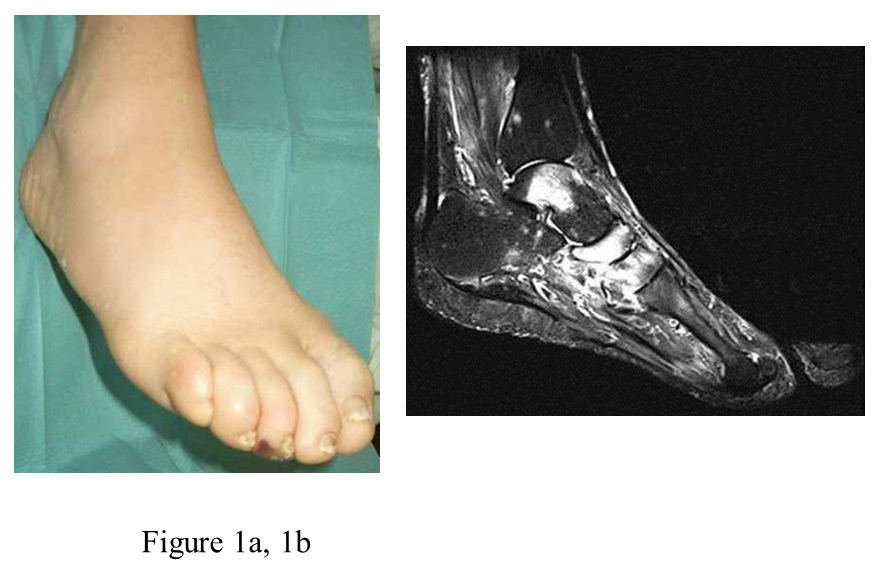
Figure 1
a: clinical aspect of an acute Charcot foot stage 0.
b: MRI (T2w, fat suppressed) showing marrow oedema in the talus, and the navicular and cuneiform bones plus adjacent soft tissue oedema (bright appearance)
DOI: https://doi.org/10.4414/smw.2013.13831
Abbreviations:
ACF acute Charcot foot
BMI body mass index
DEXA dual energy X-ray absorption
F-18 FDG PET/CT fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography
HIV human immunodeficiency virus
ICI integral classification of injuries
MRI magnetic resonance imaging
STIR short tau inversion recovery
TCC total contact cast
VPT vibration perception threshold
X-ray Roentgen radiography
BACKGROUND: Acute Charcot foot (ACF) is a skeletal breakdown associated with inflammatory swelling of a foot in patients with pain insensitivity, such as diabetic neuropathy. In ACF stage 0, skeletal pathology (e.g. osseous oedema) is visible on magnetic resonance imaging (MRI), but not on plain radiographs. Continued unprotected walking invariably causes stage 1 (complex cortical fractures). Treatment by total contact cast (TCC) is of limited benefit if X-ray-based. The benefits of MRI-based TCC treatment are unknown.
AIM: To assess the impact of MRI, all cases of ACF diagnosed by MRI between 2000 and 2012 were reviewed.
METHOD: Audit of medical charts of a single outpatient diabetic foot clinic.
RESULTS: Seventy-one cases (59 patients) were retrieved. Diagnosis of stage 0 (n = 27 cases) and stage 1 (n = 44 cases) was established one and two months (medians) after symptom onset, respectively. Unremarkable radiographs, that were not cross-checked by MRI (n = 13 cases), misled primary care physicians to postpone referral until five months after symptom onset, when cortical fractures had already occurred in 12 cases. Midfoot (Chopart- and Lisfranc-) lesions healed better in stage 0 versus stage 1 (69% versus 7% without deformities, p = 0.0012), while forefoot (metatarsal) lesions healed well in either stage (100% versus 75% without deformities). TCC-treatment lasted four to six months.
CONCLUSION: Healing of ACF was more efficient in stage 0 than in stage 1. Expeditious MR imaging was indispensable to diagnose stage 0 in a swollen foot of a neuropathic patient, while unremarkable X-rays often led to a missed diagnosis.
Charcot foot is a complication of pain insensitivity and can result in devastating bone and joint destruction with foot deformity (called Charcot’s arthropathy or neuroarthropathy). The cause of the condition is controversial among experts. There is ample evidence that the Charcot foot is triggered by post-traumatic skeletal inflammation (frequently caused by traumatic injury, but also by a local surgical procedure or infection [1]). The acute Charcot foot is more or less swollen, hot and red (fig. 1a, 2a), and may ache upon weight bearing (although not very much). Immediate offloading and immobilisation [2] resolves the inflammation and stops the acute bone and joint damage. Full blown arthropathy only develops when continued repetitive weight bearing (walking), causes fractures of bones or joints already weakened from inflammation (e.g. post injury osteoporosis [3]). Animal experiments have shown that an inflamed foot is very painful and thereby activity limiting in terms of repetitive weight bearing [4]. Hence, Charcot arthropathy does not develop in pain sensitive feet but only in pain insensitive feet (e.g. in hereditary sensory neuropathy, leprosy and syringomyelia and in neuropathy from diabetes, alcoholism or HIV [5–10]).
In 1966, Eichenholtz divided the “natural” course of the Charcot foot on the basis of X-ray findings into stage 1 (bone dissolution), stage 2 (coalescence) and stage 3 (remodelling) [11]. Stage 1 represents the damaging acute phase, stage 2 represents the repair phase and stage 3 represents the chronic, quiescent, healed phase. However, it had already been shown in 1976 by Classen et al. [12] that clinical symptoms (swelling, warmth, erythema and deep dull aching while walking) regularly precede the X-ray signs of Eichenholtz stage 1 by several weeks or months together with scintigraphic bone abnormalities. In 1984, Edmonds and Watkins reported that immobilising and offloading a foot with these early changes may prevent fractures and deformity [13]. Consistently, Shibata et al. in 1990 added a fourth stage, Charcot foot stage 0 (clinical and scintigraphic signs without X-ray abnormalities) to the conventional classification [14]. The bone pathology of stage 0 could later be identified by magnetic resonance imaging (MRI) as reactive osseous oedema, similar to stress injury [15–21].
Early detection and treatment of acute Charcot foot stage 0 has become imperative to prevent devastating skeletal destruction and permanent deformity [22]. However, studies of this stage are scarce [16–20, 23, 24], as availability of MRI is still limited. Most studies to date were X-ray based [2, 3, 5, 25–29], consequently focusing on stage 1–3. We recently reported preliminary data on the benefits of very early offloading and immobilising with a total contact cast (TCC) [23, 24, 30, 31]. In the present study, we reviewed our experiences in MRI-based management of the acute diabetic Charcot foot over a 12 year period.
A retrospective, observational, exploratory cohort study was performed, reviewing all cases of acute diabetic Charcot foot (Eichenholtz stage 0 or 1) admitted to the authors’ institution between 01.01.2000 and 31.12.2011 that were diagnosed by MRI. The patient files were assessed focusing on medical history, timing of diagnosis and treatment, regional distribution of skeletal damage, foot deformity, healing without skeletal deformity, duration of treatment, cases requiring customised footwear, adverse effects of treatment, follow-up morbidity. The study extends previous assessments approved by the local ethical committee that were published between 2005 and 2008 [23, 24, 30, 31].

Figure 1
a: clinical aspect of an acute Charcot foot stage 0.
b: MRI (T2w, fat suppressed) showing marrow oedema in the talus, and the navicular and cuneiform bones plus adjacent soft tissue oedema (bright appearance)

Figure 2
a: clinical aspect of an acute Charcot foot stage 1.
b: MRI (T1w, fat suppressed, with contrast media) showing bone marrow oedema and impact fractures in the Lisfranc joints, a fluid collection with circular structure (equivalent to synovial fluid leakage and/or fracture hematoma) in the tarsal region, and adjacent soft tissue oedema (bright appearance).

Figure 3
a: bivalved, removable total contact cast (TCC) as used in our institution, opened; leg is covered with compression stocking.
b: TCC closed.
The diagnosis of acute diabetic Charcot foot stage 0 or stage 1 was established according to the clinical and MRI criteria published previously [32]. Stage 0 was defined as swollen, reddened and hot foot with diffuse or patchy marrow oedema (indicative of trabecular microfractures) in two or more bones on MRI [32] with significant soft tissue oedema adjacent to the inured bones (grade I–III according to the grading system of Kiuru et al. [33] for stress bone lesions) in the absence of cortical fracture (on MRI or on plain X-ray) in a patient with diabetic neuropathy.
Stage 1 was defined as a swollen, reddened and hot foot with diffuse or patchy bone marrow oedema with adjacent soft tissue oedema on MRI, plus closed cortical fractures (on MRI or X-ray) comprising fracture lines on MRI (stress injury grade IV according to Kiuru et al. [33]), simple metatarsal shaft fractures and splintered fractures (predominantly affecting tarsal bones and joints) in a patient with diabetic neuropathy. Stage 1 was distinguished from Eichenholtz stage 2 according to the absence of significant soft tissue oedema in the presence of a palpable bone mass (bony protuberance, representing callus) and according to the presence of stress injuries stage V on MRI [33], and/or to callus formation on X-ray.
Cases treated and followed up by the diabetic foot clinic until healing of the acute Charcot foot were included. Cases with coexisting plantar ulceration or with possible septic skeletal pathology were not eligible.
– Diabetic neuropathy: Vibration perception threshold <5/8 at the first metatarsal head, as measured with the 64 Hz Rydel-Seiffer tuning fork in subjects with established type-1 or type-2 diabetes mellitus [34].
– Healed Charcot foot stage 0: Complete or nearly complete regression of the osseous oedema on MRI, consistent with the observations by Zampa et al. [35], following resolution of symptoms (swelling, warmth, erythema) as shown by McGill et al. [36]. Healed stage 0 is associated with a normal bone and skeletal structure (healed stage 0, restitution ad integrum).
– Healed Charcot foot stage 1: Complete or nearly complete regression of the osseous oedema on MRI, consistent with the observations by Zampa et al. [35], following resolution of symptoms (swelling, warmth, erythema) as shown by McGill et al. [36]. Healed stage 1 is associated with abnormal bone structure (e.g. subchondral cysts) and skeletal structure, thus representing stage 3 according to Eichenholtz [11].
– Healing time: Time (months) from institution of total contact cast (TCC) treatment until transition to shoes.
– Relapse: Recurrence of osseous injury (oedema or fracture) and swelling, pain and hyperthermia in a healed foot more than one month after cessation of TCC treatment was classified as a relapse. Recurrence of these signs and symptoms within one month after cessation of TCC was indicative of premature cessation of treatment; in such a case, TCC treatment was resumed and continued until permanent healing.
Complete offloading and immobilisation of the affected foot was instituted as an emergency measure on the spot, by putting the patient in a wheelchair or to bed in the university hospital (to reduce the oedema, if necessary). Subsequently, mostly within three days, patients were provided with a bivalved, removable total contact cast (TCC), very few patients temporarily received a prefabricated polypropylene ankle-foot orthosis [37], such as Rebound ® Air Walker, VACOcast®, or Aircast® boot [29, 37].
The TCC was made by orthopaedic cast technicians as follows: the leg is extended, and the foot is placed in neutral position with the hallux extended to stretch the plantar fascia. Foot and lower limb are covered with cotton tube gauze, on which a layer of Cellona® synthetic underpadding is applied and wrapped with Haftan® bandages. Subsequently, wet fibreglass tape (Cellacast Xtra®, all Lohmann & Rauscher, Neuwied/Germany) is bandaged in several layers around the limb, extending from the metatarsal heads to the tibial tuberosity. After drying out, the circular cast is cut open with a cast saw. Cellona® and Haftan® wrapping is discarded. Inside, both valves are padded with a thin layer of cotton covered rubber foam. On the outside, Velcro-straps are applied. The rear valve of the cast is furnished with a rocker bottom walking sole. To secure a balanced gait, the walking sole of contralateral shoe is augmented by about 2 cm. The TCC is checked and the rubber foam padding is augmented after some days, following reduction of the oedema. If no longer fitting properly, the TCC needs to be renewed.
The TCC served to immobilise the foot and ankle joints and to partially offload the foot during ambulation (protected weight bearing [29, 37]). It had to be used the whole day and always together with two crutches; weight bearing should be limited, but specific limits were not set. For sleeping, the TCC could be removed, to enable unloaded foot movements. All patients were provided with class I compression stockings [38, 39]; antithrombotic drugs were not prescribed. TCC was applied until healing of the acute Charcot foot.
Transition to shoes: When healing of the acute Charcot foot was assumed, patients were advised to alternate between TCC and special shoes (see below) for four to eight weeks, and preferably to use the TCC for long walking distances in the transition period. This advice was deemed necessary because osteoporosis from inactivity during TCC treatment has to be anticipated, and hence an increased fracture risk with immediate resumption of 100% physical activity.
Shoes: Patients received either a bespoke shoe with stiff, rocker bottom walking sole and customised rigid cradle, or a stock diabetic shoe furnished with a customised rigid cradle and a stiff, rocker bottom walking sole for the healed Charcot foot, according to the foot deformity. Shoes were provided by a local orthopaedic shoemaker under supervision of the physician in charge of the foot clinic (EAC, AR).
Costs: All treatment costs were covered by the patients’ sick funds.
– Patient history: Time of symptom onset, quality of symptoms (e.g. swelling, pain) and possible traumatic events, were looked up in the patients’ charts.
– Imaging studies: Radiographs and MR images were provided by various institutions (practices of radiology in the city of Düsseldorf, other hospitals, or the department of radiology of the Heinrich Heine University of Düsseldorf), to which the patients had been referred by their general practitioners or by the foot clinic, because of suspected acute Charcot foot. At the radiologists’ institutions, T1 weighted, T2 weighted and STIR imaging had been carried out, with or without contrast media, at the discretion of the radiologist in charge. MRI was repeated in each patient for monitoring of the healing process at the discretion of the diabetic foot clinic. In cases with stage 1 Charcot foot, conventional X-ray monitoring was also performed as appropriate. Descriptions of the MRI or X-ray morphology, as provided in written form by the reporting board qualified radiologists were accepted unchecked for the purposes of the study. In doubtful cases, images were re-read.
– Distribution of skeletal involvement: The MRI abnormalities were divided into three anatomical regions [29, 35, 40]. The forefoot region comprised the metatarsals including the metatarsophalangeal joints, but not the Lisfranc joint (ICI code 83.1 and 83.2 [40]). The midfoot region comprised ICI code 82, including Lisfranc and Chopart joints. The hindfoot region comprised calcaneus, talus and talocrural joint (ICI code 81). Cases with more than one region involved were classified according to the most affected region.
– Foot temperature: Skin temperature was palpated in comparison to the contralateral unaffected foot.
– Foot oedema: Subcutaneous oedema was assessed by inspection and palpation, in comparison to the contralateral unaffected foot (documented by photography).
– Foot deformity: Deformity was assessed by inspection and palpation, in comparison to the contralateral unaffected foot (documented by photography). Depression of the longitudinal arch was graded according to severity.
All patients had been followed up until transition to shoes and for variable periods of time thereafter (at the discretion of the patients or their referring physicians).
Data are presented as medians with interquartile range (IQR). Descriptive analyses were carried out, applying Fisher’s exact test, Mann-Whitney U test, Kruskal-Wallis test and Bonferroni correction, as appropriate. A two-sided p <0.05 was considered significant.
The clinical characteristics of the patients are summarised in table 1. A total of 59 patients matching the inclusion criteria were retrieved, with altogether 71 cases of acute Charcot foot stage 0 (n = 27) or stage 1 (n = 44). Included were one relapse in four type-1 diabetic patients each, two relapses in three type-1 diabetic patients each, and one contralateral acute Charcot foot in a type-1 and a type-2 diabetic patient each. The forefoot region was affected in 18 (25%) of the 71 cases, the midfoot region in 48 (68%), and the hindfoot region in five (7%).
Forty of the 59 patients reported deep dull aching upon walking on the affected foot, significantly more with type 1 than with type 2 diabetes. Patients reporting pain were more frequently able to recall a trauma preceding the onset of Charcot symptoms (24 of 49 cases), as compared to patients not feeling pain (4 of 22 cases; Fisher’s exact test p = 0.015). However, patients reporting pain did not seek medical help earlier (2.3 vs. 2.3 months), and did not receive TCC treatment earlier, than patients not reporting pain. Pain reporting was not different among cases with stage 0 or stage 1. Vibration perception threshold (VPT) was similar in patients reporting/ not reporting pain.
Treatment details are summarised in tables 2–5.All 71 cases were treated conservatively; surgical interventions were not required. Of the 27 cases of acute Charcot foot stage 0, 19 (70%) healed without deformity, compared to 14 (32%) out of the 44 cases of acute Charcot foot stage 1 (Fisher’s exact test p = 0.002). TCC treatment was required for four (2–8) months in stage 0 versus five (3.5–14) months in stage 1 (n.s.). Only one case with stage 0 of the forefoot, and one case with stage 0 of the midfoot progressed to stage 1 during TCC treatment– most likely due to occasional walking without TCC. Few adverse effects of TCC were noted: skin ulcers (9 cases), and malalignment of the cast (foot healed in fixed supination) in one case. Thromboembolic complications were not noted.
Time from onset of symptoms until institution of TCC treatment was one (0.5–2) months in stage 0, and two (0.62–3.6) months in stage 1 (n.s.). The time interval from symptom onset until treatment was affected by the diagnostic imaging strategy (see table 2). Fifty cases subjected to MRI first received TCC after one (0.5–2) months, versus 21 cases subjected to X-ray first, who received TCC after 2.5 (0.75–5) months (p <0.02 Mann-Whitney U test). In 13 cases of stage 0, the referring general practitioner had failed to cross-check a negative X-ray by MRI and subsequently the referral to the foot clinic for treatment was postponed by 4.5 months. Meanwhile, 12 of the 13 cases had progressed to stage 1 with permanent skeletal deformities, see table 2.
Treatment details are differentiated between affected foot regions and ACF stages in table 3. The time period between onset of symptoms and institution of treatment was shorter for stage 0 than for stage 1 cases. Healing achieved in stage 0 resulted less frequently in deformities than healing achieved in stage 1. Midfoot affections healed more often with deformities than forefoot or hindfoot affections. Duration of TCC treatment required for healing was not much different between stage 0 and stage 1, but differed in relation to the affected foot region (midfoot affections required longest treatment).
Severity of foot deformity before and after TCC is summarised in table 4. Only one case (stage 1) developed a rocker bottom foot, while six cases (one of stage 0, and five of stage 1) developed a flat foot.
Post-healing footwear requirement is summarised in Table 5. Rigid customised shoes with customised cradle and rocker bottom walking sole were required by 17% of forefoot stage 0 cases, 17% of forefoot stage one case, 35% of midfoot stage 0 cases, 61% of midfoot stage 1 cases, and zero hindfoot cases.
During four (2–6) years after healing, 14 of the 71 healed Charcot feet developed an ulcer, four of the 27 (15%) stage 0 feet, and ten of the 44 (23%) stage 1 feet. The ulcers were cured conservatively. Amputation or other surgery was not required.
| Table 1: Clinical features of patients with acute Charcot foot (ACF). | ||
| Diabetes mellitus | ||
| Type 1 | Type 2 | |
| Men/women, n | 8/16 | 22/13* |
| Age, yrs | 55 (48.5–59.5) | 62 (56–69)** |
| Duration of diabetes, yrs | 32 (25.5–41) | 10 (5–19)** |
| BMI, kg/m² | 24.6 (23.2–26.7) | 30.9 (27.1–33.8)** |
| VPT, x/8 | 1.5 (0–3) | 2 (1–3) |
| Pain reported, n | 21 | 19* |
| Patients with | ||
| – end stage renal disease, n | 3 | |
| – transplantation, n | 2 | 1 |
| – osteoporosis, n | 1 | |
| Patients with >1 case of ACF, n | 8 | 1* |
| Cases of ACF, n | 33 | 38 |
| Cases of ACF per pat, n | 1.4 | 1.1 |
| Cases stage 0/stage 1, n | 13/20 | 14/24 |
| Medians (IQR). * Fisher’s exact test, and ** Mann-Whitney U-Test; p <0.05 Type1 versus Type 2 Diabetes mellitus. Transplantation: kidney (n = 2), kidney-pancreas (n = 1). Osteoporosis: abnormal bone mineral density (DEXA criteria) | ||
| Table 2: Effect of imaging strategy at symptom onset (X-ray or MRI first) on detection and treatment of acute Charcot foot stage 0. | |||
| MRI first (n = 19) | X-ray first (n = 21), cross-checked by MRI | ||
| Yes (n = 8) | No (n = 13) | ||
| Detected stage 0 cases, n | 19 | 8 | |
| Institution of TCC after symptom onset, months | 1 (0.25–1.375) | 0.5 (0.375–0.875) | 5 (2.5–6)* |
| Feet with skeletal deformities at institution of TCC, n | 4 (21%) | 0 (0%) | 12 (92%)** |
| Medians (IQR), or numbers (%). TCC = total contact cast. *p <0.02 versus other groups, Mann-Whitney U test; ** two-sided p <0.0001 versus other groups, Fisher’s exact test | |||
| Table 3: Duration from symptom onset until institution of TCC treatment, duration of TCC treatment until healing, and proportion of ACF cases healing without foot deformity. | ||||||
| Affected foot region | Forefoot | Midfoot | Hindfoot | |||
| ACF stage | Stage 0 | Stage 1 | Stage 0 | Stage 1 | Stage 0 | Stage 1 |
| Cases, n | n = 6 | n = 12 | n = 20 | n = 28 | n = 1 | n = 4 |
| Time from symptom onset to institution of TCC, months | 0.6 (0.5–1) | 2 (0.5–4) | 1 (0.5–2.25) | 2 (0.6–3.25) | 1.5 | 0.9 (0.1–3) |
| Duration of TCC, months | 2.75 (2–4.5) | 4 (2.9–6) | 5.5 (3.25–8) | 6 (4–8) | 2 | 6 (3.5–9) |
| Cases healing without deformity, n | 6 (100%) | 9 (75%) | 12 (69%)* | 2 (7%)* | 1 (100%) | 3 (75%) |
| Medians (IQR), or numbers (%). ACF = acute Charcot foot. TCC = total contact cast. *Bonferroni-corrected p = 0.0012, Fisher’s exact test | ||||||
| Table 4: Distribution of deformities in acute Charcot feet, before and after TCC-treatment, according to foot region affected. | |||
| Cases (%) with foot deformity (depression of longitudinal arch) | |||
| Affected foot region | Forefoot | Midfoot | Hindfoot |
| Total number of cases, n | 18 (100%) | 48 (100%) | 5 (100%) |
| Prior to TCC treatment | |||
| No depression, n | 16 (89%) | 25 (52%) | 5 (100%) |
| Moderate depression, n | 2 | 5 | |
| Severe depression, n | 16 | ||
| Flat foot/rocker foot, n | 2 | ||
| At end of TCC treatment | |||
| No depression, n | 15 (83%) | 14 (29%) | 4 (80%) |
| Moderate depression, n | 3 | 12 | 1 |
| Severe depression, n | 15 | ||
| Flat foot/rocker foot, n | 7 | ||
| TCC = total contact cast | |||
| Table 5: Distribution of types of footwear required after healing the acute Charcot foot, according to Charcot foot stage and foot region affected. | |||
| Footwear after healing of acute Charcot foot | |||
| Affected foot region | Forefoot | Midfoot | Hindfoot |
| Total number of cases | 18 | 48 | 5 |
| TCC instituted in Stage 0, n | 6 | 20 | 1 |
| – normal footwear, n | 4 | 5 | |
| – special “diabetic” shoes, n | 1 | 8 | 1 |
| – customised rocker shoes, n | 1 | 7 | |
| TCC instituted in Stage 1, n | 12 | 28 | 4 |
| – normal footwear, n | 6 | 4 | 3 |
| – special “diabetic” shoes, n | 4 | 7 | 1 |
| – customised rocker shoes, n | 2 | 17 | |
| TCC = total contact cast | |||
| Table 6: Comparison of the present findings to published data. | ||||||
| Author (reference) | Cases (n) | ACF stage | Mode (duration) of intervention | Outcome: distribution of the major deformities | ||
| %forefoot | %midfoot | %hindfoot | ||||
| Armstrong [27] | 55 | 1 | TCC (4.5 months) | 3 | 82 | 15 |
| Sinacore [25] | 30 | 1 | TCC (3 months) | 20 | 69 | 11 |
| Fabrin [28] | 115 | 1 | Crutches (3–6 months) | 19 | 74 | 7 |
| Present series | 44 | 1 | TCC (2–9 months) | 100 | ||
| Edmonds [19] | 12 | TCC (6 months) | ||||
| Present series | 27 | TCC (2–8 months) | ||||
| ACF = acute Charcot foot. TCC = total contact cast | ||||||
The main results of our study are that TCC treatment of the acute Charcot foot should begin as early as possible, i.e. in stage 0 rather than stage 1, and that MRI is very much instrumental to achieve this goal. The diagnostic strategy to detect acute Charcot foot stage 0 appeared to have a significant impact on the treatment efficiency in our study. X-ray is generally recommended as first step imaging modality to diagnose acute Charcot foot in the case of a swollen, hot and reddened foot in a patient with diabetic neuropathy [7]. However, in our setting, this strategy has failed since most of the referring physicians did not to admit the patients to the foot clinic (to be treated by TCC), when the X-ray was negative for any skeletal trauma. It is also of concern that plain X-ray is totally insufficient to detect midfoot fractures [41, 42].
The data of our study are otherwise fairly comparable to previous series of acute Charcot foot: the type 1 diabetic patients were slightly younger, less obese, and had longer duration of diabetes than the type 2 diabetic patients [25, 27, 29]. Moreover, the type 1 diabetic patients more often had acute Charcot foot recurrences, probably related to the decreased bone mineral density that is typical in this patient population (contrary to type 2 diabetic patients). The stage 0 cases had similar outcomes as those reported by Edmonds et al. [19], showing that TCC treatment can prevent progression to stage 1 with its more severe pre- and post-treatment skeletal damage and deformities. Also, the outcomes in our stage 1 cases corroborate previous reports (see table 6); however, none of them required amputation during four years of follow up, compared to 11.8% of cases requiring transtibial amputation during follow up of 3.8 years in the series of Saltzman et al. [26].
Median healing time in our sample was on average two months shorter than the eight months period reported by Zampa et al. based on strict MRI criteria [35]. We found that the healing time was not significantly shorter in stage 0 cases than in stage 1 cases, seemingly at variance to the observations by Zampa et al. As MRI surveillance was less strict in our series, we might have missed the exact time point of healing in some of our patients. A recent study based on F-18 FDG PET/CT scan and MRI observed definite healing in stage 0 (presumably including bone remodelling) only 15 months after symptom onset [43].
The present series confirms that healing time is related to the anatomical region [25]. Chopart or Lisfranc joint affections required considerably longer offloading and immobilisation compared to forefoot affections, irrespective of stage 0 or stage 1. The numbers of hindfoot affections in this study were too small to draw any conclusions in this respect. We attribute the prolonged treatment necessary to heal midfoot Charcot arthropathy to the biomechanical features. The cancellous tarsal bones are covered with very thin compacta; they break easily once their ligamentous structures are compromised (e.g. ruptured by a sprain injury). When weight bearing is continued, the longitudinal arch collapses and rocker bottom deformity will occur [24], as Paul W. Brand has explained: “The front of a foot may be regarded as a long lever with its fulcrum at the ankle. The calf muscles will pull upwards on the heel bone and thus they force the front of the foot downwards. This downward thrust of the front of the foot gives a spring to the walk as the body is lifted forward for the next step (...) If a person takes a very strong thrust on his foot, one of the bones at the centre (usually the navicular) will crumble and break and the foot then becomes flat and will even bend so that the arch is reversed and the front of the foot becomes unable to accept any thrust at all. When that happens, the centre of the foot takes all strain and the foot may be destroyed.” [44]
In previous studies, 70–84% of patients with acute Charcot foot have reported pain [13, 27], described as “deep dull aching pain in the joints” [45] upon load bearing. In the present study 68% of patients reported deep dull foot pain upon walking, unrelated to vibration sensation or to stage 0/stage 1. These patients could recall a traumatic event as the trigger of the acute Charcot foot more frequently than patients not reporting pain. Unfortunately, this did not stimulate them to seek medical help earlier, a phenomenon that remains to be elucidated [4, 46].
Our study obviously has limitations: it was retrospective and observational, based on routine clinical records, and did not have a control group of acute Charcot feet managed on the basis of X-ray. The case management did not follow a pre-defined protocol. A learning curve has to be considered. The time points of symptom onset and healing could be assessed only approximately. Firm conclusions as to the optimal imaging strategy, the optimal time point to start treatment, the optimal offloading and immobilisation modality and duration cannot be drawn from the present data. Multicentre, prospective controlled trials are necessary in this respect. Despite these shortcomings, the present data support the following tenets:
1) The acute Charcot foot is a medical emergency. Early detection and treatment is of paramount importance. At risk are those diabetic patients with insensitivity to strong noxious punctate (pinprick) stimulation [34, 46]. Contrary to current recommendations [7], X-ray should not be the initial imaging study to assess a swollen, reddened and hot neuropathic foot. MRI should be preferred instead (see also [47]). MRI is capable of showing bone injury of a milder degree than cortical fractures, which greatly benefits from offloading and immobilisation. Unremarkable X-ray may falsely suggest absence of midfoot fractures [43, 44] or may confuse inexperienced physicians not to offload and immobilise the foot.
2) Any bone marrow oedema on MRI in a pain-insensitive, swollen foot, irrespective of its nature [48–51], is likely to progress to cortical fracture if subjected to repetitive stress (i.e. unprotected walking) and should, therefore, be regarded as indicative of acute Charcot foot stage 0 (at variance to the definition of stage 0 given in the above paragraph “diagnostic criteria”). Osseous inflammation, either post-traumatic or septic, produces transient skeletal fragility [52] thereby increasing the susceptibility to injury from normal load bearing. Hence, any bone marrow oedema in a swollen, hot, reddened pain insensitive foot is likely to benefit from immediate offloading and immobilisation and should be treated accordingly. It goes without saying that in cases of, for example. osteomyelitis or acute gout, TCC treatment is complementary to the anti-infective or gout treatment in a pain insensitive foot.
In summary, treating the acute diabetic Charcot foot very early has once again proved beneficial, confirming Classen et al. [12] who “believed that procrastination could lead only to grief”, i.e. to full blown Charcot’s arthropathy. In diabetic patients, any swollen, inflamed foot, which is insensitive to pinprick pain testing [46], should therefore immediately be offloaded and immobilised and subjected to MR imaging to reach the diagnosis of acute Charcot foot stage 0 as early as possible.
1 Aragon-Sanchez J, Lazaro-Martinez JL, Hernandez-Herrero MJ. Triggering mechanisms of neuroarthropathy following conservative surgery for osteomyelitis. Diabet Med. 2010;27:844–7.
2 Johnson JTH. Neuropathic fractures and joint injuries. Pathogenesis and rationale for prevention and treatment. J Bone Joint Surg. (Am) 1967;49-A:1–30.
3 Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Engineering B. 2008;14:179–86.
4 Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–84.
5 Onvlee GJ. The Charcot foot: a critical review and an observational study of a group of 60 patients [Thesis]. Medical Faculty. University of Leiden/The Netherlands, 1998.
6 Minde JK, Svensson O, Holmberg M, Solders G, Toolanen G. Orthopaedic aspects of familial insensitivity to pain due to a novel nerve growth factor beta mutation. Acta Orthop. 2006;77:198–202.
7 Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The Charcot foot in diabetes. Diabetes Care. 2011;34:2123–9.
8 Greider TD. Orthopaedic aspects of congenital insensitivity to pain. Clin Orthop Relat Res. 1983;172:177–85.
9 Yalcin S, Kocaoglu B, Berker N, Erol B. Conservative treatment of Charcot arthropathy in a series of spina bifida patients: the experience of one centre and review of the literature. J Pediatr Orthop B. 2007;16:373–9.
10 Rangel EB, Sa JR, Gomes SA, Carvalho AB, Melaragno CS, Gonzalez AM, et al. Charcot neuroarthropathy after simultaneous pancreas-kidney transplant. Transplantation. 2012;94:642–5.
11 Eichenholtz SN. Charcot Joints. With a foreword by Philip D. Wilson. Springfield, Illinois: Charles C. Thomas, Publisher. 1966.
12 Classen JN, Rolley RT, Carneiro R, Martire JR. Management of foot conditions of the diabetic patient. Am Surg. 1976;42:81–8.
13 Edmonds ME, Watkins PJ. The Charcot joint: understanding its natural history leads to new treatment and prevention. Abstract. Diabet Med. 1984;1:144A.
14 Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg. (Am) 1990;72-A:749–56.
15 Moore TE, Yuh WTC, Kathol MH, El-Khoury GY, Corson JD. Abnormalities of the foot in patients with diabetes mellitus: findings on MR imaging. AJR. 1991;157:813–6.
16 Greenstein AS, Marzo-Ortega H, Emery P, O’Connor P, McGonagle D. Magnetic resonance imaging as a predictor of progressive joint destruction in neuropathic joint disease. Arthritis Rheum. 2002;46:2814–6.
17 Edmonds E, Petrova NL, Elias D. The earliest magnetic resonance imaging sign of mid-foot Charcot osteoarthropathy is oedema of subchondral (subarticular) bone marrow which needs prompt therapeutic offloading. Abstract. Diabet Med. 2005;22(Suppl.2):93.
18 Edmonds ME, Petrova NL, Edmonds A, Elias A. Early identification of bone marrow oedema in the Charcot foot on MRI allows rapid intervention to prevent deformity. (Abstract) Diabet Med. 2006;23(Suppl.2):70.
19 Edmonds ME, Petrova NL, Edmonds AE, Elias DA. What happens to the initial bone marrow oedema in the natural history of Charcot osteoarthropathy? Abstract. Diabetologia. 2006;49(Suppl.1): A–684.
20 Chantelau E, Richter A, Schmidt-Grigoriadis P, Scherbaum WA. The diabetic Charcot foot: MRI discloses bone stress injury as trigger mechanism of neuroarthropathy. Exp Clin Endocrinol Diabetes. 2006;114:118–23.
21 Halstead J, Bergin D, Keenan AM, Madden J, McGonagle D. Ligament and bone pathologic abnormalities more frequent in neuropathic joint disease in comparison with degenerative arthritis of the foot and ankle. Arthritis Rheum. 2010;62:2353–8.
22 Sella EJ, Barrette C. Staging Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg. 1999;38:34–40.
23 Chantelau E, Richter A, Ghassem-Zadeh N, Poll L. “Silent” stress injuries in the feet of diabetic patients with polyneuropathy-a report on 12 cases. Arch Orthop Trauma Surg. 2007;127:171–7.
24 Kimmerle R, Chantelau E. Weight-bearing intensity produces Charcot deformity in injured neuropathic feet in diabetes. Exp Clin Endocrinol Diabetes. 2007;115:360–4.
25 Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications. 1998;12:287–83.
26 Saltzman CL, Hagy ML, Zimmerman M, Estin M, Cooper R. How effective is non-operative initial treatment of patient with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res. 2005;435:185–90.
27 Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:35763.
28 Fabrin J, Larsen K, Holstein PE.Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23:796–800.
29 Christensen TM, Gade-Rasmussen B, Pedersen LW, Hommel E, Holstein PE, Svendsen OL. Duration of off-loading and recurrence rate in Charcot osteo-arthropathy treated with less restrictive regimen with removable walker. J Diabetes Compl. 2012;26:430–4.
30 Chantelau E. The perils of procrastination: effects of early vs. delayed detection and treatment of incipient Charcot fracture. Diabet Med. 2005;22:1707–12.
31 Ghassem-Zadeh N. MRI-guided early treatment of stress bone injuries at the foot in diabetic polyneuropathy (MRT-gesteuerte Frühtherapie knöcherner Stressverletzungen am Fuss bei diabetischer Polyneuropathie). Thesis [in German]. Medical Faculty of the Heinrich- Heine-University Düsseldorf/Germany 2008.
32 Chantelau E, Poll LW. Evaluation of the diabetic Charcot foot by MR imaging or plain radiography- an observational study. Exp Clin Endocrinol Diabetes. 2006;114:428–31.
33 Kiuru MJ, Pihlajamaki HK, Ahovuo JA. Bone stress injuries. Acta Radiol. 2004;45:317–26.
34 Wienemann T, Chantelau EA. The diagnostic value of measuring pressure pain perception in patients with diabetes mellitus. Swiss Med Wkly. 2012;142:w13682.
35 Zampa V, Bargellini I, Rizzo L, Turini F, Ortori S, et al. Role of dynamic MRI in the follow-up of acute Charcot foot in patients with diabetes mellitus. Skeletal Radiol. 2011;40:991–9.
36 McGill M, Molyneaux L, Bolton T, Ioannou K, Uren R, Yue DK. Response of Charcot’s arthropathy to contact casting: assessment by quantitative techniques. Diabetologia. 2000;43:482–4.
37 Cook JJ, Cook EA. Protected weight bearing during treatment of acute Charcot neuroarthropathy: a case series. The Foot and Ankle Online Journal. 2011;4(7):1.
38 Sims DS, Cavanagh PR, Ulbrecht JS. Risk factors in the diabetic foot. Recognition and management. Phys Ther. 1988;68:1887–903.
39 Wu SC, Crews RT, Najafi B, Slone-Rivera N, Minder JL, Andersen CA. Safety and efficacy of mild compression (18–25 mm Hg) therapy in patients with diabetes and lower extremity oedema. J Diabetes Sci Technol. 2012;6:641–7.
40 Zwipp H, Baumgart F, Cronier P, Jorda E, Klaue K, et al. Integral classification of injuries (ICI) to the bones, joints and ligaments- application to injuries of the foot. Injury, Int J Care Injured. 2004;35:S-B3- S-B9.
41 Haapamaki VV, Kiuru MJ, Koskinen SK. Ankle and foot injuries: analysis of MDCT findings. Am J Roentgenol. 2004;183:615–22.
42 Peicha G, Preidler KW, Lajtai G, Seibert FJ, Grechenig W. Diagnostische Wertigkeit von Nativröntgen, Computer- und Magnetresonanztomographie beim akuten Hyperflexionstrauma des Fusses. [Article in German] Unfallchirurg. 2001;104:1134–9.
43 Ruotolo V, Giurato L, Meloni M, Masala S, Schillaci O, Bergamini A, et al. A new natural history of Charcot foot: clinical evolution and final outcome of stage 0 Charcot’s arthropathy in a tertiary care foot clinic. F-18 FDG PET/CT scan: a useful tool in diagnosis and follow-up of acute Charcot foot. Abstract O4. Diabetic Foot Study Group. Meeting 2012, Potsdam/Germany.
44 Brand PW. Insensitive feet. A practical handbook on foot problems in leprosy.1966, revised 1977. The Leprosy Mission, London. p 52–53.
45 Bailey CC, Root HF. Neuropathic foot lesions in diabetes mellitus. N Engl J Med. 1947;236:397–401.
46 Chantelau E, Wienemann T, Richter A. Pressure pain thresholds in the diabetic Charcot-foot: an exploratory study. J Musculoskelet Neuronal Interact. 2012;12:95–101.
47 American College of Radiology. ACR Appropriateness Criteria®: Suspected Osteomyelitis of the Foot in Patients with Diabetes mellitus. Last review date 2012. http://www.acr.org/Quality-Safety/Appropriateness-Criteria , accessed 9.1.2013.
48 Weishaupt D, Schweitzer ME, Alam F, Karasick D, Wapner K. Imaging of inflammatory joint diseases of the foot and ankle. Skeletal Radiol. 1999;28:663–9.
49 Weishaupt D, Schweitzer ME. MR imaging of the foot and ankle: patterns of bone marrow signal abnormalities. Eur Radiol. 2002;12:416–26.
50 Rios AM, Rosenberg ZS, Bencardino JT, Rodrigo SP, Theran SG. Bone marrow oedema patterns in the ankle and hindfoot: distinguishing MRI features. AJR. 2011;197:W720–W729.
51 Teh J, Suppiah R, Sharp R, Newton J. Imaging in the assessment and management of overuse injuries in the foot and ankle. Semin Musculoskelet Radiol. 2011;15:101–14.
52 Eyres KS, Kanis JA. Bone loss after tibial fracture. J Bone Joint Surg. [Br] 1995;77-B:473–8.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.