients from transplantation [33–35].
In our series, we transplanted two recipients with Burkholderia cepaciacomplex. One of them died of bronchiolitis obliterans syndrome (BOS) 5 years after transplantation. The other patient is clinically stable and BOS-free at 9 years post-transplant.
The survival rates for patients undergoing lung transplantation for CF (n = 107) in Zurich were 86%, 74% and 70% at 1, 3 and 5 years, respectively. Survival rates for this group of patients since 2000 were 89%, 78% and 74.1% at 1, 3 and 5 years, respectively (fig. 6).
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is the most common indication for which lung transplantation is performed [23]. Referral for transplantation in COPD patients should be considered only for patients who continue to deteriorate despite optimal medical and surgical therapy, including smoking cessation, maximal bronchodilator treatment, rehabilitation, long-term oxygen therapy, and endoscopic or surgical lung volume reduction where feasible. The definition of the appropriate timing for transplantation is complicated because very symptomatic COPD patients may have a relatively good prognosis. Therefore, the question of whether it may be justified to perform a transplant primarily for quality-of-life reasons arises frequently in these patients [1].
Listing criteria for COPD patients are: body-mass index, airflow obstruction, dyspnoea and exercise capacity(BODE) index of 7 to 10, or at least one of the following: history of hospitalisation for exacerbation associated with acute hypercapnia (PCO2 exceeding 50 mmHg), pulmonary hypertension, forced expiratory lung volume in 1 second (FEV1) less than 20% of predicted, and either diffusing capacity of the lung for carbon monoxide (DLCO) less than 20% of predicted or homogenous distribution of emphysema [1, 36].
In our clinic we performed 86 lung transplantations for COPD. One-, 3- and 5-year survival rates were 85%, 76% and 71%, respectively. Survival rates for COPD patients since 2000 were 86%, 77% and 74.7% at 1, 3 and 5 years, respectively (fig. 6). Similar survival rates were also reported from the St. Louis Lung Transplant Program [37]. They reported 88.6% 1-year, 75.6% 3-year and 70.4% 5-year survival rates in 140 recipients transplanted between1995 and 2000.
Idiopathic pulmonary fibrosis
Worldwide, idiopathic pulmonary fibrosis (IPF) patients have the worst prognosis of all patients undergoing lung transplantation [23]. In addition, IPF patients have the highest mortality rates on the waiting list [23]. Early referral of these patients is very important to reduce pretransplant and perioperative mortality.
Survival for 65 patients transplanted for IPF was 83%, 65.9% and 54.5% at 1, 3 and 5 years, respectively. Since the year 2000, 1-, 3- and 5- year survival rates were 86.8%, 67.3% and 50.4%, respectively (fig. 6). The recent ISHLT Registry reported 74% 1-year, 58% 3-year and 46% 5-year survival rates for IPF recipients [23].
Primary pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a progressive and severely disabling disorder induced by an increase in pulmonary vascular resistance and ultimately leading to right heart failure and death [1]. For these patients, severe dyspnoea on exertion, of New York Heart Association (NYHA) class III or IV (which reflects severely limited daily activities), is the referral criterion. Persistent NYHA class III or IV dyspnoea on medical treatment, failure to respond to intravenous medical treatment, mean pulmonary artery pressure (mPAP) >50 mm Hg, cardiac index <2 l/min/m2, and right atrial pressure >15 mm Hg are criteria for transplantation [1].
The survival rates for patients undergoing lung transplantation for primary pulmonary arterial hypertension (PPH) (n = 22) were 63.6%, 54.2% and 48.8% at 1, 3 and 5 years, respectively. Since the year 2000, 1-, 3- and 5-year survival rates were 81%, 68% and 60.6%, respectively (fig. 6). The recent ISHLT Registry reported 69.8% 1-year, 58.8% 3-year and 50.2% 5-year survival for PPH recipients [23].
Special considerations
Perioperative extracorporeal membrane oxygenation implementation
Extracorporeal membrane oxygenation (ECMO) is currently used in lung transplantation either to bridge patients prior to transplantation or to treat post-transplant severe primary graft dysfunction [38].
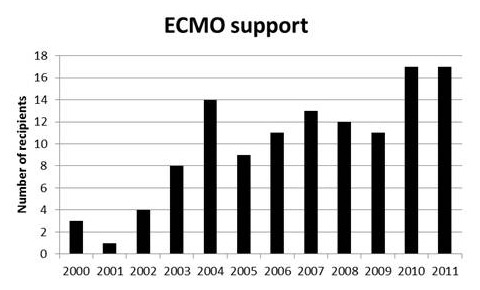
Figure 7
The number of patients who underwent lung transplantation exclusively under extracorporeal membrane oxygenation support since 2000 at our centre.
In our programme we have steadily increased the number of transplantations performed with ECMO support. We transplanted 128 patients (39%) with ECMO support or cardiopulmonary bypass. Since 2000 we have preferred using ECMO rather than cardiopulmonary bypass during lung transplantation. Figure 7 shows patients transplanted with ECMO support since 2000. In 11 of these ECMO was used as a bridging procedure. The decision to perform the operation with ECMO is made according to preoperatively diagnosed pulmonary arterial hypertension, gas exchange status including one-lung ventilation, temporary clamping of the ipsilateral pulmonary artery, and/or after transplantation of one side in the case of graft dysfunction. If graft dysfunction occurs immediately after reperfusion in the operating room or the recipient has preoperatively diagnosed severe pulmonary arterial hypertension we continue ECMO support for a couple of days on the ICU. For venoarterial (VA) cannulation, we prefer a femoral vein and the ascending aorta or a femoral artery. If we know that we will continue the ECMO support in the postoperative period we use a subclavian artery or contralateral femoral artery, connecting the cannula with a vascular graft. Heparin is administered as 50 IU/kg at the time of ECMO implantation and then titrated to maintain an activated clotting time of 150–180 seconds.
ECMO as a bride to lung transplantation is increasingly used worldwide. Recently, Hoopes et al. reported 93% 1-year and 66% 5-year survival rates comparable to those transplanted without ECMO as a bridging procedure [39]. In our series we had 11 patients bridged to transplantation on ECMO. Our 1-year and 2-year survival rates were 64% and 45%, respectively.
Size-reduced lung transplantation
For transplant recipients requiring bilateral lung transplantation, the donor-to-recipient supply and demand mismatch is even greater [40]. Owing to the increasing scarcity of donors, in particular for smaller recipients, advanced operative strategies have been developed [41–46]. Size-reduced lung transplantation is increasingly performed, often in urgent cases. Peripheral segmental resection is the most common method for downsizing, whereas lobar and split-lung transplants are other options performed [41–46].
Whenever size-reduced lung transplantation is undertaken, appropriate size-matching of donor organs and the recipient is vital. Oversized lung grafts can potentially lead to atelectasis and impaired airway clearance due bronchial anatomy distortion. Undersized grafts cause lung hyperexpansion and might limit exercise tolerance as a result of haemodynamic compromise. Recently we have shown that, in size-reduced lung transplantation, the recipient’s best postoperative FEV1 could be predicted from the donor’s calculated and corrected FEV1, adjusted for the number of transplanted segments. Moreover, our data demonstrated that size-reduced lung transplantation can be performed safely with peri- and post-operative complications and post-transplant outcome comparable to standard lung transplantation [47, 48].
Lobar lung transplantation
The limitation of lung transplantation due to size-mismatch, especially in smaller adults and paediatric recipients, could be overcome by utilising lobar lung transplantation. Since January 2000, 75 recipients received down-sized lungs. Of these, 23 patients underwent bilateral lobar lung transplantation (BLLT) with anatomic lobectomy (9% of the total lung transplantations in this period) [49]. Indications for BLLT were cystic fibrosis (n = 10), pulmonary fibrosis (n = 8), emphysema (n = 3), and primary pulmonary hypertension, progressive systemic sclerosis and bronchiolitis (for each n = 1). Sixteen patients were female, with a median age of 41 years (range 13‒66 years). The decision to perform size-reduced lung transplantation was made in the operating theatre during implantation on the basis of visual assessment of the size discrepancy. Our data demonstrated that bilateral lobar lung transplantation has short- and long-term outcomes comparable to standard bilateral lung transplantation.
Donation after cardiac death (DCD) programme
One of the solutions for lack of suitable donor lungs is to use donation after cardiac death (DCD) donors. Mid- and long-term results from DCD donors are encouraging, with survival rates comparable to those from brain death donors [50–53]. Recently, Levvey et al. from Australia reported an excellent 5-year survival rate of 90% from their cohort of 72 patients [51].
Zurich University Hospital started a DCD programme from category 3 donors in October 2011. Since 2012, we have performed three DCD lung transplantations, which are not included in this paper.
Future directions
Ex-vivo lung perfusion
Ex-vivo lung perfusion (EVLP) was proposed originally to assess the function of the lung donated after a cardiac death , as an interim evaluation of the graft prior to transplantation [54, 55]. The concept of EVLP is not only to evaluate lungs before transplantation, but also to recondition lungs of inferior quality outside the cadaver and to extend the cold ischaemic time by intermittent warm reperfusion [56]. Similar early outcomes have been reported in recipients who underwent transplantation after EVLP to those with conventionally selected and transplanted lungs [56–59]. Based on our extensive research in this field in the last 7 years we also have started an EVLP programme in our transplant centre [60–63]. We performed EVLP in two marginal donors. These lungs did not fulfil the implantation criteria and were not transplanted.
Lung engineering
Advances in stem-cell and tissue engineering research resulted recently in the creation of a bioartificial lung, which was able to maintain gas exchange at room air after in-vivo transplantation [64].
The donor lungs were decellularised with detergent perfusion and yielded scaffolds with acellular vasculature, airways and alveoli. To regenerate gas exchange tissue, scaffolds were seeded with epithelial and endothelial cells. To establish function, cell-seeded constructs were perfused and ventilated in a bioreactor simulating the physiologic environment of developing lungs. By day 5, constructs could be perfused with blood and ventilated at physiological pressures, generating gas exchange comparable to that of isolated native lungs. To show in-vivo function, regenerated lungs were transplanted into the orthotopic position in an animal model. After transplantation, constructs were perfused by the recipient's circulation and ventilated by means of the recipient’s airway and respiratory muscles, providing gas exchange in vivo for up to 6 hours after extubation [63].
Conclusion
The additional improvement in post-transplant survival of lung transplant recipients in the recent era at our centre is most likely due to a number of factors: better surgical techniques, organ preservation and intensive-care management probably played a role. In addition, careful post-transplant long-term management by transplant pulmonologists, including rigorous treatment of airway infections, sinus surgery and routine nasal care (CF recipients), long-term therapy with macrolide antibiotics for (BOS), and extracorporeal photopheresis in selected recipients with BOS and recurrent acute allograft rejection might have contributed to improved outcomes at our centre as well [27, 32].
The two main problems are limited organ supply and failure to ensure long-term allograft function with the current immunosuppression strategies. Increased use of DCD donors, utilisation of EVLP to assess and recondition marginal donor organs, and advances in tissue engineering research could overcome at least the limited supply of eligible donor lungs for transplantation.
References
1 Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update – a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55.
2 Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22(11):1183–200.
3 Gabbay E, Williams TJ, Griffiths AP, et al. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med. 1999;160:265–71.
4 Straznicka M, Follette DM, Eisner MD, et al. Aggressive management of lung donors classified as unacceptable: excellent recipient survival one year after transplantation. J Thorac Cardiovasc Surg. 2002;124:250–8.
5 Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421–8.
6 Lardinois D, Banysch M, Korom S, et al. Extended donor lungs: eleven years experience in a consecutive series. Eur J Cardiothorac Surg. 2005;27(5):762–7.
7 Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg. 2009;35:293–8.
8 Inci I, Weder W. Airway complications after lung transplantation can be avoided without bronchial artery revascularization. Curr Opin Organ Transplant. 2010;15(5):578–81.
9 Schuurmans MM, Benden C, Inci I. Practical approach to early postoperative management of lung transplant recipients. Swiss Med Wkly. 2013;143:w13773.
10 Speich R, Nicod LP, Aubert JD, et al. Ten years of lung transplantation in Switzerland: results of the Swiss Lung Transplant Registry. Swiss Med Wkly. 2004;134(1-2):18–23.
11 Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg. 1995;110(5):1424–32.
12 Wildevuur C.R.H., Benfield J.R. A review of 23 human lung transplants by 20 surgeons. Ann Thorac Surg. 1970;9:489–515.
13 Ruttmann E, Ulmer H, Marchese M, et al. Evaluation of factors damaging the bronchial wall in lung transplantation.J Heart Lung Transplant. 2005;24(3):275–81.
14 Schroder C, Scholl F, Daon E, et al. A modified bronchial anastomosis technique for lung transplantation. Ann Thorac Surg. 2003;75(6):1697–704.
15 Alvarez A, Algar J, Santos F, et al. Airway complications after lung transplantation: a review of 151 anastomoses. Eur J Cardiothorac Surg. 2001;19(4):381–7.
16 Choong CK, Sweet SC, Zoole JB, et al. Bronchial airway anastomotic complications after pediatric lung transplantation: incidence, cause, management, and outcome. J Thorac Cardiovasc Surg. 2006;131(1):198–203.
17 Herrera JM, McNeil KD, Higgins RS, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg. 2001;71:989–93.
18 Aigner C, Jaksch P, Seebacher G, et al. Single running suture – the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg. 2003;23(4):488–93.
19 Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg. 2007;84(2):401–9.
20 Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg. 2007;31(4):703–1.
21 Takao M, Katayama Y, Onoda K, et al. Significance of bronchial mucosal blood flow for the monitoring of acute rejection in lung transplantation. J Heart Lung Transplant. 1991;10:956–67.
22 Schmid RA, Boehler A, Speich R, et al. Bronchial anastomotic complications following lung transplantation: still a major cause of morbidity? Eur Respir J. 1997;10(12):2872–5.
23 Christie JD, Edwards LB, Kucheryavaya AY, et al; International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012 ;31(10):1073–86.
24 Oto T, Okada Y, Bando T, et al; Japanese Society of Lung and Heart–Lung Transplantation. Registry of the Japanese society of lung and heart-lung transplantation: the official Japanese lung transplantation report 2012. Gen Thorac Cardiovasc Surg. 2013;61:208–11.
25 Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–22.
26 Meachery G, De Soyza A, Nicholson A, et al. Outcomes of lung transplantation for cystic fibrosis in a large UK cohort. Thorax. 2008;63:725–31.
27 Hofer M, Benden C, Inci I, et al. True survival benefit of lung transplantation for cystic fibrosis patients: the Zurich experience. J Heart Lung Transplant. 2009;28:334–9.
28 Aurora P, Whitehead B, Wade A, et al. Lung transplantation and life extension in children with cystic fibrosis. Lancet. 1999;354:1591–3.
29 Burton CM, Milman N, Carlsen J, et al. Copenhagen National Lung Transplant Group. The Copenhagen National Lung Transplant Group: survival after single lung, double lung, and heart-lung transplantation. J Heart Lung Transplant. 2005;24:1834–43.
30 de Perrot M, Chaparro C, McRae K, et al. Twenty-year experience of lung transplantation at a single center: Influence of recipient diagnosis on long-term survival. J Thorac Cardiovasc Surg. 2004;127:1493–501.
31 Aaron SD, Ferris W, Henry DA, et al. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am J Respir Crit Care Med. 2000;161:1206–12.
32 Inci I, Stanimirov O, Benden C, et al. Lung transplantation for cystic fibrosis: a single center experience of 100 consecutive cases. Eur J Cardiothorac Surg. 2012:41:435–40.
33 Egan TM, Detterbeck FC, Mill MR, et al. Long-term results of lung transplantation for cystic fibrosis. Eur J Cardiothorac Surg. 2002;22:602–9.
34 Chaparro C, Maurer J, Gutierrez C, et al. Infection with Burkholderia cepaciain cystic fibrosis: outcome following lung transplantation. Am J Respir Crit Care Med. 2001;163:43–8.
35 De Soyza A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet. 2001;358:1780–1.
36 Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12.
37 Cassivi SD, Meyers BF, Battafarano RJ, et al. Thirteen-year experience in lung transplantation foremphysema. Ann Thorac Surg. 2002;74:1663–9.
38 Aigner C, Wisser W, Taghavi S, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg. 2007;31(3):468–73.
39 Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145:862–8.
40 Barr ML, Schenkel FA, Cohen RG, et al. Recipient and donor outcomes in living related and unrelated lobar transplantation. Transplant Proc. 1998;30:2261–3.
41 Aigner C, Jaksch P, Taghavi S, et al. Donor total lung capacity predicts recipient total lung capacity after size-reduced lung transplantation. J Heart Lung Transplant. 2005;24:2098–102.
42 Aigner C, Winkler G, Jaksch P, et al. Size-reduced lung transplantation: an advanced operative strategy to alleviate donor organ shortage. Transplant Proc. 2004;36:2801–5.
43 Shigemura N, Bermudez C, Hattler BG, et al. Impact of graft volume reduction for oversized grafts after lung transplantation on outcome in recipients with end-stage restrictive pulmonary diseases. J Heart Lung Transplant. 2009;28:130–4.
44 Artemiou O, Wieselthaler G, Zuckermann A, et al. Downsizing of the donor lung: peripheral segmental resections and lobar transplantation. Transplant Proc. 1997;29:2899–900.
45 Aigner C, Mazhar S, Jaksch P, et al. Lobar transplantation, split lung transplantation and peripheral segmental resection – reliable procedures for downsizing donor lungs. Eur J Cardiothorac Surg. 2004;25:179–83.
46 Santos F, Lama R, Alvarez A, et al. Pulmonary tailoring and lobar transplantation to overcome size disparities in lung transplantation. Transplant Proc. 2005;37:1526–9.
47 Inci I, Irani S, Kestenholz P, et al. Donor predicted post-operative forced expiratory volume in one second predicts recipients’ best forced expiratory volume in one second following size-reduced lung transplantation. Eur J Cardiothorac Surg. 2011;39:115–9.
48 Benden C, Inci I, Weder W, Boehler A. Size-reduced lung transplantation in children – an option worth to consider! Pediatr Transplant. 2010;14(4):529–33.
49 Inci I, Schuurmans MM, Kestenholz P, et al. Long-term outcomes of bilateral lobar lung transplantation. Eur J Cardiothorac Surg. 2012;43:1220–5.
50 Cypel M, Sato M, Yildirim E, et al. Initial experience with lung donation after cardiocirculatory death in Canada. J Heart Lung Transplant. 2009;28:753–8.
51 Levvey BJ, Harkess M, Hopkins P, et al. Excellent Clinical Outcomes from a National Donation-After-Determination-of-Cardiac-Death Lung Transplant Collaborative. Am J Transplant. 2012;12:2406–13.
52 Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant. 2008;8:1282–9.
53 De Vleeschauwer S, Van Raemdonck D, Vanaudenaerde B, et al. Early outcome after lung transplantation from non-heart-beating donors is comparable to heart-beating donors. J Heart Lung Transplant. 2009;28:380–7.
54 Steen S, Söberg T, Pierre L, et al. Transplantation of lungs from a non-hear-beating donor. Lancet. 2001;357:825–9.
55 Krueger T, Berutto C, Aubert JD. Challenges in lung transplantation. Review article. Swiss Med Wkly. 2011;141:w13292.
56 Wierup P, Haraldsson A, Nilsson F, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81:460–6.
57 Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–6.
58 Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;14;364:1431–40.
59 Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion – pushing the limits. Am J Transplant. 2012;12:1839–47.
60 Inci I, Arni S, Acevedo C, et al. Surfactant alterations following donation after cardiac death donor lungs. Transpl Int. 2011;24(1):78–84.
61 Inci I, Ampollini L, Arni S, et al. Ex vivo reconditioning of marginal donor lungs injured by acid aspiration. J Heart Lung Transplant. 2008;27(11):1229–36.
62 Inci I, Arni S, Inci D, et al. Impact of topical cooling solution and prediction of pulmonary graft viability from non-heart-beating donors. J Heart Lung Transplant. 2008;27(9):1016–22.
63 Inci I, Zhai W, Arni S, et al. Fibrinolytic treatment improves the quality of lungs retrieved from non-heart-beating donors. J Heart Lung Transplant. 2007;26:1054–60.
64 Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–33.






