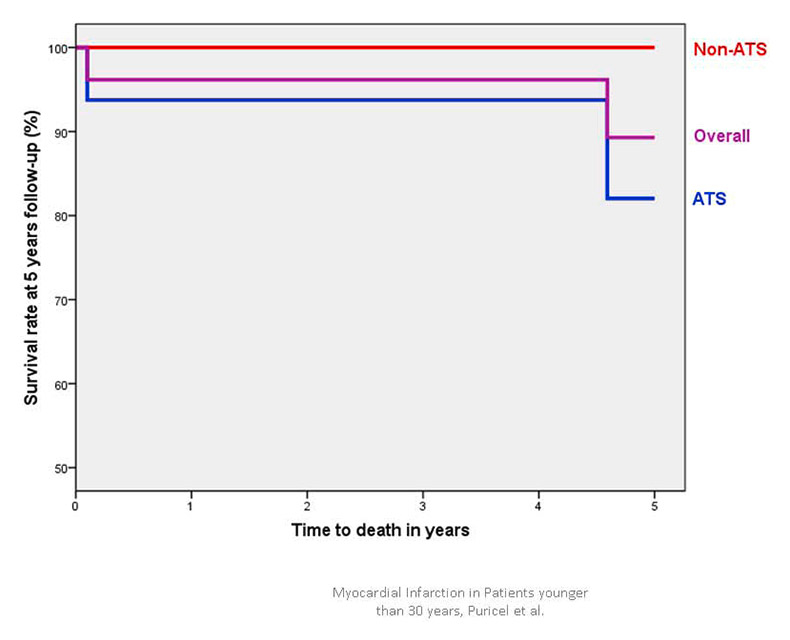
Figure 1A
Cumulative survival in patients <30 years presenting with acute coronary syndrome.
ATS = atherosclerosis; non-ATS = patients without angiographic evidence of atherosclerosis.
DOI: https://doi.org/10.4414/smw.2013.13816
Abbreviations
ACS acute coronary syndrome
ATS atherosclerosis
CABG coronary artery bypass graft surgery
CAD coronary artery disease
CD cardiac death
IQR interquartile range
MACE major adverse cardiac event
MI myocardial infarction
PCI percutaneous coronary intervention
STEMI ST-elevation myocardial infarction
TLR target lesion revascularisation
TVR target vessel revascularisation
BACKGROUND: Coronary atherosclerosis begins early in life, but acute coronary syndromes in adults aged <30 years are exceptional. We aimed to investigate the rate of occurrence, clinical and angiographic characteristics, and long-term clinical outcome of acute coronary syndrome (ACS) in young patients who were referred to two Swiss hospitals.
METHODS: From 1994 to 2010, data on all patients with ACS aged <30 years were retrospectively retrieved from our database and the patients were contacted by phone or physician’s visit. Baseline, lesion and procedural characteristics, and clinical outcome were compared between patients in whom an underlying atypical aetiology was found (non-ATS group; ATS: atherosclerosis) and patients in whom no such aetiology was detected (ATS group). The clinical endpoint was freedom from any major adverse cardiac event (MACE) during follow-up.
RESULTS: A total of 27 young patients with ACS aged <30 years were admitted during the study period. They accounted for 0.05% of all coronary angiograms performed.
Mean patient age was 26.8 ± 3.5 years and 22 patients (81%) were men. Current smoking (81%) and dyslipidaemia (59%) were the most frequent risk factors. Typical chest pain (n = 23; 85%) and ST-segment elevation myocardial infarction (STEMI; n = 18 [67%]) were most often found. The ATS group consisted of 17 patients (63%) and the non-ATS group of 10 patients (37%). Hereditary thrombophilia was the most frequently encountered atypical aetiology (n = 4; 15%). At 5 years, mortality and MACE rate were 7% and 19%, respectively.
CONCLUSION: ACS in young patients is an uncommon condition with a variety of possible aetiologies and distinct risk factors. In-hospital and 5-year clinical outcome is satisfactory.
Acute coronary syndrome (ACS) is rarely encountered in young adults and may have unusual causes. Data on incidence, risk factors and clinical outcome of ACS in this particular subset are limited. On currently available evidence, young patients represent 0.4%–19% of all ACS cases, depending on the cut-off age used [1–13]. Most studies define young patients as aged less than 45 years. Alioglu et al. found that, in 2,400 patients suffering from ACS, 2% of patients were aged <35 years [12]. One study reported that 0.4% of patients with ACS were aged <30 years [13]. As classic coronary atherosclerotic plaque rupture is considered to be rare during the early decades of life, uncommon aetiologies may be considered. In line with this, cardiovascular risk factors, the extent of coronary artery disease and clinical outcome after ACS might be different from those of older patients [14–20].
Therefore, we investigated the incidence, baseline characteristics and long-term clinical outcome of ACS in patients younger than 30 years who were referred to our institutions. We also sought to compare the aforementioned variables between patients in whom an underlying atypical aetiology was found and patients in whom no such aetiology was detected.
From 1994 to 2010, data for all patients with ACS aged <30 years who had been admitted to two Swiss hospitals (University Hospital Bern and Hospital Fribourg) were retrospectively retrieved from our database. This was a prospective longitudinal study. All patients were identified in the catheter laboratory databases and prospectively followed up. Inclusion criteria were age ≥18 years and ≤30 years at the time of ACS, diagnosis of ACS in accordance with the consensus paper from the ESC-ACC-AHA-WHF joint taskforce [21], willingness to provide informed consent and to participate in follow-up (ESC: European Society of Cardiology; ACC: American College of Cardiology; AHA: American Heart Association; WHF: World Heart Federation). Patients <18 or >30 years of age and those unable or unwilling to provide informed consent or to participate in follow-up were excluded from the study. The study complied with the Declaration of Helsinki regarding investigations in humans and was approved by the Institutional Ethics Committee.
Medical records reviewed included prehospitalisation and paediatric medical history, drug abuse, physical examination, homocysteinaemia, antiphospholipid antibody titre, other thrombophilic conditions, blood lipids, coronary angiography and ventriculography, 12-lead electrocardiogram (ECG), percutaneous coronary intervention (PCI) or coronary artery graft bypass surgery (CABG) reports, as well as postprocedural echocardiography. Dyslipidaemia was defined in accordance with the reports of the National Cholesterol Education Programme (Adult Treatment Panels II and III) [22, 23]. Hyperhomocysteinaemia was considered present when plasma concentrations were >15 µmol/l.
Coronary angiograms were recorded at baseline and immediately after the intervention. Digital angiograms were analysed at the angiographic core laboratory at Bern University Hospital, Switzerland, with the use of an automated edge-detection system using the CMS-GFT algorithm (MEDIS™, Leiden, The Netherlands). Quantitative measurements included the diameter of the reference vessel, minimum luminal diameter and percentile diameter of the stenosis (defined as the diameter of the reference vessel minus the minimal luminal diameter, divided by the reference diameter and multiplied by 100).
Patients were followed up for up to 15 years (range 0.1–15 years). Information regarding clinical status was collected at clinic visits or by means of telephone interview and was obtained 1, 2 and 5 years after the ACS that had led to study inclusion. All patients were contacted finally by telephone prior to data analysis. When the patient was not accessible, data were retrieved from the referring physician, hospital electronic database or Municipal Civil Registries. The clinical endpoint was freedom from any major adverse cardiac event (MACE) during follow-up. MACE was defined as the occurrence of cardiac death (CD), nonfatal myocardial infarction (MI), or target vessel revascularisation (TVR) during the follow-up period. Target lesion revascularisation (TLR) was defined as any repeat percutaneous or surgical intervention, or other complication of the target lesion. The target lesion was defined as the treated segment from 5 mm proximal to the stent to 5 mm distal to the stent. TVRwas defined as any repeat PCI of any segment of the target vessel, defined as the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself. Any death due to a proximate cardiac cause, all unwitnessed deaths and deaths of unknown causes were considered cardiac. Definite, probable and possible stent thromboses were determined in accordance with the Academic Research Consortium (ARC) definitions [24]. Stent thrombosis was defined as early, late or very late if the event occurred within 30 days, <1 year or >1 year, respectively, after the procedure.
Statistical analysis was performed using SPSS software 18.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis was primarily descriptive and focused on reporting the incidence of risk factors, clinical presentation, the revascularisation strategy employed and the outcome. Continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR). Categorical variables are presented as counts and percentages.
In a univariate analysis, baseline, lesion and procedural characteristics as well as clinical outcome were compared between patients with and without an underlying atypical aetiology. For continuous variables the parametric t-test or the nonparametric Mann-Whitney U test were employed according to their distribution. Contingency tables were created for categorical variables and the chi-square and the Fisher’s exact test were used to account for differences.
A total of 27 young patients with ACS aged <30 years were admitted during the study period, and accounted for 0.05% of all coronary angiographies performed at our institutions during this period. Baseline clinical and procedural characteristics are summarised in table 1. Mean patient age was 26.8 ± 3.5 years and 22 patients (81%) were men. Current smoking (81%), dyslipidaemia (59%) and positive family history (44%) were the most frequently encountered risk factors.

Figure 1A
Cumulative survival in patients <30 years presenting with acute coronary syndrome.
ATS = atherosclerosis; non-ATS = patients without angiographic evidence of atherosclerosis.
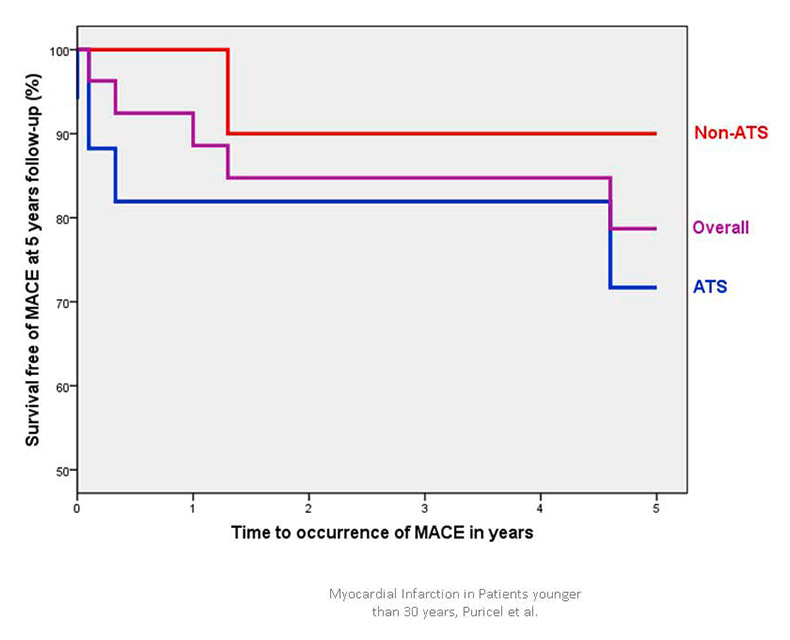
Figure 1B
Cumulative survival free of major cardiac adverse events (MACE) in patients <30 years presenting with acute coronary syndrome.
ATS = atherosclerosis; non-ATS = patients without angiographic evidence of atherosclerosis.
At presentation, typical chest pain occurred in 23 patients (85%), syncope in 3 patients (11%) and isolated dyspnoea in 2 patients (7%). ST-elevation myocardial infarction (STEMI) was present in 18 patients (67%) and cardiogenic shock in 2 patients (7%). Initial mean left ventricular ejection fraction was 48% ± 11%.
Coronary angiography was performed in all patients. Single vessel disease was found in 19 patients (70%) and the left anterior descending artery was involved in 22 cases (81%). Mean stenosis was 91% ± 22%. PCI was performed in 19 patients (70%), of whom 15 (56%) were stented. Table 2 shows further lesion and procedural characteristics.
ACS was due to an unusual condition in ten patients (37%, nonatherosclerosis [non-ATS] group): hereditary thrombophilia with or without patent foramen ovale in four patients (15%), cocaine abuse in two (7%), Kawasaki and familial Mediterranean fever in two (7%), emboli in infective endocarditis in one (4%), and antiphospholipid antibody syndrome in one (4%). None of the eight patients with plasma homocysteine measurements (10.5 ± 2.2 µmol/l) had values that were above the homocysteinaemia cut-off value (15 µmol/l).
In the remaining 17 patients (63%), atherosclerosis was present (ATS group). In these patients, 75% had at least two classical risk factors for coronary artery disease (median 2, IQR 2‒3) whereas patients with an unusual condition presented these risk factors less frequently (median 1.5, IQR 1‒3; p = 0.34).
No statistically significant difference regarding risk factors, or clinical or angiographic presentation between patients with or without an uncommon condition could be detected.
In-hospital MACE occurred in two patients (7%): One patient died and another had nonfatal MI as a result of early definite stent thrombosis (one acute; one subacute).
Of the 26 initial survivors, one patient was lost during follow-up. Median follow-up duration was 5.1 years (IQR 2.6–9.6 years). Table 3 indicates in-hospital and 5-year outcomes. Figure 1 depicts the Kaplan-Meier curve for 5-year survival (1A) and survival free of MACE (1B). Overall, the MACE rate was found to be 19% at 5 years follow-up. Two patients (7%) died within the 5 years succeeding the ACS that had led to study inclusion.
| Table 1:Baseline characteristics and clinical presentation on admission. | ||||
| Overall(n = 27) | Non-ATS(n = 10) | ATS(n = 17) | p-value | |
| Baseline characteristics | ||||
| Age in years, mean ± SD | 26.8 ± 3.5 | 27.1 ± 3.8 | 26.6 ± 3.5 | 0.96 |
| Male, n (%) | 22 (81) | 7 (70) | 15 (88) | 0.33 |
| Dyslipidaemia, n (%) | 16 (59) | 6 (60) | 10 (59) | 1 |
| Hypertension, n (%) | 4 (15) | 1 (10) | 3 (18) | 1 |
| Smoking, n (%) | 22 (81) | 8 (80) | 15 (82) | 0.63 |
| Family history, n (%) | 12 (44) | 3 (30) | 9 (53) | 0.25 |
| Cumulative RF, median (IQR) | 2 (1–3) | 1.5 (1–3) | 2 (2–3) | 0.34 |
| Previous MI, n (%) | 3 (11) | 2 (20) | 1 (6) | 0.56 |
| Previous PCI, n (%) | 3 (11) | 2 (20) | 1 (6) | 0.56 |
| Clinical presentation | ||||
| Chest pain, n (%) | 23 (85) | 10 (100) | 13 (76) | 0.5 |
| Shortness of breath, n (%) | 2 (7) | 2 (20) | 0 (0) | 0.16 |
| Syncope, n (%) | 3 (11) | 0 (0) | 3 (18) | 0.25 |
| Ventricular fibrillation, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.5 |
| STEMI, n (%) | 18 (67) | 6 (60) | 11 (65) | 0.69 |
| Pain to needle time in hours median (IQR) | 10 (3–36) | 4 (1–31) | 26 (6–38) | 0.16 |
| NSTEMI, n (%) | 6 (22) | 2 (20) | 4 (24) | 1 |
| Cardiogenic shock, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.53 |
| LVEF as percentage, mean ± SD | 48 ± 11 | 41 ± 13 | 53 ± 7 | 0.02 |
| Stenosis as percentage, mean ± SD | 91 ± 22 | 96 ± 8 | 88 ± 26 | 0.36 |
| ATS = atherosclerosis; IQR = interquartile range; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NSTEMI = non ST-elevation myocardial infarction: PCI = percutaneous coronary intervention; RF = risk factor; SD = standard deviation, STEMI = ST-elevation myocardial infarction | ||||
| Table 2:Lesion and procedural characteristics. | ||||
| Overall(n = 27) | Non-ATS(n = 10) | ATS(n = 17) | p-value | |
| LAD, n (%) | 22 (81) | 9 (90) | 13 (76) | 0.62 |
| LCX, n (%) | 7 (26) | 1 (10) | 6 (35) | 0.2 |
| RCA, n (%) | 9 (33) | 1 (10) | 8 (47) | 0.09 |
| Intermediate, n (%) | 1 (4) | 1 (10) | 0 (0) | 0.37 |
| Single vessel, n (%) | 20 (70) | 9 (90) | 10 (59) | 0.19 |
| Multivessel, n (%) | 8 (30) | 1 (10) | 7 (41) | 0.19 |
| PCI, n (%) | 19 (70) | 6 (60) | 13 (77) | 0.42 |
| Stent, n (%) | 15 (56) | 4 (40) | 11 (65) | 0.26 |
| BMS, n (%) | 6 (22) | 2 (20) | 4 (24) | 1 |
| DES, n (%) | 8 (30) | 2 (20) | 6 (35) | 0.67 |
| POBA, n (%) | 6 (19) | 3 (30) | 2 (12) | 0.33 |
| Thrombolysis, n (%) | 4 (15) | 1 (10) | 3 (18) | 1 |
| CABG, n (%) | 5 (19) | 2 (20) | 3 (18) | 1 |
| ATS = atherosclerosis; BMS = bare metal stent; CABG = coronary artery bypass graft surgery; DES = drug-eluting stent; LAD = left anterior descending artery; LCX = circumflex artery; POBA = plain old balloon angioplasty; RCA: right coronary artery | ||||
| Table 3:In-hospital and 5-year outcomes. | ||||
| Overall(n = 27) | Non-ATS(n = 10) | ATS(n = 17) | p-value | |
| In-hospital MACE, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| In-hospital death, n (%) | 1 (4) | 0 (0) | 1 (6) | 1 |
| In-hospital cardiac death, n (%) | 1 (4) | 0 (0) | 1 (6) | 1 |
| In-hospital MI, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| In-hospital stent thrombosis, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| 5-year MACE, n (%) | 5 (19) | 1 (10) | 4 (24) | 0.62 |
| 5-year death, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| 5-year cardiac death, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| 5-year MI, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| 5-year TLR, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| 5-year TVR, n (%) | 3 (11) | 1 (10) | 2 (12) | 1 |
| 5-year revascularisation, n (%) | 3 (11) | 1 (10) | 2 (12) | 1 |
| 5-year stent thrombosis, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.52 |
| ATS = atherosclerosis; MI = acute myocardial infarction; MACE = major adverse cardiac events; TLR = target lesion revascularisation; TVR: target vessel revascularisation; ST: stent thrombosis | ||||
ACS in patients under the age of 30 is a rare entity. We present the largest series reported to date. Our findings are as follows. Firstly, at least one in three patients suffer from an atypical underlying condition of which hereditary thrombophilia is the most common. Secondly, early arthrosclerosis is more frequently encountered in this population than we suspected. Of these patients, approximately three of four will have at least two classical risk factors for the development of CAD. Thirdly, this part of the population is prone to present with single vessel disease – especially in the left anterior descending coronary artery. Finally, in-hospital outcome is acceptable and was darkened solely by procedure-related complications, specifically early stent thrombosis.
With regard to risk factors, the high prevalence of smoking in this particular patient subset is of particular interest. Our findings are in line with a previously reported prevalence of 77% in Swiss patients aged ≤35 years by Schoenenberger and colleagues [25]. Smoking is the most crucial risk factor for the development of ACS in this part of the population. The literature reports smoking by up to 82% of young patients suffering from ACS [7, 26]. Case-control studies indicate that smoking is an independent risk factor for the development of ischaemic heart disease in young patients [27]. An interesting finding is reported by Huang and colleagues who found that the rate of smoking in patients aged ≤35 years and suffering from MI was significantly higher than in patients >65 years old suffering from the same condition.[28] Smoking persistence is associated with the occurrence of secondary events after MI in young patients [29]. Taken together, these data underline the importance of primary and secondary prevention specifically in this subset of patients.
At 5 years, around 90% of the patients were alive and around 80% were free from any MACE. This satisfactory long-term outcome has already been suggested previously. Possible explanations are the lesser extent of CAD in comparison with older patients, more preserved left ventricular function, and a higher ability of young patients to modify their lifestyle. In line with this, Ralldis and colleagues showed that decreased left ventricular ejection fraction at presentation and the continuation of smoking after myocardial infarction were most predictive for further MACEs in young patients [29].
Regarding the incidence of stent thrombosis, young age is usually considered to be protective. On the one hand, one might assume that since adverse events are rare in this population such procedure-related complications become more evident. On the other hand, patient compliance was not assessed in the present study and might affect this finding.
The main limitation of this study is the small number of patients. Although we neither performed an a priori nor an a posteriori sample size calculation, statistical power is likely to be low. External validity is threatened by the lack of statistical significance with regard to type II errors for the endpoints. However, the results of the present study could help determine the sample size for further research. As some of the data collection was retrospective our study could be subject to recall or information bias. Generalisations must therefore be made with caution.
ACS in young patients is an uncommon condition with a variety of possible aetiologies and distinct risk factors. The latter should be vigorously controlled – especially in patients with a positive family history of premature CAD. In-hospital and 5-year clinical outcome is acceptable. Further research is needed to corroborate the findings of the present study.
1 Chen YL, Bhasin A, Youssef AA, Wu CJ, Yang CH, Hsieh YK, et al. Prognostic factors and outcomes in young chinese patients with acute myocardial infarction undergoing primary coronary angioplasty. International Heart Journal. 2009;50(1):1–11.
2 Chan MY, Woo KS, Wong HB, Chia BL, Sutandar A, Tan HC. Antecedent risk factors and their control in young patients with a first myocardial infarction. Singapore Med J. 2006;47(1):27–30.
3 Morillas P, Bertomeu V, Pabon P, Ancillo P, Bermejo J, Fernandez C, et al. Characteristics and outcome of acute myocardial infarction in young patients. The PRIAMHO II study. Cardiology. 2007;107(4):217–25.
4 Hoit BD, Gilpin EA, Henning H, Maisel AA, Dittrich H, Carlisle J, et al. Myocardial infarction in young patients: an analysis by age subsets. Circulation. 1986;74(4):712–21.
5 Doughty M, Mehta R, Bruckman D, Das S, Karavite D, Tsai T, et al. Acute myocardial infarction in the young ‒The University of Michigan experience. Am Heart J. 2002;143(1):56–62.
6 Rumboldt Z, Rumboldt M, Pesenti S, Polic S, Miric D. Peculiarities of myocardial infarction at young age in Southern Croatia. Cardiologia. 1995;40(6):407–11.
7 Teixeira M, Sa I, Mendes JS, Martins L. Acute coronary syndrome in young adults. Rev Port Cardiol. 2010;29(6):947–55.
8 Hamsten A, Norberg R, Bjorkholm M, de Faire U, Holm G. Antibodies to cardiolipin in young survivors of myocardial infarction: an association with recurrent cardiovascular events. Lancet. 1986;1(8473):113–6.
9 Kanitz MG, Giovannucci SJ, Jones JS, Mott M. Myocardial infarction in young adults: risk factors and clinical features. J Emerg Med. 1996;14(2):139–45.
10 Siwach SB, Singh H, Sharma D, Katyal VK. Profile of young acute myocardial infarction in Harayana. J Assoc Physicians India. 1998;46(5):424–6.
11 Ueda Y, Okada K, Ogasawara N, Oyabu J, Hirayama A, Kodama K. Acute myocardial infarction without disrupted yellow plaque in young patients below 50 years old. J Interv Cardiol. 2007;20(3):177–81.
12 Wolfe MW, Vacek JL. Myocardial infarction in the young. Angiographic features and risk factor analysis of patients with myocardial infarction at or before the age of 35 years. Chest. 1988;94(5):926–30.
13 Gotsman I, Lotan C, Mosseri M. Clinical manifestations and outcome of acute myocardial infarction in very young patients. Isr Med Assoc J. 2003;5(9):633–6.
14 von Eyben FE, Bech J, Madsen JK, Efsen F. High prevalence of smoking in young patients with acute myocardial infarction. J R Soc Health. 1996;116(3):153–6.
15 Veludo ET, Marques VC, Simoes MV, Furuta MS, Figueiredo GL, Viviani LF, et al. Clinical profile, coronary angiography findings and early outcome in young patients with acute myocardial infarction in the thrombolytic era. Arq Bras Cardiol. 1997;68(6):401–5.
16 Garoufalis S, Kouvaras G, Vitsias G, Perdikouris K, Markatou P, Hatzisavas J, et al. Comparison of angiographic findings, risk factors, and long term follow-up between young and old patients with a history of myocardial infarction. Int J Cardiol. 1998;67(1):75–80.
17 Ghosh K, Khare A, Shetty S. Fasting plasma homocysteine levels are increased in young patients with acute myocardial infarction from Western India. Indian Heart J. 2007;59(3):242–5.
18 Ogawa M, Abe S, Saigo M, Biro S, Toda H, Matsuoka T, et al. Homocysteine and hemostatic disorder as a risk factor for myocardial infarction at a young age. Thromb Res. 2003;109(5-6):253–8.
19 Nikfardjam M, Graf S, Hornykewycz S, Zorn G, Huber-Beckmann R, Wojta J, et al. Homocysteine plasma levels in young patients with coronary artery disease. Relation to history of acute myocardial infarction and anatomical extent of disease. Thromb Res. 2001;103(Suppl 1):S35–39.
20 Patrizi R, Pasceri V, Sciahbasi A, Summaria F, Rosano GM, Lioy E. Evidence of cocaine-related coronary atherosclerosis in young patients with myocardial infarction. J Am Coll Cardiol. 2006;47(10):2120–2.
21 Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–95.
22 National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation. 1994;89(3):1333–445.
23 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
24 Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51.
25 Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Niedermaier G, et al. Acute coronary syndromes in young patients: presentation, treatment and outcome. Int J Cardiol. 2011;148(3):300–4.
26 Chua SK, Hung HF, Shyu KG, Cheng JJ, Chiu CZ, Chang CM, et al. Acute ST-elevation myocardial infarction in young patients: 15 years of experience in a single center. Clin Cardiol. 2010;33(3):140–8.
27 Hbejan K. Smoking effect on ischemic heart disease in young patients. Heart Views. 2011;12(1):1–6.
28 Huang J QH, Li ZZ, Zhang JM. Comparison of Clinical Features and Outcomes of Patients With Acute Myocardial Infarction Younger Than 35 Years With Those Older Than 65 Years. Am J Med Sci. 2013. Epub 2013 Jan 16.
29 Rallidis LS, Lekakis J, Panagiotakos D, Fountoulaki K, Komporozos C, Apostolou T, et al. Long-term prognostic factors of young patients (<or=35 years) having acute myocardial infarction: the detrimental role of continuation of smoking. Eur J Cardiovasc Prev Rehabil. 2008;15(5):567–71.
Funding / potential competing interests: This work was supported by the Fonds Scientifique Cardiovasculaire (Fribourg).
Authors’ contribution:Serban Puricel participated in the conception and design, analysis and interpretation of data and in drafting the manuscript. Stéphane Cook was involved in the conception and design, analysis and interpretation, acquisition of data, drafting and revising the manuscript critically for important intellectual content. Mario Togni, Tobias Rutz, Aris Moschovitis, Bernhard Meier, Peter Wenaweser, Stephan Windecker and Jean-Christophe Stauffer participated in the acquisition of data and revised the manuscript critically for important intellectual content. Cedric Lehner, Markus Oberhaensli and Mathieu Stadelmann participated in the analysis and interpretation of data as well as in the critical revision of the manuscript for important intellectual content. All authors have given their final approval of the version to be published.